Serum extracellular vesicle-derived ASS1 is a promising predictor for the occurrence of HEV-ALF
Ze Xiang, Chun Jiang, and Jiajia Yang contributed equally to this study.
Abstract
Development of biomarkers for predicting the occurrence of hepatitis E virus related-acute liver failure (HEV-ALF) is conducive to prevention and early intervention. Serum samples from 250 HEV-ALF patients, 250 patients with acute hepatitis E (AHE) and 250 health controls (HCs) were collected. We assessed the predictive ability of extracellular vesicle (EV)-derived argininosuccinate synthase 1 (ASS1) levels for HEV-ALF occurrence. Serum EVs were successfully isolated. EV-derived ASS1 levels in the HEV-ALF patients were significantly higher than those in the AHE patients and HCs. In HEV-ALF patients, EV-derived ASS1 levels were positively correlated with the number of failed organs and disease progression. The logistical regression showed that EV-derived ASS1 level is an independent risk factor for HEV-ALF, and orthogonal partial least squares discriminant analysis (OPLS-DA) also suggested that EV-derived ASS1 level has high predictive capability. Besides, the area under the curve (AUC) of EV-derived ASS1 level to predict HEV-ALF occurrence was 0.728 (0.684–0.772) with the sensitivity and specificity being 72.80% and 64.80%, which had a high decision-making ability. Furthermore, there existed no significant difference between the age ≥60 and age <60 groups in EV-derived ASS1 levels. Serum EV-derived ASS1 level is a promising predictor for the occurrence of HEV-ALF.
Abbreviations
-
- ALF
-
- acute liver failure
-
- ASS1
-
- argininosuccinate synthase 1
-
- AUC
-
- area under the curve
-
- DCA
-
- decision curve analysis
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- EVs
-
- extracellular vesicles
-
- HCs
-
- health controls
-
- HEV
-
- hepatitis E virus
-
- NTA
-
- nanoparticle tracking analysis
-
- OPLS-DA
-
- orthogonal partial least squares discriminant analysis
-
- TEM
-
- transmission electron microscopy
1 INTRODUCTION
Hepatitis E is a liver disease caused by hepatitis E virus (HEV), which is one of the main causes of acute viral hepatitis in the world.1 HEV is a positive-sense, single strand RNA virus that is primarily transmitted via fecal-oral route.2 HEV subtypes 1/2/3/4/7 can infect human beings, and its host range is very wide.3, 4 HEV infection is usually a self-limiting disease, and the majority of infections are asymptomatic. However, 5%–30% of HEV-infected patients present acute symptoms,5 and 0.5%–4% will develop acute liver failure (ALF), especially in pregnant women, the elderly or people with underlying liver diseases.6 In pregnant women, the risk of developing ALF increases to 15%–25%.7
Liver failure can be caused by a variety of factors that damage the liver, including hepatitis virus infection, drugs, hepatotoxic substances and so on.8 Early diagnosis, effective treatment and active prevention of complications are essential in ALF patients, and accurate prediction of the ALF occurrence is conducive to prevention and early intervention.9 Nevertheless, specific predictive biomarkers for HEV-ALF have not been effectively studied. Hence, the development of biomarkers for predicting the HEV-ALF occurrence is of great significance.
Argininosuccinate synthase 1 (ASS1) is a key rate-limiting enzyme in the production of arginine, urea and nitric oxide.10 The ASS1 gene, located on chromosome 9 and encoding arginine succinate synthetase, is expressed in many tissues, with the highest levels in liver and kidney.11 In recent years, significance progress has been made in the relationship between ASS1 and liver diseases. Xu et al.12 confirmed that MANF supplementation can significantly improve ethanol-induced liver steatosis in mice by enhancing the ASS1 activity and activating the AMPK pathway. Du and colleagues summarized the metabolic dysregulation and emerging therapeutic targets for hepatocellular carcinoma (HCC), they also mentioned that the expression of ASS1 was significantly decreased in HCC patients, and the stable silencing of ASS1 promoted HCC cell migration and invasion. It was indicated that ASS1 can serve as a potential target for HCC diagnosis and treatment.13 ASS1 can also be used as a classification and clinical diagnostic tool for hepatocellular adenomas.14, 15 Besides, ASS1 gene mutation can cause citrullinemia type I,16 and the occurrence of liver failure was reported in cases with citrullinemia type I.17, 18 Therefore, there may exist a specific link between ASS1 and liver failure although it has not been fully studied.
Extracellular vesicles (EVs) are secreted by many cells, and their derivatives have been confirmed to play a role in prediction, diagnosis and treatment for ALF.19 In this study, we assessed the value of serum EV-derived ASS1 levels in predicting the HEV-ALF occurrence, which may provide new insights into the prediction of HEV-ALF.
2 PATIENTS AND METHODS
2.1 Study populations
From June 1, 2017 to May 31, 2022, a total of 250 HEV-ALF patients and 250 AHE patients were retrospectively screened from Suzhou Municipal Hospital, the First Affiliated Hospital of Zhejiang University School of Medicine, Linyi Traditional Hospital, the First People's Hospital of Yancheng City, the Second People's Hospital of Yancheng City and the Fifth People's Hospital of Wuxi. A total of 250 health controls (HCs) were all from the First People's Hospital of Yancheng City.
AHE inclusion criteria: (1) serum anti-HEV IgM positive, and/or more than two-fold increased titers in anti-HEV IgG; (2) HEV RNA positive with clinical symptoms, showing elevated liver enzymes and/or jaundice and/or nonspecific symptoms, fatigue, itching, and nausea included. Definition of HEV-ALF: (1) abnormal liver function (prothrombin activity ≤40% or international normalized ratio [INR] ≥1.5), jaundice and hepatic atrophy in 2 weeks; (2) stage 2 or 3 encephalopathy complicating end-stage disease; and (3) without chronic liver disease. Exclusion criteria: (1) co-infection with other hepatitis virus, and/or human immunodeficiency virus (HIV); (2) with other liver disease not caused by viral hepatitis; (3) incomplete data.20, 21
Serum samples of AHE and HEV-ALF patients were collected when they were enrolled, and the samples were frozen at −80°C for testing. Clinical data of recruited patients were extracted from hospital medical records. Follow-up data collection was through medical records or telephone interviews with patients and their families. All patients or their families provided the informed consents.
2.2 Isolation of serum EVs
Serum samples were placed on ice and centrifuged at 4°C to remove sediment after they were thawed in the 25°C water bath. 500 μl serum supernatant was collected and added into qEV column (qEVoriginal/35 nm). More buffers were added when the final sample entered under the tube via the column top, and a 1.5 ml EV solution was obtained with high purity after collecting the void volume. Further, samples were added to a new ultrafiltration tube pretreated with 1 × PBS. The ultrafiltration tube was then centrifuged and a certain amount of 1 × PBS was added. Subsequently, the ultrafiltration inner tube was vortexed for 3 min for downstream experiments. We resuspended the isolated EV samples in phosphate buffer to obtain the concentrated EV samples with the volume of 200 μl. and placed them in cryopreservation tubes. Finally, the labeled samples were frozen at −80°C.
2.3 Transmission electron microscopy (TEM)
On the copper wire, 5 μl EV samples were dropped. The samples were incubated at room temperature for 5 min, and after incubation, we carefully absorbed the excess liquid with filter paper. Afterwards, we added a drop of 2% uranium dioxyacetate to the copper web. The samples were incubated at room temperature for 1 min, and we also used filter paper to absorb the excess liquid. The samples were dried at room temperature for 20 min, and at last, we observed the samples under the TEM (HT-7700, Hitachi).
2.4 Nanoparticle tracking analysis (NTA)
We thawed the frozen samples in the water bath at 25°C and next placed them on ice.
After diluted with 1 × PBS (1: 800), the samples can be directly used for NTA under the nano-particle tracking analyzer (ZetaVIEW S/N 20-602, PARTICLE METRIX).
2.5 Western blot
EV samples were resuspended using 1 × PBS, and lysed by adding RIPA lysate of equal volume. EV proteins were separated by SDS-PAGE gel and then transferred to PVDF membrane. PVDF membrane was further blocked in the skim milk for 1 h after transfer, and it was next incubated with primary antibody overnight at 4°C. Secondary antibody was added after washing to remove unbound primary antibody, and the membrane was incubated for 30 min at 25°C. The antibodies used were following: CD9 (Abcam, ab92726), CD63 (Abclonal, A5271), TSG101 (Abcam, ab125011), APOA 1 (Santa Cruz, sc-376818), APOB (Novus Biologicals 20737), and ALB (Santa Cruz, sc-271605).
2.6 Detection of anti-HEV antibodies and HEV RNA
In all patients, anti-HEV IgM and IgG antibody presence was determined using the HEV enzyme-linked immunosorbent assay (ELISA) kit (Wantai). The optical density (OD) >1.1 was considered as positive, while ≤1.1 was considered negative. Real-time reverse transcription-quantitative PCR (RT-qPCR) was used for the detection of HEV RNA. Total viral RNA from the samples was extracted using the viral nucleic acid extraction kit (Aikang).
2.7 ELISA
The levels of EV-derived ASS1 were detected using the ELISA kit (MSKBIO, No. 201908). The concentrated EVs were diluted 1:500 using 1 × PBS. We used 100 μl RIPA lysate to precipitate the EV samples on ice for 30 min. The samples were then diluted 1:3 with 1 × PBS. The blank control and standard substance were added to a microplate, and they were coated with ASS1 antibody. 100 μl diluted samples were added to the microplate. The plate was incubated for 1 h at 37°C, after which we discarded the liquid in the microplate and dried the plate. Then, 100 μl liquid A was added and incubated for 1 h at 37°C. After washing for 3 times, liquid B of equal volume was added and incubated for 30 min. The plate was also washed for 3 times. Next, 100 μl substrate solution was added. The plate was incubated in the dark at 37°C for 20 min, and 50 µl stop solution was added to terminate the reaction. At last, the plates were read at 450 nm wavelength with a microplate reader.
2.8 Statistical analysis
In this study, SPSS 26.0, GraphPad Prism 9 and MedCalc were used for statistical analysis. If the data is normally distributed, mean ± standard deviation was used, and t-test was performed to compare between two groups. One-way ANOVA was conducted among three groups, and LSD method was used for pairwise comparison among three groups. If not, median (quartile) was used, and Mann–Whitney U test was performed between two groups. Kruskal–Wallis H test was conducted among three groups, and Bonferroni method was used for pairwise comparison among three groups. The relationship between serum EV-derived ASS1 levels (Y) and HEV-related parameters (X) was analyzed using the linear regression. To determine the independent risk factors for the occurrence of HEV-ALF, we conducted the univariate and multivariate logistic regression analyses. Area under the curve (AUC) with 95% confidence interval was calculated by MedCalc, and the sensitivity and specificity were also given. Decision curve analysis (DCA) was used by the R rmda package to evaluate the benefit of serum EV-derived ASS1 levels in assisting decision-making at different threshold probabilities. Besides, we analyzed all data for orthogonal partial least squares discriminant analysis (OPLS-DA) through SIMCA (Version 14.1.0.2047, 64-bit, MKS Umetrics). A p < 0.05 was statistically significant.
3 RESULTS
3.1 Characteristics of isolated serum EVs
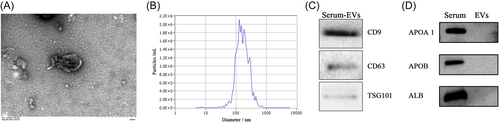
Under TEM, EVs isolated from serum were found to be rounded or round-like in shape with a diameter of 40–100 nm, which had a complete envelope and clear background (Figure 1A). NTA was used to measure the particle size and distribution. It was revealed that the particle size was concentrated between 65.9 and 249.3 nm, and the maximum distribution peak was 157.6 nm (Figure 1B). Through western blot, the expression of several marker proteins was shown, including CD9, CD63, and TSG101 (Figure 1C), while the expression of APOA 1, APOB, and ALB was not detected (Figure 1D).
3.2 Expression of serum EV-derived ASS1
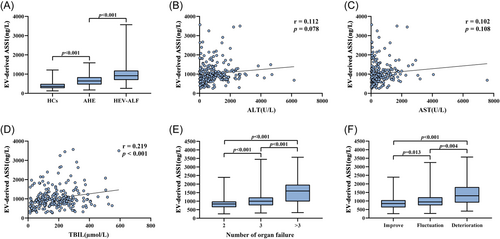
The serum EV-derived ASS1 levels were detected in 250 HCs, 250 AHE patients and 250 HEV-ALF patients using ELISA. It was found that the levels of serum EV-derived ASS1 in the HEV-ALF group were significantly higher than those in the AHE group [HEV-ALF: 915.28 (691.96–1186.58) ng/L vs. AHE: 643.09 (457.68–841.68) ng/L, p < 0.001]. The serum EV-derived ASS1 levels in the AHE group were significantly higher than those in the HCs group [AHE: 643.09 (457.68–841.68) ng/L vs. HCs: 359.96 (269.92–492.90) ng/L, p < 0.001; Figure 2A]. The difference in the serum EV-derived ASS1 expression among three groups showed that the higher serum EV-derived ASS1 levels, the more severe the condition of patients with HEV infection, indicating the HEV-ALF occurrence.
3.3 Relationship between serum EV-derived ASS1 levels and dynamic changes in HEV-ALF patients
To clarify the relationship between serum EV-derived ASS1 levels and the condition of HEV-ALF patients, the correlation between serum EV-derived ASS1 levels and HEV-related parameters was assessed. The serum EV-derived ASS1 levels positively correlated with the levels of TBIL (r = 0.219, p < 0.001; Figure 2D), while neither significant difference between serum EV-derived ASS1 levels and ALT levels was discovered (r = 0.112, p = 0.078; Figure 2B), nor significant difference between EV-derived ASS1 levels and AST levels (r = 0.102, p = 0.108; Figure 2C).
Organ failure often occurs in HEV-ALF patients during the disease period. According to the number of failed organs, HEV-ALF patients were divided into the number 2 group (n = 150), number 3 group (n = 59) and number >3 group (n = 41). The levels of serum EV-derived ASS1 in the number 2 group were significantly lower than those in the number 3 group [Number 2: 835.02 (646.83–985.58) ng/L vs. Number 3: 997.94 (756.29–1221.75) ng/L, p < 0.001], and the levels of serum EV-derived ASS1 in the number 3 group were significantly lower than those in the number >3 group [Number 3: 997.94 (756.29–1221.75) ng/L vs. Number >3: 1593.55 (981.92–1966.08) ng/L, p < 0.001; Figure 2E].
Furthermore, based on the dynamic changes of HEV-ALF patients' condition, we divided HEV-ALF patients into the improvement group (n = 131), fluctuation group (n = 86) and deterioration group (n = 33). It was discovered that the levels of serum EV-derived ASS1 in the improvement group were significantly lower than those in the fluctuation group [Improvement: 849.69 (623.99–1050.75) ng/L vs. Fluctuation: 943.12(743.26–1219.18) ng/L, p = 0.013], and the levels of serum EV-derived ASS1 in the fluctuation group were significantly lower than those in the deterioration group [Fluctuation: 943.12 (743.26–1219.18) ng/L vs. Deterioration: 1305.33 (906.00–1816.94) ng/L, p = 0.004; Figure 2F].
3.4 Serum EV-derived ASS1 level is an independent risk factor for the HEV-ALF occurrence
We described the clinical characteristics of 250 HCs, 250 AHE patients and 250 HEV-ALF patients through Table 1. The mean age of the HEV-ALF group was 56.50 ± 11.58 years, that of the AHE group was 53.68 ± 12.39 years, and that of the HCs group was 54.70 ± 11.67 (p = 0.028). There was no statistically significant difference in gender among the three groups (p > 0.05). In the HEV-ALF group, 61.20% of patients were male, 54.80% in the AHE group were male, and 51.60% in the HCs group were male (p > 0.05). Besides, no significant difference existed in BMI among the three groups (p > 0.05).
| Variables | HCs (n = 250) | AHE (n = 250) | HEV-ALF (n = 250) | p Value |
|---|---|---|---|---|
| Age (year) | 54.70 ± 11.67 | 53.68 ± 12.39 | 56.50 ± 11.58a | 0.028 |
| Males (%) | 129 (51.60) | 137 (54.80) | 153 (61.20) | 0.089 |
| BMI | 24.07 ± 2.95 | 23.63 ± 3.25 | 23.86 ± 3.08 | 0.290 |
| EV-derived ASS1 (ng/L) | 359.96 (269.92–492.90) | 643.09 (457.68–841.68)b | 915.28 (691.96–1186.58)b,a | <0.001 |
| WBC (×109/L) | 6.96 (5.70–8.15) | 5.88 (4.67–7.23)b | 6.04 (4.60–7.30)b | <0.001 |
| RDW | 13.60 (12.40–15.10) | 14.20 (13.00–16.00)b | 14.10 (13.10–16.00)b | <0.001 |
| CRP (mg/L) | 6.20 (4.08–8.32) | 8.08 (3.76–13.66)b | 11.55 (5.76–18.51)b,a | <0.001 |
| PLT (109/L) | 201.50 (164.75–239.00) | 195.00 (144.75–257.25) | 162.50 (112.75–223.00)b,a | <0.001 |
| PT (s) | 12.00 (11.60–12.50) | 16.55 (13.08–19.00)b | 17.80 (14.45–20.82)b,a | <0.001 |
| ALT (U/L) | 16.00 (12.00–19.25) | 522.50 (268.25–828.50)b | 641.50 (246.75–1328.00)b | <0.001 |
| AST (U/L) | 21.00 (16.00–26.00) | 204.50 (115.50–283.25)b | 356.10 (109.00–755.25)b,a | <0.001 |
| GGT (U/L) | 25.30 (19.25–32.02) | 147.75 (80.00–260.02)b | 142.00 (78.00–261.82)b | <0.001 |
| TP (g/L) | 70.85 (66.90–74.82) | 65.05 (60.58–69.90)b | 61.15 (55.28–65.35)b,a | <0.001 |
| ALB (g/L) | 43.46 ± 4.68 | 37.74 ± 5.39b | 34.71 ± 5.63b,a | <0.001 |
| TBIL (μmol/L) | 11.70 (8.30–14.18) | 29.90 (21.30–104.52)b | 170.10 (95.30–252.72)b,a | <0.001 |
| UREA (mmol/L) | 4.86 (3.36–5.84) | 4.49 (3.48–5.56) | 4.38 (3.46–5.82) | 0.621 |
| CR (μmol/L) | 75.90 (67.18–82.52) | 64.00 (52.08–77.70)b | 65.75 (55.00–78.10)b | <0.001 |
| TG (mmol/L) | 1.07 (0.82–1.28) | 1.88 (1.22–2.72)b | 1.98 (1.21–2.98)b | <0.001 |
| TCH (mmol/L) | 4.25 (3.66–4.78) | 3.74(3.10–4.79)b | 3.05 (2.43–3.93)b,a | <0.001 |
| CHE (U/L) | 15286.50 (11725.75–19594.00) | 6183.00 (4477.00–7782.00)b | 4559.00 (3484.25–5459.50)b,a | <0.001 |
| Total T3 (nmol/L) | 2.36 (2.08–2.68) | 1.44 (1.09–1.72)b | 1.19 (0.84–1.57)b,a | <0.001 |
| Total T4 (nmol/L) | 100.44 (88.98–116.65) | 110.42 (83.90–136.56)b | 107.56 (79.71–138.46) | 0.042 |
| TSH (mIU/L) | 2.32 (1.65–2.83) | 1.44 (0.80–2.05)b | 1.09 (0.53–1.65)b,a | <0.001 |
| AFP (ng/ml) | 3.50 (2.30–4.72) | 6.20 (3.18–12.85)b | 9.65 (4.10–24.15)b,a | <0.001 |
| FER (ng/ml) | 77.00 (44.00–107.00) | 769.50 (345.75–1266.50)b | 2498.50 (957.00–4581.50)b,a | <0.001 |
- Abbreviations: AHE, acute hepatitis E; HCs, health controls; HEV-ALF, hepatitis E virus related-acute liver failure.
- a Compared with AHE group, p < 0.05.
- b Compared with HCs, p < 0.05.
All patients in the HCs, AHE and HEV-ALF groups underwent laboratory tests. When comparing the levels of laboratory parameters among the three groups, we identified significant differences in serum EV-derived ASS1, WBC, RDW, CRP, PLT, PT, ALT, AST, GGT, TP, ALB, TBIL, CR, TG, TCH, CHE, TT3, TT4, TSH, AFP, and FER levels (all p < 0.05). In contrast, there existed no significant difference in UREA level (all p > 0.05).
Univariate and multivariate logistic regression analyses were performed to determine risk factors of the HEV-ALF occurrence (Table 2). Univariate logistic regression analysis showed that age and the levels of serum EV-derived ASS1, PLT, PT, ALT, AST, TP, ALB, TBIL, TCH, CHE, TSH, and FER were significantly different between the AHE group and the HEV-ALF group (all p < 0.05). Moreover, multivariate logistic regression analysis suggested that serum EV-derived ASS1, TP, TBIL, CHE, TSH, and FER were independent risk factors for the occurrence of HEV-ALF (all p < 0.05).
| Variables | AHE (n = 250) | HEV-ALF (n = 250) | p Value | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | ||||
| Age (year) | 53.68 ± 12.39 | 56.50 ± 11.58 | 0.009 | 1.020 (1.005–1.035) | 0.009 | ||
| Males (%) | 137 (54.80) | 153 (61.20) | 0.147 | 1.301 (0.911–1.857) | 0.147 | ||
| BMI | 23.63 ± 3.25 | 23.86 ± 3.08 | 0.410 | 1.024 (0.968–1.082) | 0.409 | ||
| EV-derived ASS1 (ng/L) | 643.09 (457.68–841.68) | 915.28 (691.96–1186.58) | <0.001 | 1.003 (1.002–1.003) | <0.001 | 1.002 (1.001–1.003) | <0.001 |
| WBC (×109/L) | 5.88 (4.67–7.23) | 6.04 (4.60–7.30) | 0.429 | 1.075 (0.988–1.169) | 0.093 | ||
| RDW | 14.20 (13.00–16.00) | 14.10 (13.10–16.00) | 0.921 | 1.022 (0.940–1.111) | 0.608 | ||
| CRP (mg/L) | 8.08 (3.76–13.66) | 11.55 (5.76–18.51) | <0.001 | 1.003 (0.993–1.013) | 0.578 | ||
| PLT (109/L) | 195.00 (144.75–257.25) | 162.50 (112.75–223.00) | <0.001 | 0.995 (0.992–0.997) | <0.001 | ||
| PT (s) | 16.55 (13.08–19.00) | 17.80 (14.45–20.82) | <0.001 | 1.105 (1.056–1.155) | <0.001 | ||
| ALT (U/L) | 522.50 (268.25–828.50) | 641.50 (246.75–1328.00) | 0.003 | 1.001 (1.000–1.001) | <0.001 | ||
| AST (U/L) | 204.50 (115.50–283.25) | 356.10 (109.00–755.25) | <0.001 | 1.002 (1.001–1.002) | <0.001 | ||
| GGT (U/L) | 147.75 (80.00–260.02) | 142.00 (78.00–261.82) | 0.722 | 1.000 (0.999–1.000) | 0.325 | ||
| TP (g/L) | 65.05 (60.58–69.90) | 61.15 (55.28–65.35) | <0.001 | 0.902 (0.876–0.929) | <0.001 | 0.950 (0.909–0.993) | 0.022 |
| ALB (g/L) | 37.74 ± 5.39 | 34.71 ± 5.63 | <0.001 | 0.906 (0.876–0.937) | <0.001 | ||
| TBIL (μmol/L) | 29.90 (21.30–104.52) | 170.10 (95.30–252.72) | <0.001 | 1.010 (1.008–1.012) | <0.001 | 1.005 (1.002–1.007) | <0.001 |
| UREA (mmol/L) | 4.49 (3.48–5.56) | 4.38 (3.46–5.82) | 0.992 | 1.053 (0.989–1.121) | 0.106 | ||
| CR (μmol/L) | 64.00 (52.08–77.70) | 65.75 (55.00–78.10) | 0.343 | 1.002 (0.999–1.004) | 0.204 | ||
| TG (mmol/L) | 1.88 (1.22–2.72) | 1.98 (1.21–2.98) | 0.365 | 1.128 (0.974–1.305) | 0.108 | ||
| TCH (mmol/L) | 3.74 (3.10–4.79) | 3.05 (2.43–3.93) | <0.001 | 0.552 (0.465–0.656) | <0.001 | ||
| CHE (U/L) | 6183.00 (4477.00–7782.00) | 4559.00 (3484.25–5459.50) | <0.001 | 1.000 (0.999–1.000) | <0.001 | 1.000 (1.000–1.000) | <0.001 |
| Total T3 (nmol/L) | 1.44 (1.09–1.72) | 1.19 (0.84–1.57) | <0.001 | 1.004 (0.991–1.016) | 0.554 | ||
| Total T4 (nmol/L) | 110.42 (83.90–136.56) | 107.56 (79.71–138.46) | 0.575 | 1.001 (0.997–1.005) | 0.676 | ||
| TSH (mIU/L) | 1.44 (0.80–2.05) | 1.09 (0.53–1.65) | <0.001 | 0.674 (0.552–0.823) | <0.001 | 0.920 (0.704–1.203) | 0.542 |
| AFP (ng/ml) | 6.20 (3.18–12.85) | 9.65 (4.10–24.15) | 0.002 | 1.001 (1.000–1.002) | 0.102 | ||
| FER (ng/ml) | 769.50 (345.75–1266.50) | 2498.50 (957.00–4581.50) | <0.001 | 1.001 (1.001–1.001) | <0.001 | 1.001 (1.000–1.001) | <0.001 |
- Abbreviations: AHE, acute hepatitis E; HEV-ALF, hepatitis E virus related-acute liver failure.
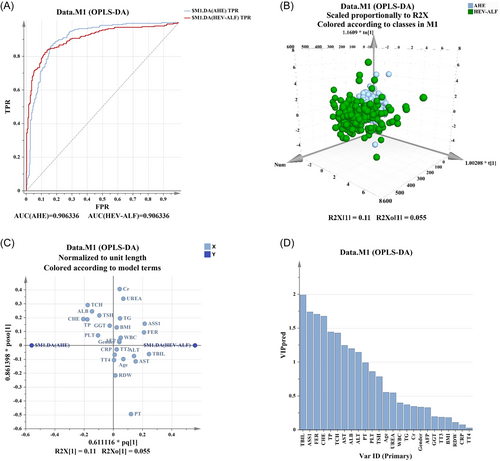
In addition, OPLS-DA was carried out to further rank and evaluate the prediction ability of these factors. The results suggested these factors can unambiguously distinguish patients in the two groups (Figure 3A,B). The serum EV-derived ASS1 level was considered as the main indicator, with the second highest predictive capability only to the level of TBIL (Figure 3C,D).
3.5 Prediction ability of serum EV-derived ASS1 levels for the occurrence of HEV-ALF
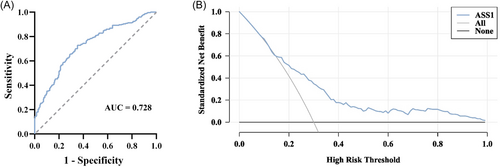
The ability of serum EV-derived ASS1 levels to predict the occurrence of HEV-ALF was evaluated. According to ROC curve analysis, the AUC of serum EV-derived ASS1 levels to predict the HEV-ALF occurrence was 0.728 (0.684–0.772), and the sensitivity and specificity were 72.80% and 64.80%, respectively (Figure 4A). Additionally, DCA results found that the levels of serum EV-derived ASS1 had a high decision-making ability in predicting the occurrence of HEV-ALF (Figure 4B).
3.6 Expression of serum EV-derived ASS1 in the elderly with HEV-ALF
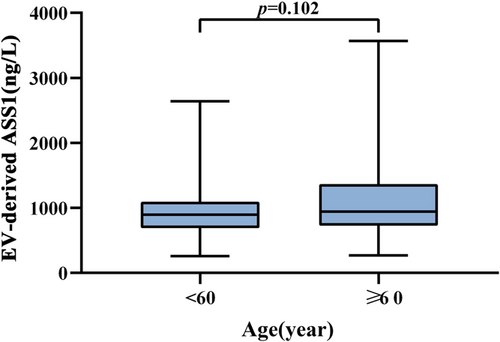
If infected with HEV, pregnant women, the elderly and people with underlying liver diseases are more likely be severe. In our study, patients with other liver diseases have been excluded and no pregnant women were found, therefore the levels of serum EV-derived ASS1 of the elderly were explored. Patients with HEV-ALF were divided into the age <60 group (n = 147) and age ≥60 group (n = 103) depending on whether they were elderly or not. The levels of serum EV-derived ASS1 in the age ≥60 group were higher than those in the age <60 group, but no significant difference was found (Age ≥60: 943.31 (727.94–1367.38) ng/L vs. Age <60: 896.22 (688.97–1094.48) ng/L, p = 0.102; Figure 5).
4 DISCUSSION
ALF is a life-threatening disease with sudden severe hepatic dysfunction. Symptoms of ALF include jaundice, fever, nausea, and vomiting. If patients with ALF do not receive timely treatment, the condition will deteriorate rapidly, thereby resulting a high mortality rate.22, 23 In developed countries, the common causes of ALF include paracetamol toxicity, ischemia, drug-induced liver injury, HBV and autoimmunity, and in developing countries, ALF is mainly caused by viral hepatitis.24 ALF caused by HEV infection has gained much attention in recent years, but specific biomarkers that can predict the HEV-ALF occurrence have not been sufficiently studied. In previous studies, urea cycle disorders have been proved to be closely associated with liver diseases.25 As a key rate-limiting enzyme of urea cycle, ASS1 has been confirmed to play a pivotal role in liver steatosis and HCC.12, 13 Therefore, we aimed to explore the prediction ability of ASS1 for the occurrence of HEV-ALF. This study was the first to explore the clinical value of serum EV-derived ASS1 levels in predicting the HEV-ALF occurrence, which fully indicated that serum EV-derived ASS1 is a promising predictor for the occurrence of HEV-ALF.
In this study, serum EVs were successfully isolated from the samples of patients. TEM clearly revealed the shape of isolated EVs. NTA was used to measure the particle size and distribution, and the particle size of EVs was concentrated between 65.9 and 249.3 nm, with the maximum distribution peak being 157.6 nm. Besides, EV biomarkers such as CD9, CD63, and TSG101 were identified through the western blot, while the expression of APOA 1, APOB, and ALB was not detected. In general, TEM, NTA, and western blot results confirmed the integrity and purity of isolated EVs.
Subsequently, the levels of serum EV-derived ASS1 were detected in all enrolled patients. It was observed that serum EV-derived ASS1 levels in the HEV-ALF group was significantly higher than those in the AHE group, and serum EV-derived ASS1 levels in the AHE group were significantly higher than those in the HCs group. Hence, we speculated that the higher serum EV-derived ASS1 levels in the HEV-infected patients, the more severe the patients' condition and the greater the risk of developing HEV-ALF.
We further assessed the relationship between serum EV-derived ASS1 levels and the condition of HEV-ALF patients, a positive linear correlation existed between serum EV-derived ASS1 and TBIL levels. The levels of TBIL can be used to evaluated the condition of HEV-ALF patients, which suggested that the levels of serum EV-derived ASS1 can also be closely associated with the severity of HEV-ALF patients. In addition, the higher the level of serum EV-derived ASS1, the greater the number of failed organs in patients with HEV-ALF, therefore the number of failed organs in the HEV-ALF patients was positively correlated with serum EV-derived ASS1 levels. Of note, the levels of serum EV-derived ASS1 in the improvement group were significantly lower than those in the fluctuation group, and the levels of serum EV-derived ASS1 in the fluctuation group were significantly lower than those in the deterioration group. Thus, in terms of the dynamic changes of the disease, an increase of serum EV-derived ASS1 levels in the HEV-ALF patients can reflect the worsening of the disease.
Moreover, serum EV-derived ASS1, TP, TBIL, CHE, TSH, and FER were found to be independent risk factors for the HEV-ALF occurrence. OPLS-DA results demonstrated that these factors can well distinguish patients in the AHE group and HEV-ALF group, and serum EV-derived ASS1 level had the high predictive capability. The AUC of serum EV-derived ASS1 levels was 0.728 (0.684–0.772), with the sensitivity and specificity being 72.80% and 64.80%. Additionally, the levels of serum EV-derived ASS1 were proved to have a great decision-making ability to predict the occurrence of HEV-ALF. This suggested that serum EV-derived ASS1 levels own promising clinical value in predicting the occurrence of HEV-ALF.
Finally, we analyzed the expression of serum EV-derived ASS1 in the elderly with HEV-ALF. The elderly infected with HEV are at higher risk of developing ALF. In our study, the levels of serum EV-derived ASS1 in the age ≥60 group were higher than those in the age <60 group, although there existed no significant difference. The results also indicated that the levels of serum EV-derived ASS1 are correlated with the severity of HEV-infected patients, further confirming the value of serum EV-derived ASS1 level as a predictor of the HEV-ALF occurrence.
The role of ASS1 in liver injury has been preliminarily discussed. Our study found that the higher the levels of serum EV-derived ASS1 in patients infected with HEV, the greater the risk of the HEV-ALF occurrence. This was consistent with the conclusion that a decrease in serum arginine levels is associated with the reduction of liver tissue damage in chronic HBV infection.26 In addition, Qin et al.27 verified that the increased levels of ASS1 can effectively predict drug-induced liver injury, whose effect is better than ALT and AST. Similarly, Vazquez et al.28 also showed that ASS1 can be used as a sensitive biomarker to detect liver injury in mice and humans. However, how serum EV-derived ASS1 levels reflect the condition of HEV-infected patients remains unclear, and its mechanism needs further investigation.
There are several limitations in this study. First, although this study has demonstrated the value of serum EV-derived ASS1 levels in predicting the occurrence of HEV-ALF, more patients are needed to verify the conclusion. Second, we only analyzed the serum EV-derived ASS1 levels in the elderly with HEV-ALF, the expression of serum EV-derived ASS1 in pregnant women and people with underlying liver diseases should be explored. Third, it is still unclear whether serum EV-derived ASS1 levels have prognostic value in HEV-ALF patients. More clinical data needs to be collected to explore its prognostic value.
5 CONCLUSION
In conclusion, serum EV-derived ASS1 levels can predict the occurrence of HEV-ALF and are closely related to the severity of HEV-infected patients. This study will facilitate early intervention of HEV-infected patients who may become severe and prevent further deterioration of the disease, thus promoting better diagnosis and treatment of HEV-ALF patients.
AUTHOR CONTRIBUTIONS
Guarantor of this work: Jian Wu. Experimental design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, statistical analysis: Jian Wu, Ze Xiang, Chun Jiang, and Jiajia Yang. Conducted the main experiments: Lan Huang and Bin Jiang. Collected serum and completed the follow-up: Xuanli Wang, Ce Gao, Mo Li, Yiling Meng, Ling Tong, and Bai Ling. Critical revision of the manuscript: Ying Wang and Jian Wu.
ACKNOWLEDGMENTS
The authors thank all the doctors and scientists in the CCSHE open cohort study for their selfless dedication and help to complete the study successfully. This study was supported by the National Natural Science Foundation of China (No. 82272396) and Suzhou Medical and Health Science and Technology Innovation Project (No. SKY2022057).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of Suzhou Municipal Hospital (Approval number: K-2022-080-H01).
Open Research
DATA AVAILABILITY STATEMENT
All data relevant to the study are included in the article.




