The presence of resistance-associated mutations in reverse transcriptase gene is associated with cerebrospinal fluid HIV-1 escape: A multicentric retrospective analysis
Abstract
Higher risk of cerebrospinal fluid escape (CVE) has been associated with the use of specific antiretroviral (ARV) classes, such as protease inhibitors. We assessed whether archived resistance-associated mutations (RAMs) can mediate this relationship by identifying patients treated with incompletely active antiretroviral regimens. A retrospective multicentric study on 282 adult people with HIV on antiretroviral therapy (ART) and available historical plasma genotype resistance testing (HGRT) for reverse transcriptase (RT) and protease genes between 2001 and 2021. The odds ratio for demographic, clinic-, and ART-related variables and CVE was estimated by multivariable modeling. HGRT-adjusted central nervous system effectiveness penetration (CPE) score was computed in modeling the risk. Median age, plasma VL, and CD4 count were 49 years, <50 copies/mL, and 310 cells/μL. CVE was detected in 51 participants (17.0%). No difference in CVE prevalence was observed according to ART type, number of ARVs or ARV classes. Participants with CVE had more frequently plasma (52.9% vs. 32.1%, p = 0.005) and CSF RAMs in RT (n = 63, 57.1% vs. 28.6%, p = 0.029), but not in protease gene. The presence of plasma RAMs in RT associated with increased odds of CVE in adjusted analyses (aOR 3.9, p < 0.001) and in models restricted to plasma viral load ≤50 copies/mL (n = 202; aOR 4.3, p = 0.003). CVE risk decreased by 40% per each point increase in HGRT-adjusted CPE score in multivariable models (p < 0.001). Rather than the type of ARV classes or of ART regimens, functional mono or dual regimens caused by the presence of RAMs affecting ART components may explain the majority of cases of CVE.
1 INTRODUCTION
Modern combination antiretroviral therapy (ART) is highly effective at suppressing viral replication in plasma compartment with HIV persisting in body reservoirs.1 In some cases, cerebrospinal fluid (CSF) viral load (VL) can be significantly above the concentration quantified in plasma. This entity is known as CSF viral escape (CVE) and has been described in 2%–20% of patients in several cohorts worldwide.2-6 Neurologically symptomatic CVE is a rare clinical syndrome that has been often described in the presence of CSF drug resistance associated mutations (RAMs) in HIV genes.7-9 On the contrary, asymptomatic CVE has been more commonly detected in clinical studies of asymptomatic participants undergoing lumbar puncture (LP), where selection bias may exist.8, 10, 11 The long-term implications of CVE remain unclear: longitudinally, patients with detectable CSF HIV RNA may have higher inflammation but no emergence of neurological symptoms.11
Several risk factors for CVE have been reported: the duration of HIV infection, suboptimal adherence, low CD4+ T-cell count nadir, reduced central nervous system (CNS) penetration of antiretrovirals (ARVs), and the use of specific ARVs.4, 12, 13 Regarding the last issue, CVE has been reported more frequently among patients on protease inhibitors (PIs), especially atazanavir-based ART, in Indian and US cohorts,12, 14, 15 and in few more recent cases from Uganda.16 Among PIs, atazanavir does do not consistently achieve in vitro 50% inhibitory concentrations for wild-type HIV in the CSF, while better performance has been achieved by boosted darunavir and lopinavir.17-21 PI-including regimens are usually recommended as second-line ART in many of the above countries after failure to first-line regimens that included non-nucleos(t)ide reverse transcriptase inhibitors (NNRTIs); in these settings, RAMs have already been selected and patients' adherence may had been suboptimal. Indeed, PI-based regimens have been associated to a low risk of RAMs selection when given to ART-naïve patients.22
The possible driver of CVE could be the presence of RAMs and the association with PIs use would just be a consequence rather than the cause of worse pharmacological profile generated by prior virological failures. Nevertheless, to date, no large cohort has ever assessed the role of RAMs in mediating such findings nor the prevalence of cases of CVE that could be explained by ART regimens functionally equivalent to mono or dual therapy after adjusting for the impact of RAMs on the antiretroviral components. Despite nowadays most patients are on INSTI-based regimens that may show reduced risk of CVE, a better clarification of drivers of this compartmentalized virologic failure, especially in relation to the number of active antiretrovirals within the CNS, is still of current concern as it may elucidate potential limitations of modern dual therapies and of salvage regimens in individuals with archived RAMs. Therefore, the aim of our work was to determine the impact of plasma RAMs in reverse transcriptase (RT) and protease (PT) genes on CVE risk in a multicentric European setting.
2 MATERIALS AND METHODS
2.1 Study design and participants
This retrospective cross-sectional study included data from adult PWH with available plasma historical genotype resistance testing (HGRT) in RT and PT genes performed prior LP, attending five Italian Clinics (Amedeo di Savoia Hospital, Turin; L. Spallanzani IRCCS, Rome; Spedali Civili General Hospital, Brescia; Santi Paolo e Carlo Hospital, Milan; Umberto I Hospital, Rome) and one Clinic in the UK (University Hospital Sussex NHS Foundation Trust, Brighton) between January 2001 and December 2021.
Eligible participants were age ≥18 years with at least one paired plasma and CSF VL and on ART since at least 1 year. The temporal criterion of ART duration was chosen to reduce the contribution of CSF slow suppressor (higher CSF VL compared to plasma VL due to slower suppression in CNS after the beginning of ART) to CVE. CVE was defined as (a) plasma VL undetectable by clinical diagnostic assay (100% with lower limit of quantification of 50 copies/mL) and CSF VL ≥ 51 copies/mL; (b) detectable plasma VL with concurrent CSF VL of at least 0.5 Log10 cp/mL higher than plasma.10 PWH with any active inflammatory and/or infectious CNS disorder that could transiently increase CSF HIV RNA (such as CNS infections, autoimmune diseases or conditions increasing cells trafficking across the blood-brain barrier, as previously associated with secondary CVE23-26) were excluded to focus on etiologic mechanisms underlying primary CVE. In case of availability of multiple CSF/plasma pairs per participant, the first pair only was included unless collected on different ART regimens at least 1 year apart. In case of multiple episodes of CVE per participant only the first one was included to avoid selection and sampling bias. The institutional review board at each involved site approved the research.
2.2 Historical genotype resistance testing and HGRT-adjusted ART
Any plasma genotype resistance result recorded before CSF collection was sum up and included in the cumulative HGRT for each participant. A subgroup of patients had resistance testing performed also in CSF; CSF results were described when available but not included in multivariable modeling due to the limited sample size and the likely selection bias.
HGRT was performed with direct full-population sequencing of plasma/CSF samples on the ABI PRISM 3130xl genetic Analyzer (Applied Biosystem) using the ViroSeq HIV-1 genotyping system (Abbott) modified in that a nested PCR step was introduced to enhance the sensitivity for samples with HIV-1 RNA < 1000 copies/mL.27 After nucleic acid extraction from 1 mL of plasma/CSF, sequences of HIV-1 RT and PT regions were constructed for each sample with seven different primers targeting the majority of HIV-1 PT and RT genes, then analyzed by ViroSeq HIV-1 genotyping software vs2.8 (Celera Diagnostics).
Stanford Drug resistance database (http://hivdb.stanford.edu) and the International AIDS Society RAM mutation list (https://www.iasusa.org/resources/hiv-drug-resistance-mutations/) were used to evaluate the impact of RAMs on ART regimen at the time of LP.
The effectiveness of ART at the time of LP was estimated for each participant by adjusting the CNS penetration and effectiveness (CPE) score (a proxy of effectiveness and pharmacokinetics of ARVs within the CNS) for plasma HGRT, as previously suggested.12, 28 Briefly, genotypic susceptibility scores were calculated using GRT results and assigning a score of 0 for resistant, of 0.5 for intermediate resistance, and of 1 for susceptible profile to each drug; HGRT-adjusted CPE scores were thereafter calculated by multiplying the CPE value by the genotypic susceptibility score for each drug regimen and summing the scores.
2.3 Statistical analyses
CVE was compared to demographic, disease, ART, and HGRT variables. Data were analyzed by nonparametric tests (Kruskal–Wallis, Mann–Whitney test, χ2 test for trend). Continuous and categorical variables were reported as medians (interquartile range) and as absolute numbers (proportion). The intrasubject median change in CPE score after adjustment for HGRT was analyzed by paired Wilcoxon test reporting Cohen's D as effect size measure. Univariate and multivariable regressions (logistic regression for binary outcomes, entry method) were run to calculate unadjusted and adjusted odds ratios (OR) of the outcome CVE. Multivariable models included variables with relevant biological significance and those that showed univariate p < 0.05. Sensitivity analyses were conducted in participants with plasma VL < 50 cp/mL. Analyses were performed using StataSE 17 (StataCorp LLC).
3 RESULTS
3.1 Study population
A total of 300 plasma/CSF paired VL from 282 participants met the eligibility criteria. Demographic and HIV-related characteristics of the study population are described in Table 1. Participants were mostly male (67.0%), with a median age of 49 years. Plasma and CSF VL were<50 cp/mL in 202 (67.3%) and 195 (65.0%) participants. Current CD4+ T-cell count was 310 cells/µL with a high prevalence of subjects with nadir CD4+ T-cell count below 200 (75.7%; Table 1). The median duration of any ART was 5 years (30.7% started ART within 2 years) and of current ART was 19 months. ART consisted of standard NRTI-backbone-based triple regimens in 74.7% and less conventional regimens in 25.3% of the cases (three different ARVs classes, dual therapy, monotherapy and ≥4 drugs; see Supporting Information: Table 1); 36.7% of participants were on their first ART regimen. Participants underwent LP in 16.7% of cases due to enrollment in prospective studies involving HIV infection of CNS with no CNS confounding or neurological signs or symptoms, in 46.7% due to clinical indications without eventual CNS involvement (to rule out CNS infections/disorders, due to complaints of neurological signs or symptoms, or due to peripheral lymphomas without meningeal/CNS involvement) and in 36.6% due to self-reported or objectified neurocognitive impairment; no differences in LP indications was observed between participants with and without CVE (Table 1).
| Parameters at LP | Study population (n = 300) | CSF escape (n = 51) | No CSF escape (n = 249) | p Value |
|---|---|---|---|---|
| Age, years | 49 (43–55) | 48 (39–52) | 50 (43–56) | 0.006 |
| Male sex, n | 201 (67.0%) | 28 (54.9%) | 173 (69.5%) | 0.044 |
| Enrollment site, n | 0.075 | |||
| Torino | 113 (37.7%) | 20 (39.2%) | 93 (37.3%) | |
| Spallanzani | 89 (29.6%) | 23 (45.1%) | 66 (26.5%) | |
| Brescia | 52 (17.3%) | 5 (9.8%) | 47 (18.9%) | |
| Milano | 23 (7.7%) | 3 (5.9%) | 20 (8.0%) | |
| Brighton | 14 (4.7%) | 0 | 14 (5.6%) | |
| Sapienza | 9 (3.0%) | 0 | 9 (3.6%) | |
| Calendar year, n | 0.012 | |||
| 2016–2021 | 116 (38.7%) | 13 (25.5%) | 103 (41.4%) | |
| 2011–2015 | 144 (48.0%) | 27 (52.9%) | 117 (47.0%) | |
| 2006–2010 | 30 (10.0%) | 7 (13.7%) | 23 (9.2%) | |
| 2001–2005 | 10 (3.3%) | 4 (7.8%) | 6 (2.4%) | |
| LP indication, n | 0.969 | |||
| RP | 50 (16.7%) | 10 (19.6%) | 40 (16.1%) | |
| CC without CNS involvement | 140 (46.7%) | 21 (41.2%) | 119 (47.8%) | |
| NCI | 110 (36.6%) | 20 (39.2%) | 90 (36.1%) | |
| Plasma HIV-RNA < 50 cp/mL, n | 202 (67.3%) | 35 (68.6%) | 167 (67.1%) | 0.829 |
| Plasma HIV-RNA, Log10 cp/mL | ||||
| Overall | 1.69 (1.69–2.15) | 1.69 (1.69–2.16) | 1.69 (1.69–2.14) | 0.958 |
| When detectable | 2.85 (2.16–4.51) | 2.44 (2.18–3.10) | 3.19 (2.15–5.28) | 0.172 |
| CSF HIV-RNA < 50 cp/mL, n | 195 (65.0%) | 0 | 195 (78.3%) | - |
| CSF HIV-RNA, Log10 cp/mL | ||||
| Overall | 1.69 (1.69–2.10) | 2.18 (1.97–3.62) | 1.69 (1.69–1.69) | <0.001 |
| When detectable | 2.54 (2.07–3.49) | 2.18 (1.97–3.62) | 2.63 (2.16–3.45) | 0.201 |
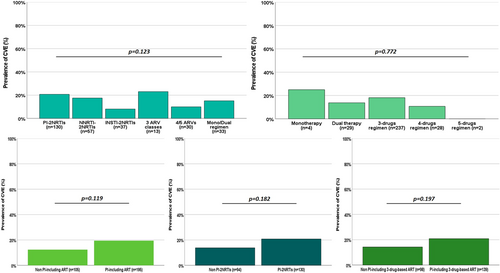
| Type of ART regimen, n | 0.123 | |||
| PI-2NRTIs | 130 (43.3%) | 27 (52.9%) | 103 (41.4%) | |
| nNRTI-2NRTIs | 57 (19.0%) | 10 (19.6%) | 47 (18.9%) | |
| INSTI-2NRTIs | 37 (12.3%) | 3 (5.9%) | 34 (13.6%) | |
| 3 classes-based regimens | 13 (4.3%) | 3 (5.9%) | 10 (4.0%) | |
| ≥4 drugs-based regimens | 30 (10.0%) | 3 (5.9%) | 27 (10.8%) | |
| Mono-dual regimens | 33 (11.0%) | 5 (9.8%) | 28 (11.2%) | |
| PI-including ART, n | 195 (65.0%) | 38 (74.5%) | 157 (63.0%) | 0.119 |
| PI-based mono/dual regimens, n | 29/33 (87.9%) | 5/5 (100%) | 24/28 (85.7%) | 0.999 |
| PI-2NRTIs, n | 130/224 (58.0%) | 27/40 (67.5%) | 103/184 (56.0%) | 0.181 |
| PI-based three-classes regimens, n | 12/13 (92.3%) | 3/3 (100%) | 9/10 (90.0%) | 0.999 |
| PI-based ≥4 drugs-based regimens, n | 24/30 (80.0%) | 3/3 (100%) | 21/27 (77.8%) | 0.999 |
| Number of ARVs | 3 (3–3) | 3 (3–3) | 3 (3–3) | 0.412 |
| Number of ARVs, n | ||||
| 1–2 drugs | 33 (11.0%) | 5 (9.8%) | 28 (11.2%) | 0.765 |
| 3 drugs | 237 (79.0%) | 43 (84.3%) | 194 (77.9%) | 0.307 |
| 4–5 drugs | 30 (10.0%) | 3 (5.9%) | 27 (10.8%) | 0.283 |
| On first ART regimen, n | 110 (36.7%) | 14 (27.4%) | 96 (38.6%) | 0.135 |
| Current CD4+ T-cells count, cells/µL | 310 (139–554) | 310 (215–567) | 310 (122–553) | 0.958 |
| Nadir CD4+ T-cells count, cells/µc | 86 (26–199) | 80 (13–166) | 97 (28–205) | 0.138 |
| Nadir CD4+ T-cells count, n | ||||
| <100 | 159 (53.0%) | 32 (62.7%) | 127 (51.0%) | 0.084 |
| 100–200 | 68 (22.7%) | 10 (19.6%) | 58 (23.3%) | |
| 201–350 | 43 (14.3%) | 8 (15.7%) | 35 (14.1%) | |
| 351–500 | 20 (6.7%) | 0 | 20 (8.0%) | |
| >500 | 10 (3.3%) | 1 (2.0%) | 9 (3.6%) | |
| CD4/CD8 ratio | 0.4 (0.2–0.8) | 0.4 (0.3–0.8) | 0.4 (0.2–0.7) | 0.879 |
| Duration of ART, years | 5 (2–8) | 4 (2–6) | 5 (2–9) | 0.036 |
| Duration of ART, n | ||||
| 1–2 years | 92 (30.7%) | 18 (35.3%) | 74 (29.7%) | 0.030 |
| 3–5 years | 74 (24.7%) | 17 (33.3%) | 57 (22.9%) | |
| 6–10 years | 82 (27.3%) | 14 (27.4%) | 68 (27.3%) | |
| >10 years | 52 (17.3%) | 2 (3.9%) | 50 (20.1%) | |
| Duration of current ART, months | 19 (10–43) | 14 (9–28) | 19 (10–46) | 0.219 |
| Duration of current ART, n | ||||
| <1 year | 144 (48.0%) | 27 (52.9%) | 117 (47.0%) | 0.434 |
| 1–2 years | 82 (27.3%) | 13 (25.5%) | 69 (27.7%) | |
| 3–10 years | 74 (24.7%) | 11 (21.6%) | 63 (25.3%) | |
| Plasma HGRT, n | ||||
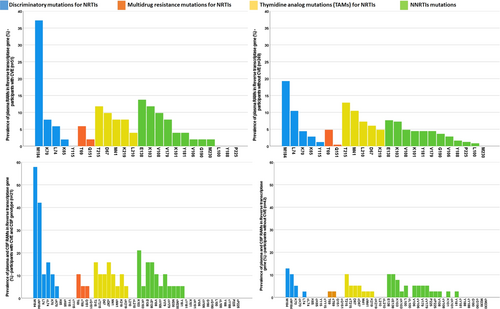
| RT RAMs | 107 (35.7%) | 27 (52.9%) | 80 (32.1%) | 0.005 |
| PI RAMs | 31 (10.3%) | 9 (17.6%) | 22 (8.8%) | 0.060 |
| Total number of plasma RAMs | ||||
| RT | 0 (0–2) | 1 (0–3) | 0 (0–1) | 0.190 |
| PI | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.156 |
| CSF HGRT, n | ||||
| RT RAMs | 24/63 (38.1%) | 12/21 (57.1%) | 12/42 (28.6%) | 0.029 |
| PI RAMs | 8/63 (12.7%) | 5/21 (23.8%) | 3/42 (7.1%) | 0.063 |
| Total number of CSF RAMs | ||||
| RT | 0 (0–1) | 1 (0–3) | 0 (0–1) | 0.177 |
| PI | 0 (0–0) | 0 (0–1) | 0 (0–0) | 0.141 |
| CPE score | 7 (7–8) | 7 (7–8) | 7 (7–8) | 0.921 |
- Abbreviations: ART, antiretroviral regimen; ARVs, antiretroviral drugs; CC, clinical conditions; CNS, central nervous system; CPE, Central nervous system penetration effectiveness score; CSF, cerebrospinal fluid; HGRT, historical cumulative genotype resistance test; INSTI, integrase strand transfer inhibitors; LP, lumbar puncture; NCI, neurocognitive impairment; NNRTIs, non-nucleos(t)ide reverse transcriptase inhibitors; NRTIs, nucleos(t)ide reverse transcriptase inhibitors; PI, protease inhibitors; RAMs, resistance associated mutations; RP, research purposes; RT, reverse transcriptase gene.
3.2 CSF escape
CVE was observed in 51 unique participants (17.0%). CVE was detected a median of 14 months (9–28) after starting current ART (which was the first regimen in 27.4% of participants), and 4 years (2–6) from the first ART. Plasma VL was undetectable in 68.6% (Table 1). The distribution of regimens is shown in Table 1: 38 (74.5%) were on a PI-including regimen with boosted darunavir (35.3%), lopinavir (21.6%) and atazanavir (13.7%) as the most frequent PIs; four participants were on dual regimens: two on dolutegravir plus boosted-darunavir, and two on raltegravir plus either boosted-lopinavir and fosamprenavir; one participant was on monotherapy with boosted-darunavir. As shown in Table 1, prevalence of plasma RAMs in the RT (52.9% vs. 32.1%) and, to a lower extent, in the PT gene (17.6% vs. 8.8%) was higher in participants with CVE. CSF RAMs followed a similar pattern among participants with available data (n = 63; Table 1).
Univariate analysis showed two-fold greater odds of CVE in participants with HGRT positive for plasma RAMs in RT (OR 2.38, p = 0.005). Furthermore, participants with CVE were more likely to be of female sex and to belong to the 2001–2005 period, and less likely to be on ART from more than 10 years (Table 2). No other variable in Table 1 resulted associated with CVE risk. Despite CVE was more common in PI-including regimens, according to any ART classification and comparison, no statistically significant association between CVE and PIs use was observed (Figure 1). Similarly, no difference in CVE prevalence was observed comparing ART regimens by number of ARVs or pooled groups (mono/dual regimens, 3 classes regimens, PI-2NRTIs, NNRTI-2NRTIs, INSTI-2NRTIs and 4/5 ARVs regimens), nor between standard 3 drugs NRTI-backbone-based regimens versus less conventional regimens, nor by third class among standard 3 drugs NRTI-backbone-based regimens (data not shown).
| Covariate | OR (95% CI) | p Value | aOR (95% CI)a | p Value |
|---|---|---|---|---|
| Male sex (female ref.) | 0.535 (0.289–0.988) | 0.046 | 0.543 (0.273–1.080) | 0.082 |
| Calendar year | ||||
| 2016–2021 | Ref. | - | Ref. | - |
| 2011–2015 | 1.828 (0.897–3.729) | 0.097 | 1.960 (0.852–4.507) | 0.113 |
| 2006–2010 | 2.411 (0.866–6.714) | 0.092 | 1.502 (0.471–4.785) | 0.492 |
| 2001–2005 | 5.282 (1.315–21.217) | 0.019 | 2.151 (0.453–10.208) | 0.335 |
| Duration of ART | ||||
| 1–2 years | Ref. | - | Ref. | - |
| 3–5 years | 1.226 (0.581–2.589) | 0.593 | 1.254 (0.540–2.913) | 0.598 |
| 6–10 years | 0.846 (0.391–1.832) | 0.672 | 1.098 (0.454–2.652) | 0.836 |
| >10 years | 0.164 (0.037–0.740) | 0.019 | 0.142 (0.028–0.710) | 0.017 |
| RAMs in RT gene at HGRT | ||||
| Absent | Ref. | - | Ref. | - |
| Present | 2.38 (1.29–4.38) | 0.005 | 3.933 (1.881–8.225) | <0.001 |
- Abbreviations: 95% CI, 95% confidence interval; aOR, adjusted odds ratio; ART, antiretroviral regimen; HGRT, historical cumulative genotype resistance test; OR, odds ratio; RAMs, resistance associated mutations; RT, reverse transcriptase gene.
- a Multivariable binary logistic regression adjusted for age, type of regimen, duration of current ART, CD4+ T-cell nadir, CPE score, and plasma HIV RNA.
Multivariable modeling showed almost four-fold increased odds of CVE (aOR 3.93, p < 0.001) in participants with plasma RAMs in RT after accounting for age, sex, calendar year, duration of current and any ART, type of regimen, CPE score, plasma VL and CD4+ nadir. Duration of ART (aOR 0.14, p = 0.017 for more than 10 years vs. 1–2 years) was an additional independent risk factor (Table 2).
The association between the presence of plasma RAMs in RT and CVE was confirmed in sensitivity analyses limited to participants with plasma VL ≤ 50 copies/mL (n = 202: aOR 4.35, p = 0.003); in this subgroup, duration of ART was not an independent predictor of CVE, while CD4+ nadir (aOR 0.59 per every 100 cells/µL more, p = 0.021) and age (aOR 0.65 per every 10 years more, p = 0.047) were independently associated with CVE (Supporting Information: Table 2).
3.3 HIV-1 genotyping and RAMs
We hypothesized that archived or recently selected RAMs could impair ART efficacy in pharmacologically hard-to-reach anatomic sites (such as the CNS) thus increasing CVE risk.
The type and prevalence of plasma and CSF RAMs in RT in participants with and without CVE are reported in Figure 2. M184V/I was the most common being detected in plasma in 37.2% and 19.3% of participants with and without CVE (p = 0.005). Similarly, in CSF M184V/I was detected in 42.8% and 16.7% of participants with and without CVE (n = 63; p = 0.026).
At LP, 28 participants with CVE (54.9%) were on ART affected by one or more of the RAMs in RT and/or PI gene, while this was the case for 47 participants without CVE (18.9%, OR 5.232 [2.769–9.887], p < 0.001). After accounting for plasma HGRT, the median number of fully active ARVs was significantly lower, but the change had relevant effect size in participants with CVE only (Figure 3B). Adjusting by HGRT, the median CPE score was lower in both groups, but the change had relevant effect size in the CVE group only (Figure 3C). CVE risk decreased by almost 30% per each point increase in HGRT-adjusted CPE score (OR 0.73 [0.62–0.86], p < 0.001).
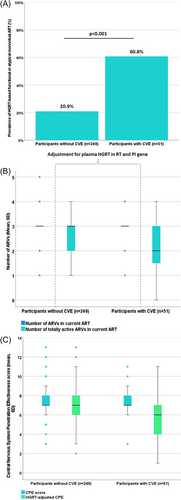
Among the remaining participants with CVE and ARVs unaffected by HGRT (n = 23), three more subjects were on atypical mono/dual regimen: boosted-darunavir, raltegravir plus boosted-lopinavir, and fosamprenavir plus raltegravir. Therefore, after accounting also for atypical regimens, 31 (60.8%) and 52 (20.9%) participants with and without CVE were on functional or atypical mono/dual regimens which significantly associated with increased risk of CVE (OR 5.872 [3.097–11.134], p < 0.001; Figure 3). The presence of plasma RAMs in PT did not contribute relevantly to this association, since results did not change after excluding the two cases of isolated RAMs in PT affecting current ART from the analysis (data not shown).
The substitution of CPE score and plasma RAMs in RT with HGRT-adjusted CPE score as covariate at multivariable modeling supported an independent association between CVE and the impairment of ARVs within the CNS mediated by the resistance phenotype (aOR 0.60 [0.48–0.75], p < 0.001; Supporting Information: Table 3).
Sensitivity analyses among participants with VL < 50 cp/mL were in line with the previous results as the associations between CVE and being on ART regimens affected by plasma RAMs in RT (OR 5.742 [2.622–12.574], p < 0.001), being on HGRT-related functional or atypical mono/dual regimens (OR 6.089 [2.799–13.427], p < 0.001) and with HGRT-adjusted CPE score were retained (OR 0.77 [0.63–0.94], p = 0.009).
3.4 Cases of CVE not associated with HGRT-related functional or atypical mono/dual therapy
Twenty cases (39.2%) of CVE were not associated with HGRT-related functional or atypical mono/dual therapy, so we sought to assess in which features they differ from the other CVE. No statistical differences in demographic, ART- and HIV-related characteristics were found (n = 20 vs. 31; Supporting Information: Table 4). Overall, these 20 participants started the last ART regimen within a year in 70% of cases (vs. 41.9%), despite similar overall duration on any ART; they had higher proportion of plasma suppression (75.0% vs. 64.5%), lower CSF VL, and lower CD4 count, suggesting that at least part of them could have been CSF slow suppressor after viral failure requiring ART change. Thirteen participants had no GRT testing in CSF at LP, and we cannot rule out potential CSF RAMs explaining CVE. The remaining seven (13.7%) cases of CVE without plasma and CSF RAMs affecting ART are described in Supporting Information: Table 5. Among these, three could be alternatively explained as CSF blip or CSF slow suppression, and four cases (7.8% of the CVE cases) remained etiologically elusive.
4 DISCUSSION
In this retrospective multicentric study of PWH on ART (one-third on first-line therapy and two-thirds ART-experienced), neither PIs use, nor the type of ART regimen were associated with differential odds of CVE after accounting for historical drug resistance genotype. Conversely, an almost 4-fold higher odds of CVE was found in participants with plasma RAMs in HIV-1 RT gene. In line with this finding, unadjusted CPE scores did not associate with CVE, while low HGRT-adjusted CPE scores were associated with increased risk of escape. All these findings were also confirmed by sensitivity analyses in participants with well-controlled HIV in plasma samples.
In anatomical reservoirs where drugs reach low inhibitory quotients, partially resistant viruses may replicate and favor the selection of further RAMs, eventually leading to CVE. PIs penetrate the CNS less effectively than other ARVs classes.17-21 Therefore, RAMs in the RT gene may have exposed patients to functional monotherapy leading to loss of virological control in CNS. In our study, CPE values were similar between participants with and without CVE, suggesting that unadjusted values alone may not discriminate those at risk. However, after RAMs were factored into CPE values, median adjusted CPE scores in CSF were lower in participants with CVE, suggesting that accumulation of RAMs combined with the reduced penetration of ARVs within CNS may lead to local ineffective viral control. These results are not in contradiction with previous larger and longitudinal cohorts reporting association between PI use and CVE, but may be complimentary, given the previous lack of genotype resistance assessment.12, 14, 15, 29 Indeed, when GRT was available, CVE has been associated with HIV-1 mutant viruses harboring M184 along with other major RAMs that conferred resistance to at least one ARV composing ART at the time of CVE development.12
PIs use was not associated with CVE. Compared to countries where PIs use has been repeatedly associated with CVE, in Europe PIs have been more commonly used as third companion drug also in first-line ART regimens (and specifically in patients with uncertain adherence), and therefore larger proportion of ART-naïve patients starting on PIs and individuals proactively switching to PI-based regimen have been enrolled in our study population. This could explain why no strong association between PIs use and CVE has been observed in our sample compared to setting where PIs were reserved to patients with virological failure.12, 14, 15 Indeed, in patients treated with NNRTIs plus nonactive companion drugs or with dolutegravir monotherapy, systemic virological failure has been quickly reported,30 while in patients on PI-based functional or actual monotherapy, low-level HIV replication in the CSF may occur despite peripheral viral suppression potentially leading to more frequent detections of CVE.31-34
To the best of our knowledge, this study describes the largest sample of data on genotype resistance testing in reverse transcriptase and protease genes linked to the risk of CVE. It is also the largest data set that assessed factors associated with primary CVE in a clinical setting that adhered to European guidelines for the treatment of HIV infection during the last two decades. While many ART regimens included in this study can be considered currently dismissed by several countries, they are still first-line regimens in many resource-limited countries. Our data should also be useful as framework to understand CVE risk in newer ARVs combinations presenting low CNS penetration as well as to evaluate the efficacy of dual regimens in hard-to-reach compartments. Dual regimens per se may be safe and effective in controlling CNS HIV infection,35 but require proper combinations accounting for virus and patients characteristics, that include historical genotype. Furthermore, while it could be debatable any ART change in CVE cases with no RAMs, we observed that most CVE cases occurred in the presence of RAMs affecting at least one of the antiretrovirals composing the current ART regimen; this finding should prompt to an intensification or change of the current ART regimen regardless of concomitant neurological signs or symptoms and of alterations in CSF analysis or in brain imaging. Based on these findings, a prompt reassessment of the HGRT and of potentially newly selected RAMs in both plasma and CSF seems also highly recommended in any case of symptomatic or asymptomatic CVE. The lack of a longitudinal design indeed does not allow us to evaluate how many of the asymptomatic cases can evolve into symptomatic CVE with no change in the ART regimen.
This study has several limitations, including the retrospective design, the long period of inclusion potentially introducing heterogeneity of regimens and clinical management, potential selection bias due to 16.7% of participants undergoing LP for research purposes, and the lack of a complete GRT assessment in CSF. The lack of data on timing of infection limited the possibility to confirm this variable as relevant for CVE and may explain why other time variables such as age or ART duration were found linked with escape. The lack of an assessment of participants ART adherence may limit the causality hypothesis; individuals with poor adherence could experience more episodes of virological failures leading to RAMs and thereby the association between CVE and RT mutations could be mediated by the lack of adherence rather than the mutations themselves. Nevertheless, our findings were confirmed in sensitivity analyses restricted to participants with plasma suppression, where adherence should be deemed of substantial degree to reach such plasma control. In this regard, it has been demonstrated that CVE can be the consequence of either replication-competent CNS reservoirs during ART or of transient viral production from cells migrating into the CNS36: the confirmed strong association between RAMs and CVE even in the subgroup of participants with undetectable plasma viremia should point toward a larger contribute of the former mechanism in the majority of CVE cases. Despite this is the largest cohort assessing the role of plasma RAMs in CVE risk, some other variables that may contribute to CVE development could have not been detected due to limited statistical power determined by the retrospective design and the relative rarity of CVE occurrence. Lastly, we could not exclude that some CVE cases were secondary to rare infections or inflammatory processes that were not routinely detected. In this regard, tailored studies are warranted to better identifying other mechanisms underlying the rarer cases of CVE (<10%) occurring in the absence of RAMs.
In conclusion, CVE was not associated with PIs use nor with any other type of ART regimen or ARV class. Viruses harboring mutations may favor CVE and the impact of single drug classes such as PIs may lose significance when adjusted for the presence and effect of specific RAMs as most CVE cases were explained by functional dual or mono-therapies due to the presence of archived RAMs in the RT gene.
AUTHOR CONTRIBUTIONS
Mattia Trunfio and Andrea Calcagno conceived and designed the study; Mattia Trunfio, Carmela Pinnetti, Stefania Arsuffi, Francesca Bai, Luigi Celani, Jaime H Vera, and Valeria Ghisetti collected the data; Mattia Trunfio performed the analyses; Carmela Pinnetti, Gabriella D'Ettorre Monforte, Jaime H Vera, Antonella D'Arminio Monforte, Emanuele Focà, Valeria Ghisetti, Stefano Bonora, Andrea Antinori, and Andrea Calcagno supervised the analyses and results interpretation; Mattia Trunfio and Andrea Calcagno wrote the original draft; all the authors reviewed the manuscript and approved the submitted version.
ACKNOWLEDGMENTS
This study was supported by Ricerca Finalizzata RF-2019-12371089 “Significance and long-term clinical and virological evolution of cerebrospinal fluid HIV viral escape,” funded by the Italian Ministry of Health.
CONFLICTS OF INTEREST STATEMENT
M. T., S. B. and A.C.'s Institution received research grants from Janssen-Cilag, MSD, Viiv and Gilead. A. C. and S. B. received speakers' honoraria and consultancy fees from Janssen-Cilag, MSD, Viiv and Gilead. M. T. received research grants by Gilead Sciences. C. P. received personal fee from Gilead Sciences and a travel grant and served on an advisory board for Janssen-Cilag. J. H. V. received travel and research grants from Merck, Janssen-Cilag, ViiV and Gilead. A. D. M. has research grants and advisory board grants by Janssen-Cilag, ViiV, MSD, Gilead. E. F. received research grants, speakers' honoraria, and consultancy fees by Viiv, MSD, Gilead, Janssen-Cilag and SOBI. A. A. has served as a paid consultant to and received payment or honoraria from Gilead Sciences, GlaxoSmithKline, Janssen-Cilag, Merck Sharp and Dohme, Roche, ViiV Healthcare. The remaining authors declare no conflict of interest.
ETHICS STATEMENT
The institutional review board at each involved site approved the research. Informed consent from participants was waived due to the retrospective and minimal risk nature of the study.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, M. T., upon reasonable request.




