A study on the morbid histopathological changes in COVID-19 patients with or without comorbidities using minimally invasive tissue sampling
Abstract
COVID-19 causes morbid pathological changes in different organs including lungs, kidneys, liver, and so on, especially in those who succumb. Though clinical outcomes in those with comorbidities are known to be different from those without—not much is known about the differences at the histopathological level. To compare the morbid histopathological changes in COVID-19 patients between those who were immunocompromised (Gr 1), had a malignancy (Gr 2), or had cardiometabolic conditions (hypertension, diabetes, or coronary artery disease) (Gr 3), postmortem tissue sampling (minimally invasive tissue sampling [MITS]) was done from the lungs, kidney, heart, and liver using a biopsy gun within 2 hours of death. Routine (hematoxylin and eosin) and special staining (acid fast bacilli, silver methanamine, periodic acid schiff) was done besides immunohistochemistry. A total of 100 patients underwent MITS and data of 92 patients were included (immunocompromised: 27, malignancy: 18, cardiometabolic conditions: 71). In lung histopathology, capillary congestion was more in those with malignancy, while others like diffuse alveolar damage, microthrombi, pneumocyte hyperplasia, and so on, were equally distributed. In liver histopathology, architectural distortion was significantly different in immunocompromised; while steatosis, portal inflammation, Kupffer cell hypertrophy, and confluent necrosis were equally distributed. There was a trend towards higher acute tubular injury in those with cardiometabolic conditions as compared to the other groups. No significant histopathological difference in the heart was discerned. Certain histopathological features were markedly different in different groups (Gr 1, 2, and 3) of COVID-19 patients with fatal outcomes.
1 INTRODUCTION
Coronavirus disease 2019 (COVID-19) has emerged as a global threat to public health since its first documented case from China in late December 2019. COVID-19 is caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV2), which is a highly contagious virus that spreads via the respiratory route.1 The virus has undergone several mutations and has given rise to several variants over the years, including the recent Omicron variant (B.1.1.529), which was first reported in November 2021.2
COVID-19 is postulated to enter the respiratory tract and is primarily a respiratory illness with COVID-19 pneumonia being the most commonly encountered serious clinical manifestation.3, 4 It can range from a mild, self-limiting pulmonary insult to frank acute respiratory distress syndrome (ARDS) requiring invasive mechanical ventilation with poor clinical outcomes5 and various complications.6-10 Other organ systems are also affected apart from the respiratory system with protean manifestations. Involvement of the cardiovascular system, gastrointestinal tract, kidneys, and liver have been reported ranging from asymptomatic laboratory aberrations to multiorgan failure related to cytokine storm.11 The main mechanism postulated for viral invasion into host cells is the high affinity of the virus for membrane-bound ACE2 (angiotensin-converting enzyme) receptors expressed on them,12 which has also been demonstrated in postmortem samples.13, 14 ACE2 expression has been reported in the lungs, heart, kidneys, small intestine, adrenal gland, and testes among others.15 In addition, the course of COVID-19 can be complicated by the development of a multisystem inflammatory catastrophe with widespread organ manifestations. Drugs used in the treatment of COVID-19, notably the immunomodulating ones like steroids, tocilizumab, and so on, have also been linked to numerous complications, indicating a complex relationship between the agent (virus), host (humans), and environment (drugs, comorbidities, etc.).7, 16
Our understanding about this novel virus and its transmission-virulence characteristics have improved greatly since the inception of the pandemic owing to the large amount of clinical data available. However, histopathological data regarding various changes at the tissue level is equally important and may have therapeutic implications. Autopsy remains the gold standard in this regard but poses a challenge considering the risk of transmission. Minimally invasive tissue sampling (MITS) offers a quicker, safer, and easier alternative in this regard.
Certain histopathological features which have been commonly reported in prior autopsy/biopsy studies include diffuse alveolar damage (DAD), superimposed pneumonia, myocarditis, hepatitis, and microthrombi formation in tissues.17 This study presents the clinical and histopathological data of 92 patients with severe COVID-19 who had a fatal outcome and provides deeper insights into the biological basis for the clinical manifestations and the extent of organ involvement.
2 METHODS
This was a single-center observational study conducted at Jai Prakash Narayan Apex Trauma Center (JPNATC), AIIMS, New Delhi, between May 2020 to August 2021. We had previously reported the postmortem histopathological features in a small subset of the patients and the present study includes the complete series.18
One hundred patients who died of COVID-19 were included. All patients were diagnosed with COVID-19 antemortem using reverse transcription polymerase chain reaction (RT-PCR)/TrueNAT/antigen testing. The study was approved by the Institute Ethics Committee (Ref No: IEC-536/05.06.2020). Ethical consent was obtained from the next of kin for postmortem tissue sampling.
Data were collected for age, gender, presenting complaints, vitals, physical signs, hospital course, relevant laboratory investigations, chest radiographs, and treatment received.
Postmortem tissue sampling was attempted from the lungs, kidney, heart, and liver using a biopsy gun within 2 hours of death. Sites for sampling were selected using surface land marks under ultrasonographic guidance, when available. The site was cleaned using chlorhexidine followed by insertion of the charged biopsy gun into the target organ. Four-six tissue specimens were collected from each organ using the same skin orifice with each core being 1–3 cm in size. Heart specimen was obtained from the left para-sternal approach, lung specimens from the midaxillary approach, kidney specimen from the lumbar or posterior approach, and liver specimens from the lower midaxillary approach.
The tissue samples were placed in vials containing freshly prepared 10% buffered formalin and transported to the laboratory on the same day at room temperature while maintaining standard precautions. The patients were divided into three groups - Group 1: Any immunocompromised state like post-transplant status, auto-immune diseases, chronic immunosuppressants use. Group 2: any solid organ or hematological maligancies. Group 3: If the patients did not qualify for either group 1 or 2 and had a cardiometabolic condition like hypertension, diabetes, or coronary artery disease as mentioned in previous treatment records/history sheet.
2.1 Histopathological processing and examination
The samples obtained by MITS were grossed in a Biosafety cabinet-2 (BSC-2). The slides obtained from paraffin-embedded blocks were stained by routine hematoxylin and eosin stain. Morphology of the organ tissue (lung, heart, kidney, liver) submitted was studied and recorded by pathologists having expertise in a particular organ system. Special staining (acid fast bacilli, silver methanamine, periodic acid schiff) and immunohistochemistry were done to supplement the basic microscopic findings when needed. Each biopsy sample was independently evaluated by two senior pathologists (D. J. and A. S. for lungs, P. S. and A. S. for liver, S. A. and A. S. for heart, G. S. and A. S. for kidney) and any differences in opinion was mutually resolved by discussion.
2.2 Data analysis
Demographic and histopathological data were entered in a preformed excel sheet and analyzed using STATA. Findings were compared in patients with or without underlying malignancy, and cardiometabolic comorbidities (diabetes mellitus, hypertension, or coronary artery disease).
3 RESULTS
3.1 Demographic characteristics
A total of 92 patients who had adequate biopsy samples (for any of the organs sampled) for a satisfactory histopathological examination were included in the study. The majority of them were males (66.3%). The mean age was 51.3 ± 18.03 years. Most of the patients had at least one comorbidity (88%) with hypertension being the most common (37%) followed by diabetes mellitus (31.5%) and malignancy (19.6%).
The median day of illness at admission was 4 days (1–60) and the median day of illness at death was 15 (2–68). The median duration of hospital stay was 9 (1–51). Most of the patients presented with severe acute respiratory illness (65.2%) with 21.7% coming with non-respiratory symptoms like altered sensorium, abdominal distension, pain, and so on. Patients with underlying malignancy were more likely to present with non-severe acute respiratory illness (SARI) complaints (61.1% vs. 28.3%, p = 0.01). At presentation, 58.7% patients were hypoxemic and 39.7% patients had tachypnoea (Table 1).
| With demographic variable | Total (n = 92) |
|---|---|
| N (%) | |
| Mean age (years) | 51.3 ± 18.03 |
| Males (%) | 61 (66.3%) |
| Comorbidities | |
| At least one comorbidity | 81 (88%) |
| Diabetes mellitus | 29 (32%) |
| Hypertension | 34 (37%) |
| Hypothyroidism | 2 (2%) |
| Malignancy | 18 (20%) |
| Chronic kidney disease | 14 (15%) |
| Chronic liver disease | 7 (8%) |
| Past pulmonary tuberculosis | 3 (3%) |
| Coronary artery disease | 9 (10%) |
| Aplastic anemia | 2 (2%) |
| Post-transplant | 2 (2%) |
| COPD | 3 (3%) |
| SLE | 2 (2%) |
| Hep B | 2 (2%) |
| ILD | 1 (1%) |
| Congenital heart disease | 1 (1%) |
| Median day of illness at admission in days (IQR) | 4 (1–60) |
| Median day of illness at death in days (IQR) | 15 (2–68) |
| Median duration of hospital stay in days (IQR) | 9 (1–51) |
| Symptoms | |
| SARI | 60 (65.2%) |
| Respiratory symptoms (not SARI) | 12 (13%) |
| Nonrespiratory symptoms | 20 (21.7%) |
| Vital signs | |
| Hypotension (MAP < 65 mmHg) | 9/78 (11.5%) |
| Hypoxemia (SpO2 < 94%) | 47/80 (58.7%) |
| Tachypnoea (RR ≥ 30/min) | 31/78 (39.7%) |
| Low GCS (≤13) | 22/75 (29.3%) |
| Laboratory parameters: | |
| Anaemia | 68/88 (77.3%) |
| Lymphopenia (ALC < 1000) | 18/36 (50%) |
| Thrombocytopenia (<100 000/mm3) | 26/87 (29.9%) |
| Renal dysfunction | 46/87 (52.9%) |
| Hyperbilirubinemia | 18/80 (22.5%) |
| Transaminitis (>2 × ULN) | 9/74 (12.2%) |
| Metabolic acidosis | 30/42 (71.4%) |
| Elevated inflammatory markers | |
| Ferritin (>500) | 29/44 (65.9%) |
| CRP (>100 mg/L) | 32/41 (78%) |
| D-dimer (>500 ng/l) | 19/21 (90.5%) |
| Chest radiograph | |
| Abnormal chest radiograph | 77/83 (92.8%) |
| Typical COVID findings | 48/83 (57.8%) |
| Area involved >50% | 66/83 (79.5%) |
| Management (at admission) | |
| Antibiotics | 91/91 (100%) |
| Anticoagulation | 66/82 (80.5%) |
| Remdesivir | 20/85 (23.5%) |
| Steroids | 82/91 (90.1%) |
- Abbreviations: ALC, absolute lymphocyte count; COPD, chronic obstructive pulmonary disease; GCS, glasgow coma scale; ILD, interstitial lung disease; MAP, mean arterial pressure; RR, respiratory rate; SARI, severe acute respiratory illness; SLE, systemic lupus erythematosus; ULN, upper limit of normal.
Complete blood count revealed anemia in 77.3% patients with lymphopenia and thrombocytopenia seen in 50% and 29.9% patients respectively. Inflammatory markers were elevated in most patients: Ferritin (65.9%), CRP (78%) and D-dimer (90.5%). Organ failure including renal dysfunction was seen in 52.9% and hyperbilirubinemia in 22.5%. Differences in presentation and laboratory parameters between patients with varying comorbidities are presented in Table 2.
| Parameter | Malignancy | Immunocompromised | Cardiometabolic comorbidities | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes (N = 18) | No (N = 74) | p Value | Yes (N = 31) | No (N = 61) | p Value | Yes (N = 41) | No (N = 51) | p Value | |
| Mean age (years) | 40.9 ± 8.7 | 50 ± 4.6 | <0.001 | 50.7 ± 6.9 | 47 ± 5.2 | 0.99 | 56.2 ± 6.6 | 42.8 ± 4.7 | 1 |
| Males (%) | 61.1% | 67.6% | 0.603 | 70.9% | 63.9% | 0.5 | 70.7% | 62.7% | 0.421 |
| Hypotension (MAP < 65) | 23.5% | 8.2% | 0.098 | 17.8% | 8% | 0.27 | 2.8% | 2.2% | 1 |
| Hypoxemia (SPO2 < 94%) | 40% | 63.1% | 0.1 | 50% | 63.5% | 0.243 | 55.6% | 38.6% | 0.131 |
| Tachypnoea (RR ≥ 30/min) | 26.7% | 42.8% | 0.197 | 32.1% | 44% | 0.305 | 37.8% | 26.8% | 0.298 |
| Low GCS (≤13) | 25% | 30.5% | 0.76 | 35.7% | 25.5% | 0.349 | 38.2% | 19.5% | 0.072 |
| SARI | 38.9% | 71.6% | 0.009 | 70.9% | 62.3% | 0.409 | 75.6% | 56.9% | 0.061 |
| Anemia | 94.4% | 72.8% | 0.05 | 93.3% | 68.9% | 0.01 | 67.5% | 85.4% | 0.05 |
| Lymphopenia (ALC < 1000) | 50% | 50% | 1 | 52.9% | 47.3% | 0.738 | 50% | 50% | 1 |
| Thrombocytopenia (<100 000) | 55.5% | 23.1% | 0.008 | 34.4% | 27.6% | 0.508 | 25% | 34% | 0.358 |
| Renal dysfunction (Creatinine > 1.2) | 38.9% | 56.5% | 0.182 | 76.7% | 43.8% | 0.003 | 58.9% | 47.9% | 0.89 |
| Hyperbilirubinemia (T bilirubin > 2) | 43.7% | 17.1% | 0.023 | 35.7% | 17.3% | 0.065 | 11.1% | 31.8% | 0.03 |
| Transaminitis (>2 × ULN) | 7% | 13.3% | 1 | 24% | 20.4% | 0.723 | 15.2% | 37.5% | 0.033 |
| Abnormal chest radiograph | 82.4% | 95.5% | 0.063 | 96.4% | 90.9% | 0.359 | 100% | 86.4% | 0.017 |
| Typical COVID findings | 47.15 | 60.6% | 0.313 | 64.35 | 54.7% | 0.407 | 56.4% | 59.1% | 0.805 |
| Area involved >50% | 64.7% | 82.1% | 0.119 | 92.6% | 78.9% | 0.117 | 82.15 | 81.8% | 0.978 |
- Abbreviations: GCS, glasgow coma scale; MAP, mean arterial pressure; RR, respiratory rate; SARI, severe acute respiratory illness; ULN, upper limit of normal.
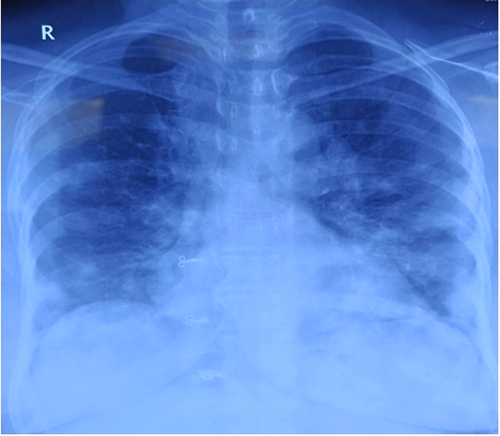
Abnormal chest radiograph was seen in 92.8% with 57.8% of them having bilateral basal subpleural opacities (Figure 1). Most of the patients were managed with steroids (90.1%) and anticoagulation was given to 80.5%, while remdesivir was administered in 23.5% patients.
3.2 Lung histopathology
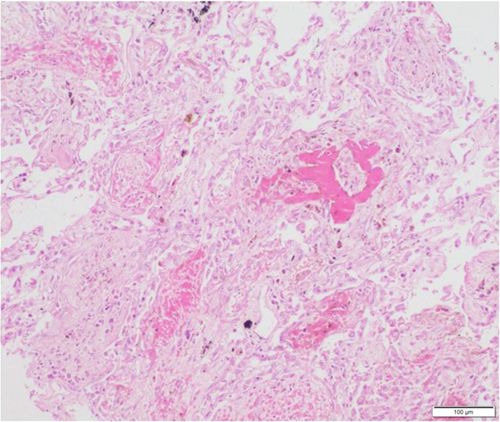
Lung biopsies were reported for 81 patients. DAD was seen in 44 of them (54.3%). Proliferative phase of DAD with hyaline membranes were present in 29 patients and 15 patients had organizing DAD showing fibroblastic proliferation (Figure 2). Immunostaining using anti-SARS-CoV-2 antibody was done in 71 cases in lung biopsies, out of which 14 came positive (patchy cytoplasmic granularity) in alveolar epithelial cells (Figure 3). Ten patients had acute lung injury. Nine patients had normal lung tissue. Associated bronchopneumonia was present in 29 patients (35.8%). Four patients (4.9%) had evidence of bacterial colonies and broad aseptate hyphae was seen in lung specimens of two patients (Table 3).

| Parameter | Immunocompromised | Malignancy | Cardiometabolic comorbidities | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes (N = 28) | No (N = 53) | p Value | Yes (N = 15) | No (N = 66) | p Value | Yes (N = 30) | No (N = 51) | p Value | |
| Acute lung injury | 3 (10.7%) | 7 (13.2%) | 1 | 4 (26.7%) | 6 (9.1%) | 0.08 | 2 (6.7%) | 8 (15.7%) | 0.31 |
| Proliferative DAD | 6 (21.4%) | 23 (43.4%) | 0.05 | 5 (33.3%) | 24 (36.4%) | 0.83 | 13 (43.3%) | 16 (31.4%) | 0.28 |
| Acute organizing DAD | 3 (10.7%) | 12 (22.6%) | 0.24 | 0 (0%) | 15 (22.7%) | 0.06 | 6 (20%) | 9 (17.7%) | 0.79 |
| Bronchopneumonia | 13 (46.4%) | 16 (30.2%) | 0.15 | 2 (13.3%) | 27 (40.9%) | 0.07 | 12 (40%) | 17 (33.3%) | 0.55 |
| Capillary congestion | 1 (3.6%) | 3 (5.7%) | 1 | 3 (20%) | 1 (1.5%) | 0.02 | 0 (0%) | 4 (7.8%) | 0.29 |
| Bacterial colonies | 2 (7.1%) | 2 (3.8%) | 0.66 | 1 (6.7%) | 3 (4.6%) | 0.57 | 0 (0%) | 4 (7.8%) | 0.29 |
| Microthrombi | 3 (10.7%) | 6 (11.3%) | 1 | 3 (20%) | 6 (9.1%) | 0.35 | 1 (3.3%) | 8 (15.7%) | 0.14 |
| Pneumocyte hyperplasia | 2 (7.1%) | 6 (11.3%) | 0.71 | 1 (6.7%) | 7 (10.6%) | 1 | 4 (13.3%) | 4 (7.8%) | 0.46 |
| Pulmonary edema | 2 (7.1%) | 5 (9.4%) | 1 | 1 (6.7%) | 6 (9.1%) | 1 | 2 (6.7%) | 5 (9.8%) | 1 |
| Alveolar hemorrhage | 2 (7.1%) | 3 (5.7%) | 1 | 1 (6.7%) | 4 (6.1%) | 1 | 1 (3.3%) | 4 (7.8%) | 0.65 |
| Pigment laden macrophages | 1 (3.6%) | 1 (1.9%) | 1 | 1 (6.7%) | 1 (1.5%) | 0.34 | 0 (0%) | 2 (3.9%) | 0.53 |
| Normal lung | 5 (17.9%) | 4 (7.6%) | 0.26 | 2 (13.3%) | 7 (10.6%) | 0.67 | 6 (20%) | 3 (5.9%) | 0.07 |
- Abbreviation: DAD, diffuse alveolar damage.
Microthrombi was visualized in nine patients (11.1%). Capillary congestion and alveolar hemorrhage were present in four and five patients respectively. Pulmonary edema was seen in seven patients.
Scattered pneumocyte hyperplasia was seen in eight patients (9.9%). Three patients showed evidence of malignancy.
Immunocompetent patients had higher prevalence of exudative DAD (43.4% vs. 21.4%, p = 0.049). Patients with underlying malignancy were more likely to have acute lung injury (26.7% vs. 9.1%, p = 0.08) and less likely to have organizing DAD (0% vs. 22.7%, p = 0.06). There was no difference in histopathological findings in patients with or without cardiometabolic comorbidities.
3.3 Liver histopathology
Liver biopsies were obtained in 83 patients. The architecture was maintained in 67 of them (80.7%). Steatosis was seen in 62 patients (74.7%), more commonly in patients with no underlying malignancy (79.4% vs. 53.3%, p = 0.04) and no difference among patients with or without cardiometabolic comorbidities (Table 4).
| Parameter | Immunocompromised | Malignancy | Cardiometabolic comorbidities | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes (N = 29) | No (N= 54) | p Value | Yes (N = 15) | No (N = 68) | p Value | Yes (N = 36) | No (N = 47) | p Value | |
| Architecture not maintained | 10 (34.5%) | 6 (11.1%) | 0.01 | 5 (33.3%) | 11 (16.2%) | 0.13 | 5 (13.9%) | 11 (23.4%) | 0.28 |
| Steatosis | 20 (69%) | 42 (77.8%) | 0.38 | 8 (53.3%) | 54 (79.4%) | 0.04 | 30 (83.3%) | 32 (68.1%) | 0.11 |
| Portal inflammation | 14 (48.3%) | 22 (40.7%) | 0.51 | 9 (60%) | 27 (39.7%) | 0.15 | 13 (36.1%) | 23 (48.9%) | 0.24 |
| Acute hepatitis | 13 (44.8%) | 19 (35.2%) | 0.39 | 6 (40%) | 26 (38.2%) | 0.90 | 13 (36.15) | 19 (40.4%) | 0.69 |
| Cholestasis | 12 (41.4%) | 15 (27.8%) | 0.21 | 6 (40%) | 21 (30.9%) | 0.50 | 6 (16.7%) | 21 (44.7%) | 0.01 |
| Kupffer cell hypertrophy | 17 (58.6%) | 31 (57.4%) | 0.92 | 8 (53.3%) | 40 (58.8%) | 0.70 | 20 (55.6%) | 28 (59.6%) | 0.71 |
| Centrizonal confluent necrosis | 4 (13.8%) | 11 (20.4%) | 0.56 | 3 (20%) | 12 (17.6%) | 1 | 6 (16.7%) | 9 (19.1%) | 0.77 |
| Vascular thrombi | 3 (10.3%) | 2 (3.7%) | 0.38 | 1 (6.7%) | 4 (5.9%) | 1 | 2 (5.6%) | 3 (6.4%) | 1 |
Portal inflammation and acute hepatitis were seen in 36 patients (43.4%) and 32 patients (38.6%) respectively. Kupffer cell hypertrophy was present in 57.8% patients. Centrizonal confluent necrosis was seen in 15 patients (18.1%). Vascular thrombi were present in five patients (6%).
Evidence of cholestasis was seen in 27 patients (32.5%), less likely in patients with cardiometabolic comorbidities (16.7% vs. 44.7%, p = 0.01).
3.4 Heart histopathology
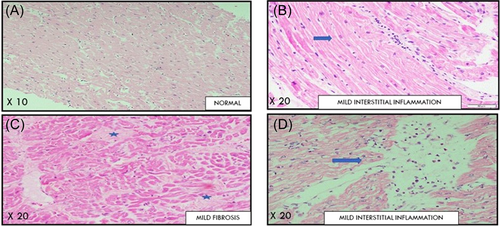
Myocardial biopsy was available for 26 patients, 3 of whom had underlying malignancy, 9 were immunocompromised, and 13 had cardiometabolic comorbidities. The myocardium was normal in 20 patients (80.0%) (Table 5). Borderline myocarditis was appreciated in a single patient. None of the specimens showed any evidence of vascular microthrombi. Background changes of cardiac hypertrophy and focal fibrosis were seen in four and two patients respectively (Figure 4).
| Parameter | Malignancy | Immunocompromised | Cardiometabolic comorbidities | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes (N = 3) | No (N = 23) | p Value | Yes (N = 9) | No (N = 17) | p Value | Yes (N = 13) | No (N = 13) | p Value | |
| Borderline myocarditis | 0 (0%) | 1 (4.3%) | 1 | 1 (11.1%) | 0 (0%) | 0.35 | 0 (0%) | 1 (7.7%) | 1 |
| Cardiac myocyte hypertrophy | 0 (0%) | 4 (17.4%) | 1 | 1 (11.1%) | 3 (17.6%) | 1 | 2 (15.4%) | 2 (15.4%) | 1 |
| Focal fibrosis | 0 (0%) | 2 (8.7%) | 1 | 0 (0%) | 2 (11.8%) | 0.53 | 2 (15.4%) | 0 (0%) | 0.48 |
| Vascular thrombi | 0 (0%) | 0 (0%) | - | 0 (0%) | 0 (0%) | - | 0 (0%) | 0 (0%) | - |
| Normal myocardium | 3 (100%) | 17 (73.9%) | 1 | 7 (77.8%) | 13 (76.5%) | 1 | 11 (84.6%) | 9 (69.2% | 0.65 |
3.5 Kidney histopathology
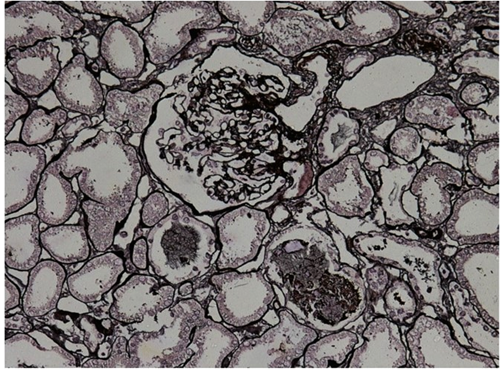
Kidney biopsies were obtained for 41 patients. Acute tubular injury was present in 35 patients (85.4%) (Figure 5). Interstitial fibrosis was seen in 11 patients (26.8%). Background changes of arteriolar hyalinosis were present in five patients (12.2%) and eight patients (19.5%) had glomerulosclerosis. Glomerular thrombi and glomerulonephritis were present in one patient each. Vacuolisation was seen in seven patients (17%). The changes were consistent when patients were compared for underlying malignancy, immunocompromised conditions or cardiometabolic comorbidities. Tubular injury was slightly more common in patients with cardiometabolic comorbidities but did not reach significance (100% vs. 77.7%, p = 0.078) (Table 6).
| Parameter | Malignancy | Immunocompromised | Cardiometabolic comorbidities | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes (N = 7) | No (N = 34) | p Value | Yes (N = 14) | No (N = 27) | p Value | Yes (N = 14) | No (N = 27) | p Value | |
| Acute tubular injury | 7 (100%) | 28 (82.4%) | 0.57 | 12 (85.7%) | 23 (85.2%) | 1 | 14 (100%) | 21 (77.7%) | 0.08 |
| Vacuolisation | 2 (28.6%) | 5 (14.7%) | 0.58 | 0 (0%) | 7 (25.9%) | 0.07 | 2 (14.3%) | 5 (18.5%) | 1 |
| Interstitial nephritis | 0 (0%) | 1 (2.9%) | 1 | 0 (0%) | 1 (3.7%) | 1 | 0 (0%) | 1 (3.7%) | 1 |
| Interstitial fibrosis | 0 (0%) | 11 (32.4%) | 0.16 | 6 (42.9%) | 5 (18.5%) | 0.14 | 5 (35.7%) | 6 (22.2%) | 0.46 |
| Arteriolar hyalinosis | 2 (28.6%) | 3 (8.8%) | 0.19 | 2 (14.3%) | 3 (11.1%) | 1 | 3 (21.4%) | 2 (7.4%) | 0.32 |
| Glomerulosclerosis | 0 (0%) | 8 (23.5%) | 0.31 | 5 (35.7%) | 3 (11.1%) | 0.10 | 4 (28.6%) | 3 (11.1%) | 0.21 |
| Glomerular thrombi | 0 (0%) | 1 (2.9%) | 1 | 1 (7.1%) | 0 (0%) | 0.34 | 0 (0%) | 1 (3.7%) | 1 |
| Glomerulonephritis | 0 (0%) | 2 (5.9%) | 1 | 0 (0%) | 2 (7.4%) | 0.54 | 2 (14.3%) | 0 (0%) | 0.11 |
4 DISCUSSION
In this study, we looked at the histopathological findings of 92 patients who died with COVID-19 in a tertiary care center in North India using MITS technique. A systematic review had showed that the pooled prevalence of mortality among hospitalized patients with COVID-19 was around 17% with older age, male gender, presence of comorbidities, and current smoking being significant risk factors.19 A review done by Sahni et al. showed that 64% of the patients had one of four comorbidities (cardiovascular disease/hypertension/diabetes mellitus/chronic lung disease) with the most prevalent being hypertension (27%). Risk of critical or fatal COVID-19 significantly increased with the presence of hypertension (odds ratio [OR]: 2.51, 2.13–2.95), diabetes (OR: 2.27, 1.87–2.74), and cardiovascular disease (3.44, 2.65–4.48).20 Our study showed a mean age of deceased patients to be 50 years with majority being males. Majority of the patients had one or more comorbidities. Comparison between the histopathological features of patients with or without comorbidities in our study demonstrated that in those immunosuppressed, exudative DAD was less common.
Pulmonary histopathology of COVID-19 patients usually shows a myriad of changes involving the epithelium, interstitium, and vasculature. DAD, alveolar edema, pneumocyte hyperplasia, metaplasia of alveolar epithelium, capillary congestion, vascular thrombosis, fibrin deposition, and interstitial fibrosis have all been described with DAD being the predominant finding.21 The proliferative phase characterized by capillary congestion, hyaline membrane formation, and alveolar edema was appreciated in 35.8% of our patients. Organizing phase of DAD characterized by fibroblastic proliferation, thickened interalveolar septa, and alveolar fibrin deposits were seen in 18.5% of patients. A review done by Menezes et al. reported a higher percentage of patients showing early phase of DAD (78.4%). Most of the patients in our study died after the second week of illness. Bronchopneumonia characterized by focal or diffuse neutrophilic infiltration was seen in more than one-third of the patients but very few of them had evidence of superadded bacterial infection. In our study, microthrombi were seen in only 11% of the patients. Studies done earlier show a higher prevalence of widespread thrombo-emboli with microangiopathy (50%). The likely reason for this discrepancy is due to the fact that most of our patients were on therapeutic anticoagulation as part of the national treatment guidelines. In contrast, most of the reported literature comes from studies done earlier before routine anticoagulation was a standard of care.22
The histological changes of COVID-19 have been reported to be similar to those seen in acute lung injury due to other viral agents (SARS coronavirus, influenza, adenovirus). Similar to previous reports, our study showed that early changes of the proliferative phase during the first week of illness were characterized by edema, epithelial damage, and endothelitis followed by the organizing/fibrotic phase, which showed septal thickening and fibrin deposits. These changes did not differ significantly between patients with different underlying host factors.23 Patients with severe COVID-19 are at an increased risk to develop secondary fungal infections with aspergillus or mucor.24-27 A review showed the prevalence of COVID-19 associated pulmonary aspergillosis (CAPA) to be around 8.6%. Destruction of the bronchial mucosa and alveolar injury, COVID-19 associated immune-dysregulation, and immunosuppressive treatment is thought to create favorable conditions for fungal growth.28 In our study, four patients had evidence of superadded bacterial colonies and in two patients broad aseptate hyphae were identified suggestive of mucormycosis. All of these patients had comorbidities or had received immunosuppressants.
Liver biopsy was obtained for 83 patients in our study with steatosis being the most common finding (77%). This is similar to the study done by Lagana et al. (75%) and the likely explanation being the high burden of cardiometabolic co-morbidities in the study population and that non-alcoholic fatty liver disease patients have higher risk of developing severe COVID-19 (OR: 2.60).29, 30 Various factors contribute to hepatic injury in COVID-19 including exaggerated immune responses, hypoxia, cardiac congestion, direct viral cytopathic effects, endothelitis, microthrombi formation, and drug-related toxicity. Hepatic dysfunction in COVID-19 most commonly manifests as mild-moderate elevations in transaminases and bilirubin, as was seen in our study (12.2% and 22.5%, respectively). Portal inflammation was appreciated in 43.4% of our patients with centrizonal confluent necrosis seen in 18.1%. Sonzogni et al. reported slightly higher but similar prevalence of such findings (portal inflammation: 66%, all mild; 35% showed confluent necrosis with 12% involving >50% of the lobule).31 They also reported a high incidence of vascular thrombosis (portal: 73%, sinusoidal: 26%), which was not seen in our study as well as other studies. Vascular thrombi were seen in a minority of patients (6% in this study and 12% as reported by Lagana et al.).29 Kupffer cell proliferation was identified in 57.8% of our patients. Other studies have also reported high numbers suggesting their possible pathogenetic role.22 Although there was no obvious bile duct injury noted, cholestasis was seen in almost one-third of our patients similar to that shown by Lagana et al.29
A systematic review done by Almamlouk et al.32 to look at postmortem myocardial histopathology identified 50 studies with a total of 548 patients. The median age was 69 years and median time of death being 9 days. The prevalence of extensive myocarditis ranged from 0% to 19.3% with median of 0%. Myocyte damage across 15 studies was only 0.6%. This was similar to our study population, where only a single patient (out of 26) had some evidence of myocarditis. Microvessel thrombi was found with a median prevalence of 36.2% (IR: 17.5%–61.7%). We did not identify any microthrombi probably due to the routine use of anticoagulation in our patients. Chronic cardiac findings were present in most of the patients, with hypertrophy being the most common (69%, interquartile range [IQR]: 46.8%–92.1%) probably due to the high burden of comorbidities present in the study population. The review also identified eight studies that showed amyloidosis with a median prevalence of 13.6% (IQR: 9.85%–17.4%). Amyloidosis was mostly senile-related (ATTR).33 We, however, did not identify any amyloid material probably due to the younger mean age group of our study and possible sampling limitations.
Renal involvement in COVID-19 is mostly secondary to terminal events and background disease. This is reflected in the high prevalence of acute tubular injury found in our study (85.4%) and is consistent with previous studies. Arterial hyalinosis and glomerulosclerosis were the second most common finding as shown in other studies (19.5% and 32.3%).22 Although we did not find any obvious evidence of viral cytotoxicity, studies using ultrastructural examination by electron microscopy have demonstrated viral particles in 14% of the patients and viral PCR positivity in 10 out of 14 cases.34 Glomerular congestion and platelet-fibrin-aggregates forming thrombi inside peritubular capillaries were seen in only one patient which was low compared to other reports (10.5%).22 Collapsing glomerulopathy has been found in majority of the native kidney biopsies done for nephrotic range proteinuria during COVID-19 with APOL1 genetic mutation being a predisposing factor. Similar findings have not been, however, replicated in postmortem biopsies.35
In this study, we also looked at the differences between patients with and without malignancies/cardiometabolic diseases/immunocompromised states. Patients with underlying malignancy who died with COVID-19 were less likely to have SARI or DAD and had higher prevalence of bicytopenia, hyperbilirubinemia, and abnormal liver architecture. This indicated that other reasons could have contributed to their mortality besides COVID-19 (e.g., sepsis due to immunosuppression). This emphasizes the need for clinicians to consider other differentials in patients with comorbidities (e.g., malignancy) as COVID-19 could be a potential bystander leading to insignificant or no health consequences. We had previously shown that a significant proportion of hospitalized patients at our facility had prior serological evidence of COVID-19 with no definitive history suggesting that asymptomatic infection is common in our population.36, 37 Patients with cardiometabolic comorbidities or immunocompromised state had almost identical histopathological findings as compared to patients without them. This indicates similar pathogenic mechanisms of COVID-19 irrespective of host immunity and the utility of a common treatment strategy for all patients with severe COVID-19.
This study adds to the growing literature of pathological findings in COVID-19 showing that lung is the primary organ involved, with DAD being the predominant finding. We compared the findings in different groups of patients (immunocompromised/cardiometabolic comorbidities/malignancies) and did not appreciate any significant intergroup differences demonstrating common mechanisms of COVID-19-related pathological injury. No other study has compared these subgroups of patients.
In our study SARS CoV-2 subtyping was not done due to resource constraints— which was a limitation of our study. However, 80% of our patients were recruited during the first wave of the pandemic. No significant differences were observed among the samples obtained during the subsequent waves indicating that the histopathological changes were similar across different waves. Further, in our cohort of COVID-19 patients, we did not routinely test for other co-infections (due to limited access to specialized tests in the COVID-19 center), which might have contributed to the pathological changes. However, in case of clinical suspicion, the concerned patients were extensively evaluated with a battery of tests—which was negative for all tested. Also, the pathological examination did not reveal any tell-tale characteristics of alternative conditions, for example, inclusion bodies (for cytomegalovirus or RSV). Hence, we believe that the histopathological changes described in the article represents largely those produced by SARS-CoV2.
5 CONCLUSION
We compared the histopathological findings in different groups of COVID-19 patients (immunocompromised/cardiometabolic comorbidities/malignancies) and found that immunocompromised patients had significantly lower prevalence of exudative DAD. Patients with underlying malignancy were more likely to have acute lung injury. No significant intergroup differences were found with regard to liver, kidney, and heart histopathology.
AUTHOR CONTRIBUTORS
The study was conceptualized and designed by Animesh Ray, Naveet Wig, and Anjan Trikha. Data acquisition was done by Amitkumar Chavan, Shubham Sahni, Baidhnath K. Gupta, Shrawan K. Raut, Shubham Agarwal, Jagbir Nehra, Bharadhan Somu, Ragu Raja, Aakansha, Chitrakshi Nagpal, Chaithra Rajanna, Anand Shahi, Anand Rajendran, Ashwin Varadrajan, Inamul Hasan, Pratheek Choppala, and Megha Priyadarshi. Histopathological examination and reporting was carried out by Deepali Jain, Arulselvi Subramanian, Sudheer Arava, Geetika Singh, Prasenjit Das, and Chitra Sarkar. Data maintenance and statistical analysis was done by Shubham Sahni, Baidhnath K. Gupta, Amitkumar Chavan, Shrawan K. Raut, and Shubham Agarwal. The manuscript was drafted by Ayush Goel. Animesh Ray, Neeraj Nischal, Manish Soneja, and Pankaj Jorwal edited and approved the final manuscript.
ACKNOWLEDGMENT
This work was supported by the All India Institute of Medical Sciences, New Delhi Intramural Research Grant (No. F.8-A-COVID-40/2020/RS dt. 02.06.2020).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




