Effects of inactivated SARS-CoV-2 vaccination on male fertility: A retrospective cohort study
Abstract
Numerous studies have revealed severe damage to male fertility from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, raising concerns about the potential adverse impact on reproductive function of the coronavirus disease 2019 (COVID-19) vaccine developed based on the virus. Interestingly, there are several researchers who have studied the impact of the COVID-19 mRNA vaccine since then but have come up with conflicting results. As a near-ideal candidate for mass immunization programs, inactivated SARS-CoV-2 vaccine has been widely used in many countries, particularly in less wealthy nations. However, little is known about its effect on male fertility. Here, we conducted a retrospective cohort study at a single large center for reproductive medicine in China between December 2021 and August 2022. Five hundred and nineteen fertile men with no history of laboratory-confirmed COVID-19 were included and categorized into four groups based on their vaccination status: unvaccinated group (n = 168), one-dose vaccinated group (n = 8), fully vaccinated group (n = 183), and booster group (n = 160). All of them underwent a semen analysis and most had serum sex hormone levels tested. There were no significant differences in all semen parameters and sex hormone levels between the unvaccinated group and either vaccinated group. To account for possible vaccination-to-test interval-specific changes, sub-analyses were performed for two interval groups: ≤90 and >90 days. As expected, most of the semen parameters and sex hormone levels remained unchanged between the control and vaccinated groups. However, participants in vaccinated group (≤90 days) have decreased total sperm motility and increased follicle-stimulating hormone level compared with the ones in unvaccinated group. Moreover, some trends similar to those found during COVID-19 infection and recovery were observed in our study. Fortunately, all values are within the normal range. In addition, vaccinated participants reported few adverse reactions. No special medical intervention was required, and no serious adverse reactions happened. Our study suggests that inactivated SARS-CoV-2 vaccination does not impair male fertility, possibly due to the low frequency of adverse effects. This information reassures young male population who got this vaccine worldwide, and helps guide future vaccination efforts.
1 INTRODUCTION
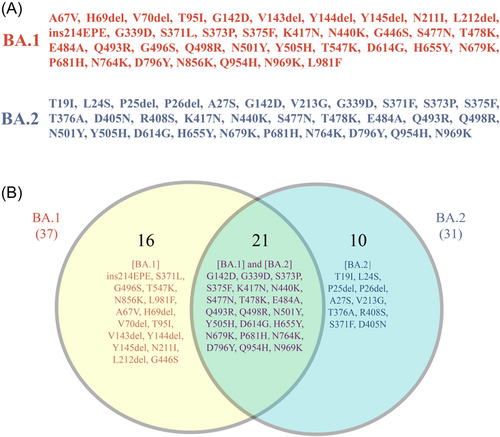
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant emerged in Southern Africa in November 2021, and became the most contagious form in the five SARS-CoV-2 variants of concern due to the high number of mutations across all spike domains. It has been divided into five distinct sublineages: BA.1, BA.2, BA.3, BA.4, and BA.5, among which BA.1 was dominantly prevalent in the early days and spread rapidly worldwide. BA.2 shared 21 amino acid substitutions (G142D, G339D, S373P, S375F, K417N, N440K, S477N, T478K, E484A, Q493R, Q498R, N501Y, Y505H, D614G, H655Y, N679K, P681H, N764K, D796Y Q954H, and N969K) with BA.1 (Figure 1), among which Q498R and N501Y are expected to enhance binding to the angiotensin-converting enzyme 2 (ACE2) receptor and H655Y, N679K, and P681H are believed to increase spike cleavage and facilitate virus transmission.1 Moreover, it contains 10 unique spike mutations (T19I, L24S, P25del, P26del, A27S, V213G, T376A, R408S, S371F, and D405N), while lacks 16 spike mutations (ins214EPE, S371L, G496S, T547K, N856K, L981F, A67V, H69del, V70del, T95I, V143del, Y144del, Y145del, N211I, L212Idel, and G446S) found in BA.1 (Figure 1). Notably, Lyngse et al.2 confirmed that all unvaccinated, fully vaccinated, and booster-vaccinated individuals had a higher susceptibility for BA.2 compared to BA.1, indicating an inherent increased transmissibility of BA.2. Therefore, it is easy to understand that the subvariant BA.2 has spread in many countries, replacing the earlier subvariant BA.1 and other variants recently.
Although either sublineage of Omicron variant is less likely to cause severe disease, it is undoubtedly that when cases increase, so do the hospitalizations and deaths. Thus, there are still unacceptably high levels of mortality in many countries currently, especially where vaccination rates are low.3 The majority of deaths remain amongst the unvaccinated individuals and those who have not received the full course of effective vaccines due to the underlying conditions.4 Therefore, the importance of getting vaccinated as soon as possible is unquestionable. However, considering the potential side effects on fertility, couples of childbearing age were more likely to refuse or delay vaccination despite availability of vaccination services. In particular, numerous studies have proven that SARS-CoV-2 infection could worsen semen parameters, lower testosterone (T) levels, and even increase the risk of erectile dysfunction,5-9 further reducing the willingness of men to receive vaccine which mimics the natural virus or contains a weakened or inactivated form. For this reason, researchers have carried out some preliminary studies in this area, but most of them focused on coronavirus disease 2019 (COVID-19) mRNA vaccines.10-13 Moreover, there are conflicting results between studies. Several studies found that there were no statistically significant changes in semen parameters before and after COVID-19 mRNA vaccination,10-12 while recently Gat et al.13 demonstrated a selective temporary decline of sperm concentration and total motile count at 3 months postvaccination. Besides, only one research studied sex hormones changes after COVID-19 mRNA vaccination, recording a significant increase of T concentration in vaccinated males after the first dose compared with the control group and a significant decrease in follicle-stimulating hormone (FSH) and T concentration in the second dose compared with the first dose.14 Changes in sex hormones have also been reported in research on the non-replicating viral vector vaccine, Sputnik V.15 The authors observed a statistically significant positive trend in the increase in the level of estradiol (E2) and a slight decrease in the level of prolactin (PRL) and total T after Sputnik V vaccination, but they hold these changes have no clinical significance due to the absence of changes in semen parameters at all stages of their study.15 In short, the jury is still out on the impact of the COVID-19 vaccine on male fertility.
As inactivated vaccines with some unique advantages such as mature manufacturing processes and ease of scaling up production and storage, Sinovac and Sinopharm vaccines have been widely used in China and also in other 110 countries to date.16 Moreover, with the rapid spread of BA.2 through parts of Asia since March this year, public anxiety and fear of contracting COVID-19 have outweighed concerns about side effects of vaccines, leading to a significant increase in the vaccination rate among reproductive-age men. Therefore, it is increasingly important to determine whether inactivated SARS-CoV-2 vaccines would impair male fertility. In this study, we comprehensively evaluated the potential effects of different doses of inactivated vaccine administration on semen parameters and sex hormone levels, and discussed in-depth the mechanism by which this phenomenon occurs from the perspective of adverse effects.
2 MATERIALS AND METHODS
2.1 Study design and setting
This was a retrospective cohort study conducted in the Center for Reproductive Medicine of the Affiliated Hospital of Jining Medical University between December 2021 and August 2022. Approval from Institutional Ethics Committee was taken before starting the study.
2.2 Participant selection
The study included 519 fertile men who visited the Center for Reproductive Medicine of the Affiliated Hospital of Jining Medical University from December 2020 to July 2022, with all of them having successfully impregnated their wives at least one time without the use of artificial reproductive technology in the past. Men who were previously infected with SARS-CoV-2 or taking medications that affect semen parameters or sex hormone levels were excluded. Based on their vaccination status, we categorized the subjects into four groups: unvaccinated group (group 1, n = 168), one-dose vaccinated group (group 2, n = 8), fully vaccinated group (group 3, n = 183), and booster group (group 4, n = 160).
- (1)
Semen analysis: Freshly ejaculated semen samples were obtained by masturbation and ejaculation into sterile containers after 2–7 days of sexual abstinence. After liquefaction for 30–60 min at room temperature, the samples were analyzed according to the World Health Organization (WHO) Laboratory Manual for the Examination and Processing of Human Semen (5th Edition). Briefly, seminal volume was determined in a graduated tube, and other semen parameters such as sperm concentration and sperm motility were evaluated by a computer-assisted sperm analysis system (SAS Medical Co., Ltd.). In addition, Diff Quick Staining Kit (Huakang Biomed Ltd.) was used for sperm morphology assessment and 200 spermatozoa were assessed per slide.
- (2)
Sex hormonal analysis: Fasting blood samples were collected in the morning from participants. Serum levels of T, luteinizing hormone (LH), FSH, E2, and PRL were determined using Unicel Dxi 800 Access Immunoassay System (Beckman Coulter Inc.). In addition, we also calculated the T/LH ratio, which is a sensitive marker of Leydig cell function.
2.3 Data collection
The baseline and clinical data of participants were obtained from pathologic records. Other details including type of vaccine administered and adverse effects following vaccination were collected via interviews.
2.4 Data analysis
All statistical analysis was performed utilizing SPSS 26.0 (SPSS Inc.). Descriptive statistics for continuous variables were expressed as mean ± standard deviation (SD), with one-way analysis of variance and Duncan's multiple range test used to examine differences between the groups. For categorical variables, data were indicated as numbers (n) and percentages (%) of the total, and compared by the χ2 test or Fisher exact test (samples with expectancy of less than 5). Among all statistical analyses, p < 0.05 was considered statistically significant.
3 RESULTS
3.1 Basic information
Table 1 described the sociodemographic characteristics and reproductive history of the participants. The mean age, BMI, age of first child, time since last birth of the whole study group were 35.30 ± 4.32 years, 26.33 ± 3.81 kg/m2, 10.05 ± 3.79, 10.02 ± 3.89 years, respectively. Most subjects were healthy with few chronic medical conditions. Pernicious habits such as smoking and alcohol consumption were rare. More importantly, there was no significant difference in any of these measures between the four groups.
| Participants' characteristics | Group 1 (n = 168) | Group 2 (n = 8) | Group 3 (n = 183) | Group 4 (n = 160) | p Value | Statistical test | |
|---|---|---|---|---|---|---|---|
| Age (years), mean ± SD | 35.16 ± 4.19a | 34.88 ± 2.75a | 35.26 ± 4.31a | 35.52 ± 4.55a | 0.619 | - | |
| BMI (kg/m2), mean ± SD | 26.68 ± 3.81a | 24.70 ± 3.74a | 26.11 ± 3.74a | 26.30 ± 3.87a | 0.075 | - | |
| Age of first child (years), mean ± SD | 10.07 ± 3.45a | 9.59 ± 2.52a | 10.24 ± 3.87a | 9.84 ± 4.10a | 0.565 | - | |
| Time since last birth (years), mean ± SD | 10.68 ± 3.49a | 9.77 ± 1.79a | 9.64 ± 4.13a | 9.75 ± 4.04a | 0.367 | - | |
| Smoking % (n/total) | 9.52% (16/168)a | 12.50% (1/8)a | 16.39% (30/183)a | 19.38% (31/160)a | 0.065 | Fisher exact test | |
| Alcohol consumption % (n/total) | 0% (0/168)a | 0% (0/8)a | 1.09% (2/183)a | 0.63% (1/160)a | 0.665 | Fisher exact test | |
| Chronic medical conditions % (n/total) | None | 100% (168/168)a | 100% (8/8)a | 99.45% (182/183)a | 98.75% (158/160)a | 0.441 | Fisher exact test |
| Hypertension | 0a | 0a | 0.55% (1/183)a | 1.25% (2/160)a | 0.441 | Fisher exact test | |
| Diabetes | 0 | 0 | 0 | 0 | - | - | |
| Hyperlipidaemia | 0 | 0 | 0 | 0 | - | - | |
- Abbreviation: BMI, body mass index.
- aThere was no significant difference in values with a common letter in their superscripts p < 0.05.
3.2 Comparison of semen parameters and sex hormone levels in control and inactivated SARS-CoV-2 vaccinated groups
Tables 2 and 3 compared the semen parameters and sex hormone levels in control and inactivated SARS-CoV-2 vaccinated groups. Fortunately, there was no difference in all semen parameters between the four groups (all p < 0.05) (Table 2). Notably, all these parameters of the participants in the control and vaccinated groups were consistent with the normal reference values of the 5th edition of the WHO guidelines except the normal forms.17 Sex hormonal analysis also showed similar results with the exception of T (Table 3). The higher T levels in the one-dose vaccinated group may be due to the smaller sample size (n = 3), and there was no difference between the unvaccinated group and fully or booster vaccinated group (3.76 ± 1.01 vs. 3.61 ± 1.02 vs. 3.90 ± 1.31 ng/ml).
| Semen parameter | Group 1 (n = 168) | Group 2 (n = 8) | Group 3 (n = 183) | Group 4 (n = 160) | p Value | Statistical test | WHO reference 5th percentile (median) |
|---|---|---|---|---|---|---|---|
| Time from vaccination to semen sample collection (days), mean ± SD | - | 87.88 ± 114.35 | 165.96 ± 78.98 | 112.66 ± 71.66 | - | - | - |
| Sexual abstinence (day), mean ± SD | 4.35 ± 1.95a | 4.38 ± 1.85a | 4.57 ± 1.93a | 4.34 ± 1.81a | 0.678 | - | 2–7 |
| Semen volume (ml), mean ± SD | 3.78 ± 1.63a | 3.29 ± 1.04a | 3.62 ± 1.33a | 3.85 ± 1.58a | 0.211 | - | 1.5 |
| Sperm concentration (106 per ml), mean ± SD | 39.09 ± 23.41a | 50.76 ± 31.99a | 40.63 ± 27.49a | 37.84 ± 27.94a | 0.096 | - | 15 |
| Total sperm count (106 per ejaculate), mean ± SD | 138.82 ± 91.20a | 151.31 ± 74.53a | 140.60 ± 102.46a | 135.35 ± 105.84a | 0.592 | - | 39 |
| Progressive motility (%), mean ± SD | 37.60 ± 14.21a | 38.43 ± 14.48a | 40.63 ± 15.41a | 39.55 ± 16.98a | 0.514 | - | 32 |
| Total motility (%), mean ± SD | 58.71 ± 18.13a | 57.91 ± 18.62a | 52.70 ± 18.06a | 50.89 ± 20.41a | 0.159 | - | 40 |
| Normal forms (%), mean ± SD | 3.53 ± 0.66a | 3.37 ± 1.13a | 3.67 ± 1.06a | 3.51 ± 1.17a | 0.299 | - | 4 |
| Men with semen volume <1.5 ml, % (n/total) | 3.57% (6/168)a | 0% (0/8)a | 2.98% (5/183)a | 1.88% (3/160)a | 0.763 | Fisher exact test | - |
| Men with asthenozoospermia, % (n/total) | 31.55% (53/168)a | 25.00% (2/8)a | 27.32% (50/183)a | 30.63% (49/160)a | 0.839 | Fisher exact test | - |
- Note: According to 5th edition of the WHO guidelines, asthenozoospermia, progressive motility <32%.
- Abbreviation: –, not applicable.
- aThere was no significant difference in values with a common letter in their superscripts p < 0.05.
| Sex hormone | Group 1 (mean ± SD) (n = 114) | Group 2 (mean ± SD) (n = 3) | Group 3 (mean ± SD) (n = 132) | Group 4 (mean ± SD) (n = 117) | p Value | Reference ranges |
|---|---|---|---|---|---|---|
| Time from vaccination to measurement (days) | - | 47.33 ± 66.76 | 170.25 ± 80.04 | 113.17 ± 42.30 | - | - |
| T (ng/ml) | 3.76 ± 1.01ab | 4.69 ± 0.24a | 3.61 ± 1.02b | 3.90 ± 1.31ab | - | 1.75−7.81 |
| LH (mIU/ml) | 4.17 ± 2.05a | 4.01 ± 0.68a | 4.39 ± 2.04a | 4.92 ± 2.24a | 0.357 | 1.24−8.62 |
| T/LH ratio | 1.10 ± 0.56a | 1.20 ± 0.24a | 0.98 ± 0.47a | 0.96 ± 0.58a | 0.350 | - |
| FSH (mIU/ml) | 5.92 ± 2.66a | 6.47 ± 1.76a | 6.14 ± 2.80a | 6.31 ± 3.84a | 0.731 | 1.27−19.26 |
| E2 (pg/ml) | 36.53 ± 16.54a | 32.19 ± 9.23a | 33.84 ± 12.59a | 32.11 ± 13.30a | 0.510 | 0-47 |
| PRL (ng/ml) | 10.77 ± 4.87a | 10.78 ± 0.49a | 10.44 ± 3.85a | 11.77 ± 5.02a | 0.540 | 2.64−13.13 |
- Abbreviations: E2, estradiol; FSH, follicle-stimulating hormone; LH, luteinizing hormone; PRL, prolactin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; T, testosterone; –, not applicable.
- ab Values without a common letter in their superscripts differ significantly p < 0.05.
In a recent article we have shown that semen quality in some individuals who have recovered from SARS-CoV-2 infection still appears to be impaired in 3 months and then returns to normal.18 Thus, additional subanalyses were performed for the results of the fully vaccinated and boosted groups according to the vaccination-to-test interval: ≤90 and >90 days (Tables 4 and 5). If participants underwent both a semen analysis and serum hormone levels testing, their vaccination-to-sex hormonal analysis interval (≤90 or >90 days) should be consistent with the vaccination-to-semen analysis interval, and those that did not meet this requirement were excluded. As expected, most of the semen parameters and sex hormone levels remained unchanged between the control and inactivated SARS-CoV-2 vaccinated groups (either ≤90 or >90 days). However, there were two special variations in groups (≤90 days): The total motility (mean ± SD) decreased from 58.71 ± 18.13% to 48.61 ± 18.06% after the booster injection (Table 4) and FSH levels increased from 5.92 ± 2.66 to 7.68 ± 3.51 mIU/ml after the full injection (Table 5).
| Semen parameter | Group 1 (n = 168) | Group 3 | Group 4 | p Value | Statistical test | ||
|---|---|---|---|---|---|---|---|
| ≤90 (n = 36) | >90 (n = 143) | ≤90 (n = 62) | >90 (n = 97) | ||||
| Time from vaccination to semen sample collection (days), mean ± SD | - | 54.11 ± 27.69 | 194.90 ± 61.16 | 44.02 ± 25.58 | 156.19 ± 55.85 | - | - |
| Sexual abstinence (day), mean ± SD | 4.35 ± 1.95a | 4.22 ± 1.90a | 4.66 ± 1.94a | 4.47 ± 1.93a | 4.25 ± 1.75a | 0.221 | - |
| Semen volume (ml), mean ± SD | 3.78 ± 1.63a | 3.71 ± 1.56a | 3.58 ± 1.25a | 3.98 ± 1.79a | 3.73 ± 1.40a | 0.157 | - |
| Sperm concentration (106 per ml), mean ± SD | 39.09 ± 23.41a | 37.59 ± 26.10a | 40.74 ± 27.60a | 35.77 ± 22.79a | 39.49 ± 30.77a | 0.314 | - |
| Total sperm count (106 per ejaculate), mean ± SD | 138.82 ± 91.20a | 131.09 ± 90.65a | 140.58 ± 103.55a | 136.68 ± 114.03a | 135.47 ± 101.00a | 0.614 | - |
| Progressive motility (%), mean ± SD | 37.60 ± 14.21a | 41.28 ± 13.68a | 40.66 ± 15.97a | 37.97 ± 15.46a | 40.64 ± 17.95a | 0.206 | - |
| Total motility (%), mean ± SD | 58.71 ± 18.13a | 55.08 ± 17.74ab | 52.31 ± 18.28ab | 48.61 ± 18.06b | 52.46 ± 21.81ab | - | - |
| Normal forms (%), mean ± SD | 3.53 ± 0.66ab | 3.48 ± 0.83ab | 3.73 ± 1.12a | 3.27 ± 0.89b | 3.68 ± 1.30a | - | - |
| Men with semen volume <1.5 ml, % (n/total) | 3.57% (6/168)a | 5.56% (2/36)a | 2.10% (3/143)a | 0a | 3.09% (3/97) a | 0.422 | Fisher exact test |
| Men with asthenozoospermia, % (n/total) | 31.55% (53/168)a | 25.00% (9/36)a | 27.27% (39/143)a | 33.87% (21/62)a | 28.87% (28/97)a | 0.809 | χ2 test |
- Note: According to 5th edition of the WHO guidelines, asthenozoospermia, progressive motility <32%.
- Abbreviation: –, not applicable.
- ab Values without a common letter in their superscripts differ significantly p < 0.05.
| Sex hormone | Group 1 (mean ± SD) (n = 114) | Group 3 (mean ± SD) | Group 4 (mean ± SD) | p Value | ||
|---|---|---|---|---|---|---|
| ≤90 (n = 22) | >90 (n = 106) | ≤90 (n = 44) | >90 (n = 72) | |||
| Number of days from vaccination to measurement (days) | - | 57.23 ± 25.69 | 197.64 ± 62.88 | 43.39 ± 22.62 | 156.65 ± 56.96 | - |
| T (ng/ml) | 3.76 ± 1.01a | 3.64 ± 1.21a | 3.62 ± 0.99a | 4.01 ± 1.46a | 3.83 ± 1.23a | 0.127 |
| LH (mIU/ml) | 4.17 ± 2.05a | 4.43 ± 2.18a | 4.34 ± 2.02a | 4.80 ± 1.87a | 5.02 ± 2.46a | 0.074 |
| T/LH ratio | 1.10 ± 0.56a | 1.02 ± 0.55a | 0.99 ± 0.45a | 1.00 ± 0.67a | 0.93 ± 0.53a | 0.179 |
| FSH (mIU/ml) | 5.92 ± 2.66ab | 7.68 ± 3.51c | 5.88 ± 2.57ab | 7.14 ± 4.71bc | 5.79 ± 3.16a | - |
| E2 (pg/ml) | 36.53 ± 16.54a | 35.40 ± 11.84a | 32.96 ± 12.24a | 35.01 ± 13.76a | 30.39 ± 12.88a | 0.051 |
| PRL (ng/ml) | 10.77 ± 4.87ab | 9.02 ± 3.14a | 10.76 ± 3.94ab | 11.11 ± 4.04b | 12.20 ± 5.55b | - |
- Abbreviations: E2, estradiol; FSH, follicle-stimulating hormone; LH, luteinizing hormone; PRL, prolactin; T, testosterone; –, not applicable.
- a–c Values without a common letter in their superscripts differ significantly p < 0.05.
3.3 Adverse reactions after inactivated SARS-CoV-2 vaccination
We investigated adverse reactions after each dose of vaccination in all vaccinated individuals and regrouped them by dose in Table 6. According to the present data, only a few participants experienced side effects after inactivated SARS-CoV-2 vaccination (1st dose: 9.7%, 2nd dose: 12.0%, booster dose: 15.0%, respectively). The main adverse reactions reported by them were injection-site pain/redness/swelling, and so forth. Systemic effects were relatively rare: the proportion of participants with fever after 1st dose vaccination, 2nd dose vaccination, booster dose vaccination was 0.3%, 0.6%, and 1.9%, respectively. Most of them disappeared in the short term, and no special medical intervention was required. No serious adverse reactions occurred in all the three groups.
| Adverse reactions | After 1st dose vaccination (n = 351) | After 2nd dose vaccination (n = 343) | After booster vaccination (n = 160) | p Value | Statistical test | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Frequency (%) | Proportion (%) | No. | Frequency (%) | Proportion (%) | No. | Frequency (%) | Proportion (%) | ||||
| Total adverse reactions | 34 | 9.7a | 100.0 | 41 | 12.0a | 100.0 | 24 | 15.0a | 100.0 | 0.212 | χ2 test | |
| Injection site adverse reactions (pain, induration, redness, swelling, or itch) | 28 | 8.0a | 82.4 | 35 | 10.2a | 85.4 | 17 | 10.6a | 70.8 | 0.502 | χ2 test | |
| Systemic adverse reactions | Muscle pain | 8 | 2.3a | 23.5 | 3 | 0.9a | 7.3 | 6 | 3.8a | 25.0 | 0.078 | Fisher exact test |
| Fatigue | 5 | 1.4a | 14.7 | 5 | 1.5a | 12.2 | 7 | 4.4a | 29.2 | 0.083 | Fisher exact test | |
| Headache, dizziness | 7 | 2.0a | 20.6 | 4 | 1.2a | 9.8 | 7 | 4.4a | 29.2 | 0.086 | Fisher exact test | |
| Fever | 1 | 0.3a | 2.9 | 2 | 0.6a | 4.9 | 3 | 1.9a | 12.5 | 0.144 | Fisher exact test | |
| Vomiting, diarrhea | 2 | 0.6a | 5.9 | 0 | 0a | 0 | 2 | 1.3a | 8.3 | 0.146 | Fisher exact test | |
| Appetite impaired, nausea | 1 | 0.3a | 2.9 | 0 | 0a | 0 | 2 | 1.3a | 8.3 | 0.092 | Fisher exact test | |
| Allergic reaction, urticaria, rash | 1 | 0.3a | 2.9 | 0 | 0a | 0 | 1 | 0.6a | 4.2 | 0.340 | Fisher exact test | |
| Stuffy, runny nose | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | |
| Cough, throat pain | 2 | 0.6a | 5.9 | 0 | 0a | 0 | 1 | 0.6a | 4.2 | 0.411 | Fisher exact test | |
| Lymphadenopathy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | |
- Abbreviations: No., number of participants with adverse reactions; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
- aThere was no significant difference in values with a common letter in their superscripts p < 0.05.
4 DISCUSSION
Based on our experience in vaccination with multiple vaccines from childhood to adulthood, there is a general consensus that it is safe to receive an approved vaccine. Moreover, as mentioned above, most studies have shown that the COVID-19 mRNA vaccine does not impair male fertility. Under these circumstances, one might feel that monitoring the effects of inactivated SARS-CoV-2 vaccines on male fertility is superfluous. In fact, the impact of vaccination on fertility has been a hot topic even before the pandemic, and more critically, available reports on this subject are conflicting. While some studies reported no negative effect of vaccination on sperm parameters and reproductive performance,19-24 others found increased incidence in sperm abnormalities.25-29 Similarly, in the earlier part of the literature, we have mentioned some studies regarding abnormal semen parameters or sex hormone levels after receiving other kind of COVID-19 vaccine. Thus, it is necessary to evaluate any effect of inactivated SARS-CoV-2 vaccination on male fertility. During our long study period (almost 16 months), several studies exploring the effects of inactivated SARS-CoV-2 vaccination on semen parameters have been published.30, 31 Compared to them, our study expanded the sample size and collection time, and included booster dose and sex hormone levels for the first time.
According to current data, there were no significant differences in most of the semen parameters and sex hormone levels between the control and inactivated SARS-CoV-2 vaccinated groups, and they were all consistent with the normal reference values. Notably, normal sperm morphology rates of vaccinated groups were below the WHO 5th percentile. It is well known that the measurement and evaluation of sperm morphology has always been a controversial subject, one of the reasons for which is that it was and still is regarded as subjective due to the fact that it has to be done by the human eye.32 Even today, most computer-assisted sperm morphology analysis systems are largely reliant on human-operator skills and suffer from the same technical problems as manual of sperm morphology evaluation. This is because all of the preparation, fixation, and staining methods of the semen smear have a great influence on the sperm morphology evaluation results. Thus, it's understandable that the average parameters in participants of the control group in our study were also below the WHO 5th percentile. Given that there were no statistically significant differences between the vaccinated and control groups either in the whole sample or additional subanalyses (Tables 2 and 4), it appears that inactivated SARS-CoV-2 vaccination did not impair sperm morphology. Additionally, after controlling for vaccination-to-test interval, some differences in total motility and FSH levels were observed between the control and vaccinated (≤90 days) groups. Fortunately, all values are within the normal range.
As mentioned above, we have reviewed and discussed the changes in semen parameters and sex hormone levels in patients during COVID-19 infection and recovery in a recent article.18 Specifically, increased FSH and LH levels and decreased sperm concentration, total motility and normal morphology were observed in patients during infection or recovery (≤90 days) when compared with uninfected ones, whose degree correlated with the severity of COVID-19 symptoms. Nonetheless, these differences gradually disappeared with the extension of the recovery period (>90 days).18 Interestingly, similar trends emerged when participants received inactivated SARS-CoV-2 vaccine (Tables 4 and 5). Whether these dramatic trends are universal remains to be clarified in larger scale studies. However, we need to reemphasize that the current evidence is not sufficient to interfere with our judgment on the safety of the inactivated SARS-CoV-2 vaccination because even the altered semen parameters and sex hormone levels are within the normal range.
It is a well-known phenomenon that the occurrence of febrile disease has a significant effect on semen quality. Moreover, even a fever of limited duration alone can alter sperm DNA integrity, induce autophagy and decrease sperm count, and/or motility and/or vitality.33-35 This is mainly due to the fact that the physiological mechanisms of scrotal heat regulation will be overwhelmed when body temperature rises by even 1°C.36 On one hand, fever was a symptom observed in over 80% of patients infected with COVID-19. On the other hand, the virus was rarely detected in their semen. Thus, in a previous study we have speculated that various reproductive system abnormalities exhibited by many male COVID-19 patients may be attributed to indirect causes such as vascular disturbances and organ-related oxidative stress induced by hyperthermia and hypoxia rather than direct viral infection.37 Similarly, if vaccination impairs fertility, it's also likely to be caused by adverse effects triggered by this vaccine. Indeed, a clear relation between vaccination, fever period, and impaired sperm quality has been observed in animal models.29 Therefore, documentation and analysis of inactivated SARS-CoV-2 vaccine-induced adverse reactions are necessary to gain a better understanding of its impact on male fertility.
Overall, the incidence rate of general reactions after any dose of vaccine was low (1st dose: 9.7%, 2nd dose: 12.0%, booster dose: 15.0%), and no serious adverse reactions or side effects were observed. This partly explains why the inactivated SARS-CoV-2 vaccine did not have a significant effect on semen parameters and sex hormones. Notably, these rates reported in our study were similar to those reported by subjects receiving either Sinovac or Sinopharm vaccine in Zeng et al.,38 Xia et al.,39 Zhang et al.,40 Kaabi et al.,41 Li et al.,42 but lower than that of several other articles such as in Cao et al.,43 Saeed et al.,44 Pasoto et al.,45 Ng et al.,46 Signorelli et al.,47 Supangat et al.,48 Medeiros-Ribeiro et al.49 (Table 7). From Table 7, it appears clearly that the incidence of adverse reactions increased significantly with the proportion of women. Thus, the low rates in our study may be due to the fact that all participants were male.
| Number of subjects (n) | Sex ratio (female vs. male) | Vaccine regimen | Incidence of adverse reactions (%) | References | ||||
|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | Booster | 1st | 2nd | Booster | |||
| 901 | 12.5%:87.5% (113 vs. 788) | BBIBP-CorV | BBIBP-CorV | BBIBP-CorV | 19.6 | 41 | ||
| 102 | 37.3%:62.7% (38 vs. 64) | CoronaVac | CoronaVac | CoronaVac | 4.9 | 42 | ||
| 50 | 40.0%:60.0% (20 vs. 30) | CoronaVac | CoronaVac | 8.0 | 42 | |||
| 144 | 52.1%:47.9% (75 vs. 69) | CoronaVac | CoronaVac | 17.4 | 6.4 | 40 | ||
| 52 | 55.8%:44.2% (29 vs. 23) | CoronaVac | CoronaVac | CoronaVac | 15.4 | 38 | ||
| 84 | 61.9%:38.1% (52 vs. 32) | WIBP-CorV | WIBP-CorV | 19.0 | 39 | |||
| 1080 | 70.4%:29.6% (760 vs. 320) | BBIBP-CorV | BBIBP-CorV | 75.6 | 86.0 | 44 | ||
| 144 | 73.6%:26.4% (106 vs. 38) | CoronaVac | CoronaVac | 38.2 | 35.4 | 48 | ||
| 41 | 75.6%:24.4% (31 vs. 10) | CoronaVac | CoronaVac | CoronaVac | 39.0 | 43 | ||
| 37 | 75.7%:24.3% (28 vs. 9) | CoronaVac | CoronaVac | 62.2 | 67.6 | 46 | ||
| 182 | 76.9%:23.1% (140 vs. 42) | CoronaVac | CoronaVac | 40.1 | 34.8 | 49 | ||
| 132 | 86.4%:13.6% (114 vs. 18) | CoronaVac | CoronaVac | 38.6 | 33.3 | 47 | ||
| 102 | 98.0%:2.0% (100 vs. 2) | CoronaVac | CoronaVac | 39.2 | 38.4 | 45 | ||
Hormonal, microbial, genetic, and behavioral differences between males and females are likely to contribute to differences in adverse reactions following immunization. It is well known that the occurrence of adverse events is related to the strength of the immune response; the stronger the immune response, the more frequency of adverse events. Sex hormones have been shown to influence immune responses and cytokine levels. Specifically, T and dihydrotestosterone increase interleukin-10 (IL10) and transforming growth factorβ (TGF-β) synthesis, causing potent anti-inflammatory responses via androgen receptor signaling.50, 51 In contrast, E2 stimulates the synthesis of proinflammatory cytokines such as IL-1, IL-6, and TNF α and inhibits the production of IL-4, IL-10, TGF-β, and interferon-γ,52-54 which are thought to be responsible for enhanced inflammation in females. Notably, since bacteria can metabolize sex hormones through hydroxysteroid dehydrogenase enzymes,55 microbiome composition may also directly influence an immune response in a sex-specific manner. Moreover, differential humoral immune responses between the sexes were observed before puberty, during the reproductive years, and after reproductive senescence,56, 57 which suggested that sex hormone is not the only mediators of sex-based differences in adaptive immune responses to vaccines. Alternatively, genetic differences might underlie these differences. The X chromosome expresses 10 times more genes than the Y chromosome, and carries many genes associated with immune mechanisms.58 These genes encode proteins, including transcriptional factors (e.g., Foxp3), cytokine receptors (e.g., Il2rg and Il13ra2), and pattern recognition receptors (e.g., Tlr7 and Tlr8), all of which play a role in the development of both pathogen-defense and autoimmunity. Furthermore, the X chromosome contains 10% of the ∼800 miRNAs in the human genome, known to modulate immunity, whereas the Y chromosome contains only two.59 These X-specific miRNAs have been shown to play a critical role in the development of autoimmune diseases such as rheumatoid arthritis and lupus that occur more frequently among women.60 Beyond these biological differences above, growing evidence supports gender-based social-cultural/behavioral differences in vaccine response, such as different societal roles, smoking and drinking habits, comorbidity rates (e.g., obesity, hypertension, and cardiovascular diseases), and educational levels.61 In general, females have a lower pain threshold and are less tolerant to painful stimuli than males.62-64 Moreover, Chinese men have higher prevalence of obesity than women,65, 66 which has been reported to lead to decreased pain sensitivity.67, 68 To date, sex has not yet emerged as a biological variable evident in the disaggregation of data and analyses in most studies on COVID-19 vaccines, with only limited studies reporting adverse events by it.44, 69, 70 Previous study revealed whole-virus trivalent inactivated influenza vaccine could induce higher levels of IgM as well as H1N1-specific IgG1 responses in female mice compared to male mice.71 More interestingly, antibody responses among women given a half dose of trivalent inactivated vaccine were similar to or greater in magnitude when compared with responses among men given a full dose.57 In view of the trend toward fewer side effects, reducing the dose may improve vaccine acceptability in some populations. Thus, further investigation related to reduced COVID-19 vaccine dosing and optimal criteria (e.g., sex, age ranges) is merited.
There are several limitations of this study that should be noted. First, although our study expanded the sample size compared to previous studies as mentioned above, it is relatively small due to its single-center retrospective study design and further studies on a larger scale are required. Moreover, particularly small sample size of group 2 was expected as the vast majority people in China swiftly received the second dose 3 weeks after the first-dose vaccination. Second, the population included in this study was men with natural fertility, thereby limiting the general applicability of the results to men with preexisting fertility problems or sex hormone disorders. Another limitation of this study was that a majority of participants underwent semen analysis and/or serum sex hormone levels test only once. Thus, it is difficult for us to use the self-controlled study design to eliminate confounding factors and selection bias.
5 CONCLUSION
Overall, based on the present data, we have comprehensively confirmed that inactivated SARS-CoV-2 vaccination is safe with no apparent detrimental effect on male fertility, which may due to the low frequency of adverse effects. Some interesting trends similar to those found during COVID-19 infection and recovery remain to be clarified in larger scale studies in the future. However, this does not mean that men receiving inactivated SARS-CoV-2 vaccine must wait 3 months before trying to conceive because all semen parameters and sex hormone levels were still within normal ranges after vaccination. Of course, due to the particular emphasis on fertility, someone may still worry about whether their fertility status has been acutely affected by the vaccination. For these people, they could undergo a semen analysis before trying to conceive within a very short period of being vaccinated, or just wait 3 months after last vaccination.
AUTHOR CONTRIBUTIONS
Yehao Dong, Fei Chen, Zewu Li, and Aijun Yang contributed to conception and design of the study. Yehao Dong and Xiaoyun Li contributed to the laboratory analysis of semen and sex hormonal. Fei Chen, Zewu Li, Yunting Zhu, Zichun Wei, Jiarui He, and Hongju Cheng contributed to the data collection and processing. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors would like to thank all working staff at the Center for Reproductive Medicine of the Affiliated Hospital of Jining Medical University for their active involvement and collaboration in project administration. Moreover, the authors are sincerely grateful for the participants in this study. This work was supported by the Natural Science Foundation of Shandong Province (Grant no. ZR2020QC100).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was approved by the Ethics Committee of the Affiliated Hospital of Jining Medical University (No. 2022C095) and was conducted in accordance with the Declaration of Helsinki. Informed contents were obtained from patients for data collection with scientific use.
Open Research
DATA AVAILABILITY STATEMENT
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request (Fei Chen; E-mail: [email protected]).




