Clinical trials on the pharmacological treatment of long COVID: A systematic review
Ying Jie Chee and Bingwen Eugene Fan contributed equally to this study.
Abstract
The postacute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (PASC), also known as post-acute coronavirus disease 19 (COVID-19) or the long COVID syndrome (long COVID) is an emerging public health concern. A substantial proportion of individuals may remain symptomatic months after initial recovery. An updated review of published and ongoing trials focusing on managing long COVID will help identify gaps and address the unmet needs of patients suffering from this potentially debilitating syndrome. A comprehensive literature search was conducted on the international databases and clinical trial registries from inception to 31 July 2022. This review included 6 published trials and 54 trial registration records. There is significant heterogeneity in the characterization of long COVID and ascertainment of primary outcomes. Most of the trials are focused on individual symptoms of long COVID or isolated organ dysfunction, classified according to cardiovascular, respiratory and functional capacity, neurological and psychological, fatigue, and olfactory dysfunction. Most of the interventions are related to the mechanisms causing the individual symptoms. Although the six published trials showed significant improvement in the symptoms or organ dysfunction studied, these initial studies lack internal and external validity limiting the generalizability. This review provides an update of the pharmacological agents that could be used to treat long COVID. Further standardization of the diagnostic criteria, inclusion of participants with concomitant chronic cardiometabolic diseases and standardization of outcomes will be essential in future clinical trials.
Abbreviations
-
- COVID-19
-
- Coronavirus disease 2019
-
- IL
-
- Interleukin
-
- MSCs
-
- Mesenchymal stem cells
-
- NICE
-
- National Institute for Health and Care Excellence
-
- PASC
-
- Postacute sequelae of SARS-CoV-2 infection
-
- PCR
-
- polymerase chain reaction
-
- PEA-LUT
-
- palmitoylethanolamide and luteolin
-
- SARS-CoV-2
-
- Severe acute respiratory syndrome coronavirus 2
-
- TGF-β
-
- transforming growth factor-beta
-
- WHO
-
- World Health Organization
1 INTRODUCTION
As of August 2022, it is estimated that 77% of the global population (6.1 billion people) have had COVID-19 at least once since the first reported cases in December 2019.1 The majority of these infections occurred following the emergence of the highly transmissible Omicron variant.2 Omicron and its subvariants caused successive outbreaks, with many countries currently experiencing their third wave.3 While infection by the Omicron is associated with less severe illness, particularly in vaccinated individuals,2 due to the high number of infections, we are now faced with a major public health threat and possibly another wave of this pandemic: the long COVID syndrome.
The long COVID syndrome is a heterogeneous clinical entity that extends beyond the acute infectious period and can last for months.4 There is now a large body of evidence showing up to 80% of hospitalized and 30% of outpatient individuals continue to experience diverse symptoms for weeks after diagnosis.4 Other terms have been used to describe this novel syndrome. These include post-acute sequelae of SARS-CoV-2 infection (PASC),5 long-haul COVID-19,6 and postacute COVID syndrome.7 The World Health Organization (WHO) and the National Institute for Health and Care Excellence (NICE) guidelines have defined post-COVID-19 syndrome as symptoms that continue or develop after the acute infection.8, 9 The trajectory of long COVID syndrome is classified into subacute if symptoms resolves within 12 weeks and chronic if the symptoms persist after that.10
Due to the heterogeneity of the long COVID syndrome, and the lack of clear diagnostic tests, establishing fixed diagnostic criteria can be challenging.11 Several groups have proposed diagnostic criteria, which include the presence of two or more symptoms from the following: constitutional, cardiovascular, respiratory, gastrointestinal, musculoskeletal, neurological,12 laboratory confirmation of COVID-19 infection, and the onset of symptoms ranging between 1 and 6 weeks after diagnosis based on the severity of initial presentation.12 Alternatively, the symptoms could be classified based on the presence of organ dysfunction, underlying comorbidities, hospitalization, treatment, or not directly accounted for by any known cause.13
More than 60 physical and psychological complaints of long COVID had been reported.11 The most common symptoms include fatigue, weakness, reduced effort tolerance, arthralgia, cognitive impairment, difficulty concentrating, chest pain, and palpitations.14 Hospital readmission attributed to post-COVID-19 symptoms had been estimated to be up to 4.5% within the first 30 days. Almost 50% of studies reported disabilities in social and family life, difficulties dealing with the workload, and loss of employment,15 with nearly a quarter of individuals still facing barriers with daily activities and meaningful work up to 7 months after the acute illness.16, 17 Moreover, emerging longitudinal data have raised alarming concerns about potential irreversibility of the adverse long-term sequelae. For example, some individuals infected with COVID-19 developed atrophy of the limbic and hippocampal regions in the brain that are involved in memory and cognitive processing, thereby raising concerns about the long-term risks of developing Alzheimer's and other forms of dementia.18 Even among individuals with mild COVID, a longitudinal study showed a substantial increase in risk spanning across a wide spectrum of incident cardiovascular diseases including ischemic heart disease, dysrhythmia and stroke that was present up to 1 year after the acute infection.19 Another cohort study reported a significant increase in the risk of incident diabetes occurring up to 12 months after the acute infection.20 While debilitating symptoms of long COVID can hinder the return to baseline function, and reduce the quality of life significantly,21 the long-term multisystemic dysfunction induced by COVID-19 could substantially increase the global burden of chronic diseases.
The implications of the post-COVID-19 syndrome can be massive and widespread. Intensive research is underway to characterize this syndrome and improve our understanding of the pathophysiology and long-term outcomes. International efforts are urgently needed to pursue rigorous clinical trials to establish effective treatments. To the best of our knowledge, there were two previously published reviews on the pharmacological management of long COVID.22, 23 The literature search for both reviews was conducted up to July 2021 and December 2021 respectively, with the latter review also including case reports, case series, and retrospective analyses.
In this review, we provide a comprehensive update on the completed and ongoing clinical trials on the management of long COVID syndrome until July 2022, expanding the literature search by more than 8 months from the latest published reports on this topic. Furthermore, this review focused specifically on pharmacological therapies, providing an in-depth appraisal of existing and novel treatments mapped according to the mechanisms of the long COVID syndrome. We also highlighted the limitations of current trials and identified gaps that need to be addressed in future interventional studies.
2 METHODS
2.1 Search strategy
A structured protocol was designed to retrieve the relevant studies, and the reporting was done following the Preferred Reporting Items for Systematic Reviews and meta-analyses (PRISMA) framework.24 A scoping literature search for published clinical trials was conducted via three databases—PubMed, EMBASE, and CINAHL from inception to 31 July 2022. The following clinical trial registries were searched for ongoing clinical trials: National Institute of Health clinicaltrials.gov, European Union Clinical Trials Register, and the WHO International Clinical Trials Registry Platform. We tapped on the search string from a previous review22 and implemented the following search terms: “long COVID” OR “post-COVID syndrome” OR “post-acute sequelae of SARS-CoV-2” OR “PASC” OR “persistent COVID” OR “post COVID” OR “enduring COVID-19” OR “long-haul COVID” OR “long-tail COVID.”
The published clinical trials and registration records of ongoing trials were reviewed independently by two authors (E. F. and C. Y. J.) based on the following inclusion and exclusion criteria.
The inclusion criteria are human subjects with long COVID or PASC aged 18-year-old and above; clinical trials involving interventions aimed at treating long COVID or PASC with status of trial as ongoing or completed at the time of literature search. The exclusion criteria are nonhuman subjects; not related to long COVID or PASC; Nonpharmacological interventions; alternative and complementary therapies; noninterventional studies (e.g., observational studies or COVID-19 registries); interventions related to pre- or postexposure prophylaxis for COVID-19; trial status being withdrawn or terminated; and studies that were non-English.
Data to be extracted included the following fields: target recruitment, diagnosis of long COVID, intervention, comparator, primary outcomes, and study design, including blinding.
3 RESULTS
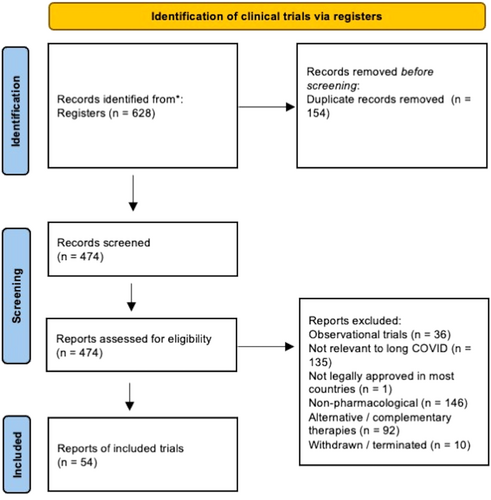
There were 230 published trials and 628 trial registration records identified. After removing duplicates, there were 206 published trials and 474 trial registration records. After reviewing the full publication or trial registration record, 6 published and 54 ongoing clinical trials were included in the review (Figures 1 and 2).
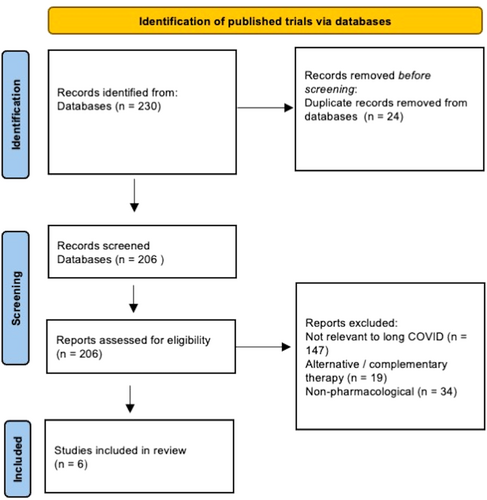
There were six published trials25-30 on long COVID interventions conducted in the following countries: Tunisia, India, Italy, and Saudi Arabia. Four of these trials were randomized27-30 and only two were double-blind.28, 30 The sample size of the published trials ranged from 36 to 290 individuals. Two trials included only premorbid healthy participants,26, 30 while another three trials included participants with at least one comorbidity such as hypertension and diabetes mellitus.25, 27, 29 The diagnosis of SARS-CoV-2 infection was made via positive polymerase chain reaction (PCR) test or SARS-CoV-2 antigen test in all six trials. None of the trials stratified participants according to vaccination status or SARS-CoV-2 variant. Only one trial conducted in Tunisia, diagnosed long COVID based on NICE guidelines and showed a positive impact of the use of sulodexide on endothelial function (post occlusive reactive hyperemia) and on symptoms of chest pain and palpitations.25 The other trials evaluated the impact of pharmacological interventions on other organ systems separately—2 trials showed that the use of anti-inflammatory and neuroprotective agent palmitoylethanolamide and luteolin (PEA-LUT) and vitamin D3 improved olfactory dysfunction28, 29 with a shorter time to recovery; one trial found that ivabradine (hyperpolarization-activated cyclic nucleotide-gated channel blocker) which acts by slowing the heart rate with increased stroke volume was superior to carvedilol (beta-blocker) which decreases the heart rate but is associated with decreased stroke volume in initial stages of treatment, for treating palpitations.26 Another trial showed that high versus low-dose prednisolone showed no difference in the outcomes of dyspnea or resting hypoxemia with exertional desaturation.27 A trial conducted in India showed that the administration of probiotics improved fatigue.30
There are 54 ongoing or recently completed trials whose results have not been published. The age of participants range from 18 to 90-year-old. Participants were planned to be randomized in 48 trials and blinding involving at least two parties in 38 trials. The diagnosis of previous SARS-CoV-2 infection was established by PCR test in 27 trials, antigen or serology in 1 trial, and the method was not detailed in the remaining 26 trials. None of the trials reported stratifying participants according to the vaccination status or SARS-CoV-2 variant. There is significant heterogeneity in the diagnosis of long COVID. One trial defined the diagnosis of long COVID according to the WHO criteria8 (NCT05047952), while two trials stated that the long COVID diagnosis would be based on expert judgment. Five trials mentioned recruiting patients with long COVID without further specification. The remaining trials looked at individual symptoms of long COVID or isolated organ dysfunction, which could be classified according to cardiovascular, respiratory and functional capacity, neurological and psychological, fatigue, and olfactory dysfunction. Twenty-four trials excluded individuals with underlying cardiovascular risk factors, including diabetes mellitus, hyperlipidemia, hypertension, renal insufficiency, and previous cardiovascular events. The interventions could be categorized into the following domains: cardiovascular and long-term survival (3 trials): NCT05096884, NCT04801940, NCT05371925; respiratory (9 trials): NCT04695704, ACTRN1262100637842 IRCT20200707048047N1, NCT04949386, NCT04607928, NCT04652518, NCT049988282, NCT04551781, NCT04880161; neurological including fatigue (9 trials): NCT04904536, NCT05047952, NCT05350774, NCT05274477, ISRCTN10665760, NCT04604704, NCT04944121, NCT04705831, EudraCT2022-000641-33; olfactory (5 trials): NCT04951362, NCT05269030, NCT05002530, NCT05184192, NCT05104424, nutraceuticals (16 trials): NCT04592354, NCT05356936, NCT04809974, NCT05311852, NCT05373043, NCT04960215, NCT05121766, NCT05080244, NCT04813718, NCT04950803, NCT05227170, NCT05371288, NCT04947488, NL9742, NCT04482595, NCT04871802; COVID-19 therapeutics and vaccines (3 trials): NCT05220280, NCT04978259, EudraCT2021-003331-28 and novel agents (9 trials): NCT03554265, NCT05126563, NCT05346120, NCT05116761, NCT04326036, NCT04842448, NCT05152849, NCT05228899, NCT0524200. These are summarized in Tables 1 and 2.
| Reference/country | Study design | Population characteristics | Intervention | Comparator | Outcomes | |||
|---|---|---|---|---|---|---|---|---|
| Sample size (N) | Long COVID diagnosis | Acute COVID diagnosis via PCR or antigen testing | Comorbidities | |||||
Charfeddine et al.25 Tunisia |
Quasi-experimental No blinding | 290 | Diagnosis based on NICE guidelines + endothelial dysfunction | Yes | 37% had hypertension; 28% had diabetes mellitus | Sulodexide | None | On Day 21, there was significantly reduced chest pain and endothelial dysfunction in the sulodexide group |
Jadhav and Jariwala26 (Abstract) India |
Open label | 24 | Persistent palpitations 7 days postdischarge | Yes | No known comorbidities | Ivabradine | Carvedilol | Ivabradine more effective in treating palpitations |
Dhooria et al.27 India |
Randomized Open label | 130 | Persistent dyspnea or resting hypoxemia or exertional desaturation; and diffuse lung parenchymal changes 3–7 weeks after acute COVID | Yes | 73% had at least one comorbidity (not specified) | High dose prednisolone (starting dose: 40 mg/day) | Low dose prednisolone (starting dose: 10 mg/day) | No differences between high versus low dose prednisolone |
Stadio et al.28 Italy |
Randomized Double-blind | 158 | Persistent olfactory dysfunction 6 months post-COVID | Yes | Excluded subjects with pre-existing olfactory disorders | PEA-LUT + olfactory training | Olfactory training only | PEA-LUT group showed greater improvement in olfactory function |
Sabico et al.29 Saudi Arabia |
Randomized Open label | 36 | Cough and gustatory loss 30 days after acute COVID | Yes | 55% of participants had hypertension; 51% had diabetes mellitus | High dose vitamin D3 | Low dose vitamin D3 | Shorter time to recovery for cough and ageusia among individuals who received high dose vitamin D |
Rathi et al.30 India |
Randomized Double-blind | 200 | Persistent fatigue 4–87 days after acute COVID | Yes | No comorbidities | Systemic and probiotic enzymes | Placebo | Greater improvement in fatigue in the intervention group |
| Trial registration number/Country | Study design | Population characteristics | Intervention | Comparator | Primary outcomes | |||
|---|---|---|---|---|---|---|---|---|
| Recruitment target (N), Age range (years) | Inclusion criteria/definition of long COVID | Method of acute COVID-19 diagnosis | Comorbidities | |||||
NCT05096884 United States of America (USA) |
Open label | 20 18–40 |
Exertional dyspnea 3–12 months | PCR | Known dysautonomia/lung diseases | Metoprolol | Nil | Improvement in dyspnea, tachycardia and 6-min walk test |
NCT04801940 United Kingdom (UK) |
Randomized Open label |
2631 >18 |
Hospitalized for COVID-19 limited to 5 days | Laboratory-confirmed (method not stated) | Not stated | Apixaban Atorvastatin |
Standard care | 12-month hospital free survival |
NCT05371925 Hungary and Mexico |
Randomized Double-blind |
200 >18 |
Convalescent phase (more than 10 days from symptom onset) | PCR | At high risk for endothelial dysfunction | Sulodexide | Placebo | Markers of endothelial dysfunction and thrombosis |
NCT04695704 Spain |
Randomized Double-blind |
284 18–80 |
Persistent respiratory symptoms exceeding 1 month from acute infection | Unclear | No underlying respiratory disorders | Montelukast | Placebo | Improvement in respiratory symptoms |
ACTRN12621000637842 Australia |
Randomized Open label |
1000 >18 |
Hospitalized for COVID-19 with hypoxia | PCR | No underlying end stage renal or hepatic impairment | Colchicine | Standard care | 6-month mortality |
IRCT20200707048047N1 Iran |
Nonrandomized Double-blind |
40 >18 |
Persistent dyspnea and/or pulmonary fibrosis at least 2 months after discharge | PCR or CT thorax findings | Not stated | Colchicine | Placebo | Changes in dyspnea rating and pulmonary CT scan at 1 and 6 months |
NCT04949386 Canada |
Randomized Double-blind |
48 18–80 |
Persistent respiratory symptoms at least 4 weeks after acute COVID-19 | PCR or serology | Excluded individuals with pre-existing evidence of cardiac ischemia | S-1226 (synthetic surfactant) | Placebo | Safety, tolerability, efficacy of S-1226 |
NCT04607928 Spain |
Randomized Double-blind |
148 >18 |
At least 5% fibrotic changes on high resolution CT thorax | Unclear | Excluded diseases such as uncontrolled ischemic cardiomyopathy | Pirfenidone | Placebo or corticosteroid | Change in lung function and degree of lung fibrosis on HRCT |
NCT04652518 USA |
Randomized Double-blind |
168 18–80 |
Persistent shortness of breath post-COVID-19 pneumonia | PCR | Excluded participants with underlying respiratory or severe cardiac insufficiency | Deupirfenidone | Placebo | Change in 6-min walk test distance |
NCT04988282 Turkey |
Randomized Open label |
642 >18 |
Post-COVID interstitial lung disease with persistent respiratory symptoms 1 month after recovery | PCR or antigen or antibody | Excluded uncontrolled DM, hypertension, cardiac ischemia | Methylprednisolone | Standard therapy | Improvement in modified Medical Research Council dyspnea scale |
NCT04551781 Egypt |
Randomized Single-blind |
450 >18 |
Persistent radiological changes on CT thorax after 14 days from acute infection | Nasopharyngeal swab (method unclear) | Excluded those with cancer, and rheumatological disorders | Prednisolone | Placebo | Resolution of chest infiltrates at 14 days |
NCT04880161 USA |
Randomized Double-blind |
32 >18 |
At least two respiratory symptoms at least 4 weeks after initial diagnosis | PCR | No underlying renal, pulmonary or cardiac disorders | Human serum albumin | Placebo | Safety and tolerability of human serum albumin |
NCT04904536 Australia |
Randomized Single-blind |
400 >18 |
Ongoing neurological symptoms attributed to COVID-19 | PCR | Excluded severe liver disease, cognitive or psychiatric disorders | Atorvastatin | Standard care | Neurological processing speed at 18 months |
NCT05047952 Canada |
Randomized Double-blind |
200 18–64 |
WHO-defined post-COVID-19 symptoms lasting at least 2 months | Clinical diagnosis or PCR | Excluded individuals with underlying psychiatric conditions | Vortioxetine | Placebo | Change in Digit Symbol Substitution Test (cognitive battery test) |
NCT05350774 USA |
Randomized Double-blind |
60 >18 |
Persistent neurological symptoms and functional impairment 3–18 months after acute COVID-19 | Patient reported antigen test, serology or PCR | Excluded individuals with DM, atherosclerotic diseases and renal impairment | IVIG or IV methylprednisolone | Placebo | Change in Health Utilities Index Mark 3–4 weeks after intervention |
NCT05274477 Switzerland |
Randomized Double-blind |
44 18–60 |
Subjective working memory impairment for at least 2 months with onset 3 months after acute COVID-19 | Antigen or serology | Excluded individuals with hypertension and BMI > 30 | Fampridine | Placebo | Digit Span backward performance (cognitive test) Other modalities of cognitive function assessment, fluency test |
ISRCTN10665760 UK |
Randomized, Open label | 4520 >18 |
Symptoms of long COVID 4 weeks after acute infection | Clinical diagnosis or positive test | No history of severe hepatic or renal dysfunction | Loratadine, famotidine, colchicine, rivaroxaban | Nil | Fatigue at baseline, 12 and 24 weeks |
NCT04705831 USA |
Randomized Double-blind |
40 >18 |
Persistent post COVID fatigue 4 weeks after acute infection | Confirmed diagnosis of COVID | Not specified | RUCONEST (C1-esterase inhibitor) | Placebo | Changes in neuropsychological measures including cognition, fatigue, function, quality of life |
EudraCT 2022-000641-33 Spain |
Randomized Double-blind |
100 >18 |
Symptoms of long COVID for at least 2 months after 90 days from initial infection | PCR or antigen or serology | Excluded decompensated heart or renal failure | Human serum albumin | Placebo | Safety profile, improvement in functional status and fatigue |
NCT04951362 Egypt |
Randomized Open label |
117 18–70 |
Post COVID anosmia | Unclear | Not specified | Intranasal ivermectin | Saline nasal spray | Regain of smell |
NCT05269030 Egypt |
Randomized Single-blind |
60 >18 |
Post COVID parosmia with acute COVID-19 diagnosed at least 3 months prior | PCR | Excluded individuals with systemic diseases such as diabetes mellitus, hypertension | Intranasal ivermectin | Local steroid spray | Improvement in parosmia |
NCT05002530 Saudi Arabia, Egypt, USA, China |
Randomized Open label |
10 000 18–70 |
Post COVID anosmia or parosmia | PCR | Excluded individuals with dyslipidemia | Aerosolized retinoic acid and 2 doses intramuscular cholecalciferol | Standard therapy | Improvement of olfaction Quality of life |
NCT05184192 USA |
Randomized Double-blind |
50 18–65 |
Olfactory dysfunction of 3 months and longer after acute COVID-19 | Unclear | Not specified | Gabapentin | Placebo | Clinical global improvement scale |
NCT05104424 Saudi Arabia |
Randomized Open label |
22 18–75 |
Persistent smell and taste dysfunction post COVID 19 | Unclear | Not specified | Intranasal insulin, zinc, gabapentin, ice cubes | Only zinc and smell training | Change in smell and taste function |
NCT04604704 USA |
Open label | 60 18–65 |
Fatigue score more than 9 using Chalder Fatigue Scale up to 4 months post COVID | PCR | Excluded individuals with significant renal, cardiac and hepatic impairment | Naltrexone and NAD+ | Nil | Reduction of fatigue up to 12 weeks of intervention |
NCT04944121 USA |
Randomized Double-blind |
70 18–65 |
Persistent fatigue determined via PROMIS fatigue score up to 6 months after acute COVID-19 | PCR | Unclear if participants with chronic diseases such as hypertension or hyperlipidemia were excluded | RSLV-132 | Placebo | Improvement in the PROMIS fatigue score |
NCT04592354 USA |
Randomized Double-blind |
70 18–75 |
Significant fatigue assessed by Fatigue Questionnaire at least 2 months post-COVID-19 | PCR | Excluded individuals with ischemic heart disease, congestive cardiac failure | Anhydrous enol-oxaloacetate | Placebo | Self-reported fatigue |
NCT05356936 USA |
Randomized Open label |
150 >18 |
Recurrent or new symptoms of long COVID more than 3 months after positive test result | PCR or antigen | Excluded participants with active malignancy | Vitamin K2 and D3 | Nil | Changes in vitamin K2, D3, inflammatory markers |
NCT04809974 USA |
Randomized Double-blind |
100 18–65 |
Persistent cognitive difficulties (“brain fog”) and at least two neurological and/or physical symptoms for at least 2 months after acute COVID | PCR | Excluded individuals with “significant systemic illness or medical condition” | Niagen (Vitamin B3) | Placebo | Change in executive function and memory composite scores up to 22 weeks |
NCT05311852 Italy |
Randomized Double-blind |
34 >18 |
Persistent cognitive difficulties and fatigue after acute COVID | PCR | Excluded individuals with neurological, psychiatric, metabolic or cardiopulmonary disorders | Palmitoylethanolamide co-ultramicronized with luteolin (dietary supplement) | Placebo | Changes in long-interval intracortical inhibition Changes in fatigue rating scale, Montreal Cognitive Assessment score |
NCT05373043 USA |
Randomized Double-blind |
300 >50 |
Diagnosis of long-COVID 3-12 months after initial infection | SARS-CoV-2 positive test | Excluded individuals with unstable coronary artery disease, peripheral artery disease, cerebrovascular events, uncontrolled hypertension, severe renal impairment | Mito-Q (coenzyme Q compound) + exercise rehabilitation | Placebo + exercise rehabilitation | Changes in flow mediated dilatation, microvascular function and cerebral vascular endothelial function |
NCT04960215 Denmark |
Randomized, Crossover quadruple-blind |
121 >18 |
Symptoms related to long COVID diagnosed by infectious disease specialists | PCR or antibody | Not stated | Coenzyme Q10 | Placebo | Self-reported symptoms of long COVID, quality of life |
NCT05121766 USA |
Randomized Double-blind |
100 18–89 |
At least one respiratory symptom or fatigue or anosmia or dysgeusia more than 12 weeks after acute infection | PCR | Not stated | Omega-3 fatty acid | Soybean oil placebo | Adherence to study protocol and retention in study; self-reported shortness of breath, cough, anosmia, ageusia, and fatigue at 12 weeks |
NCT05080244 Canada |
Randomized Double-blind |
618 >18 |
COVID-19 diagnosed less than 10 days from recruitment | Unclear | Excluded participants with active cancer and/or immunocompromised | Probiotics | Placebo | Incidence of long COVID 90 days after initial diagnosis |
NCT04813718 Austria |
Randomized Double-blind |
20 >18 |
Presence of residual symptoms of COVID-19 | Unclear | Excluded individuals with pre-existing pulmonary disorders | Omnibiotic | Placebo | Changes in microbiome, intestinal barrier composition and cytokines |
NCT04950803 Hong Kong |
Randomized Double-blind |
280 >18 |
Recovered COVID-19 patients | Unclear | Excluded immunocompromised individuals | Microbiome immunity formula | Placebo | Composite outcome including manifestations of long-COVID, future hospitalizations |
NCT05227170 USA |
Randomized Double-blind |
80 18–89 |
PASC diagnosed 30–180 days post COVID-19 diagnosis | Unclear | Excluded individuals with chronic diseases for example, renal or hepatic dysfunction and unstable angina | Lp299v (lactobacillus Plantarum) | Placebo | Changes in vascular matrices such as brachial artery flow mediated dilation and arterial stiffness |
NCT05371288 USA |
Randomized Double-blind |
50 >18 |
Mild to severe symptoms of COVID-19 (duration of symptoms not specified) | PCR or serology | Not mentioned | NAC + alpha lipoic acid + liposomal GSH | Multivitamin + magnesium | Changes in symptoms of long COVID, time to recovery and quality of life up to 4 months postintervention |
NCT04947488 Italy |
Randomized Open label |
50 20–60 |
Fatigue determined by Centre for Epidemiological Studies Scale (duration of symptoms not specified) | SARS-CoV-2 test | Excluded participants with hypertension, on immunosuppressants, nonsteroidal anti-inflammatory agents and steroidal drugs | Bioarginine C | Placebo | Changes in 6-min walk test |
NL9742 Netherlands |
Randomized Double-blind |
72 >18 |
Persistent on new symptoms of long COVID at least 12 weeks after acute COVID-19 infection | PCR or serology | Excluded individuals with COVID-19 related cardiac or pulmonary tissue damage | Lactoferrin 1200 mg/day | Placebo | Improvement of fatigue symptoms Improvement in anxiety, depressive symptoms and muscle strength |
NCT04482595 USA |
Randomized Double-blind |
66 >18 |
Had COVID-related acute respiratory distress syndrome | Unclear | Excluded individuals with severe end-organ impairment, poorly controlled diabetes mellitus, hypertension, cardiac event in preceding 6 months | BIO 300 (genistein) | Placebo | Change in diffusing capacity of lungs and 6-min Walk Test |
NCT04871802 Russia |
Open label | 100 >18 |
COVID-19 pneumonia 3 months ago | Unclear | Not mentioned | Taxifolin aqua | No | Changes in spirometry indices, echocardiogram parameters, pulse wave velocity and augmentation index |
NCT05220280 Finland |
Randomized Open label |
400 >18 |
Hospitalized for COVID-19 infection | Laboratory confirmed SARS-CoV-2 infection | Excluded individuals with severe end-organ impairment and acute myocardial infarction | Imatinib and infliximab | Standard care | Development of long-COVID symptoms up to 24 months from initial COVID-19 infection |
NCT04978259 Finland |
Randomized Open label |
202 >18 |
Hospitalized for COVID-19 infection | Laboratory confirmed SARS-CoV-2 infection | Excluded individuals with severe end-organ impairment and acute myocardial infarction | Remdesivir | None | Quality of life, fatigue, exertional dyspnea |
EudraCT 2021-003331-28 Spain |
Randomized Double-blind |
776 >18 |
Adults hospitalized for COVID-19 with persistent moderate to severe symptoms 3 months after discharge | Unclear | Not mentioned | COMIRNATY vaccine | Placebo | Change in global score of the COVID-19 symptom questionnaire at week 12 after second dose of COMIRNATY vaccine |
NCT03554265 USA |
Open label | 75 18–70 |
Score of 3 and above on the Brief Fatigue Inventory questionnaire and diagnosed with COVID-19 at least 6 months prior | PCR | Excluded individuals with significant end-organ impairment, uncontrolled diabetes | Recombinant human growth hormone | No growth hormone therapy | Change in body fat distribution, basal metabolic rate, cognitive function and global fatigue scale |
NCT05126563 USA |
Randomized Double-blind |
80 18–70 |
Clinical diagnosis with one or more neurological symptoms for at least 12 weeks after acute infection | Unclear | Excluded individuals with DM, uncontrolled hypertension, renal impairment, cardiac ischemia | Allogeneic adipose-derived mesenchymal stem cells | Placebo | Improvement in neurological symptoms for example, brain fog, headache |
NCT05346120 USA |
Randomized Double-blind |
32 18–80 |
Quick inventory of depressive symptomology score (QIDS-SR) >11 with COVID-19 diagnosis within past 90 days | Unclear | No stroke within last 3 months | Single infusion of marrow stromal cells | Placebo | Improvement in QIDS-SR within 90 days |
NCT05116761 USA |
Randomized Quadruple-blind |
60 18–85 |
At least two or more persistent symptoms of long COVID 4–20 weeks from initial infection | Confirmed previous COVID-19 infection | Excluded individuals with uncontrolled diabetes, underlying respiratory, chronic renal, hepatic impairment | Bone marrow derived extracellular vesicles | Placebo | Distance on 6-min Walk Test |
NCT04326036 USA |
Open label | 10 18–90 |
COVID-19 pneumonia | Confirmed previous COVID-19 infection | Not mentioned | Cellular stromal vascular fraction | Nil | Safety and adverse events Change in pulmonary function 6 months after intervention |
NCT04842448 Sweden |
Randomized Double-blind |
80 18–60 |
Symptoms of long COVID for at least 12 weeks | Unclear | Excluded individuals with diabetes, hypertension | Hyperbaric oxygen treatment | “Sham air” | Quality of life assessed via RAND 36 questionnaire |
NCT05152849 UK |
Randomized Double-blind |
40 18–64 |
Long COVID with fatigue predominance | PCR or serology | Excluded individuals with “uncontrolled, clinically significant disease” | AXA1125 | Placebo | Change in phosphocreatinine recovery rate after exercise |
NCT05228899 USA |
Randomized Double-blind |
30 >18 |
Prolonged fatigue or musculoskeletal complaints 6 weeks after recovery from COVID-19 | PCR or antigen | Excluded individuals with chronic renal, hepatic impairment, uncontrolled hypertension and unstable angina | Zofin | Placebo | Incidence of adverse events Changes in Fatigue Severity Score, COVID-19 related symptoms, inflammatory markers |
NCT05242003 USA |
Randomized Double-blind |
90 >18 |
Post-acute COVID-19 with moderate to severe depression | Unclear | Excluded individuals on steroids and monoclonal antibodies | MYMD1 | Placebo | Change in depressive symptoms Incidence of adverse events Changes in inflammatory markers |
- Abbreviation: PCR, polymerase chain reaction.
3.1 Mechanisms of long COVID and potential therapeutic agents
3.1.1 Chronic inflammation and endothelial dysfunction
The acute COVID-19 infection leads to a hyperinflammatory response involving cytokines interleukin (IL)-1, IL-6, and tumor necrosis factor-α.31 Mounting evidence demonstrates that low-grade chronic inflammation from delayed viral clearance could lead to a persistent catabolic state and contribute to the development of long COVID.32 Such prolonged inflammation damages the vascular endothelium and the endothelial glycocalyx barrier. Degradation of this barrier could expose the subendothelial tissue to oxidative stress,33 propagating endothelial inflammation and precipitating endothelitis.34
Restoring the damaged glycocalyx barrier is a potential strategy for attenuating endothelial dysfunction. Sulodexide, a novel agent, is a glycosaminoglycan with a significant benefit in restoring the endothelial glycocalyx. Its use has been postulated to improve endothelial function, attenuate thrombosis and inflammation. The recently published TUN-EndCOV study recruited 290 patients with long COVID symptoms and proven endothelial dysfunction.25 Approximately one-quarter of the participants had diabetes mellitus, and one-third had hypertension. After receiving oral sulodexide for 3 weeks, there was a significant improvement in endothelial function (measured as postocclusive reactive hyperemia) and this was associated with the alleviation of chest pain and palpitations. However, the quasi-experimental design of the study and the relatively short exposure to sulodexide limit the assessment of long-term outcomes and generalizability of the results. The utility of sulodexide will be further evaluated in an ongoing randomized, double-blind, placebo-controlled trial involving participants who presented with moderate to severe COVID-19 and were presumed to be at a higher risk of endothelial dysfunction. Moreover, the administration of sulodexide will be extended to 8 weeks in this trial, and the outcome measures will include a broader spectrum of thrombotic and inflammatory markers (NCT05371925).
Apart from endothelial dysfunction, persistent cardiopulmonary symptoms secondary to other mechanisms, such as residual myocardial inflammation and postural orthostatic tachycardia syndrome, are major cardiovascular sequelae to tackle. A sustained hyperadrenergic state could lead to frequent palpitations, a potentially debilitating symptom reported by nearly 70% of participants in an international survey involving more than 3700 individuals with convalescent COVID-19.17 Major cardiology societies have endorsed using low-dose beta-blocker for patients experiencing predominantly palpitations.35 However, the dose titration could be limited by the hypotensive effect, especially among individuals with orthostatic hypotension. The efficacy, tolerance, and dose titration of a beta-blocker, metoprolol, are currently being evaluated on individuals with dyspnea and tachycardia on exertion for at least 3 months after COVID-19 diagnosis in an open-label randomized controlled trial (NCT05096884). In patients with significant intolerance to beta-blocker, an alternative agent to consider is ivabradine. Ivabradine is a hyperpolarization-activated cyclic nucleotide-gated channel blocker which decreases the heart rate similar to beta-blockers.26 However, unlike beta blockers which cause a decrease in stroke volume during the initiation period and hence is associated with hypotension, ivabradine is associated with an increase in stroke volume.36 In a small trial of 24 individuals with persistent tachycardia post-COVID, ivabradine was more effective than carvedilol in treating palpitations.26 However, ivabradine is more expensive than generic beta-blockers.
3.1.2 Activation of the coagulation system
There is a bidirectional relationship between chronic inflammation and the coagulation system, implying the possibility of elevated thrombosis extending beyond the acute infectious period. Characterization of coagulation profiles in COVID-19 patients 4 months after discharge revealed persistently increased thrombin release and reduced antithrombin levels. The d-dimer levels were persistently elevated in nearly 25% of recovered COVID-19 patients 4 months after the apparent resolution of the acute infection,37 and in a separate study elevated d-dimer and Factor VIII levels were noted in 17.9% and 48.7% of patients respectively showing hypercoagulability, endothelial dysfunction, and inflammation were still detectable in patients approximately 1 year after recovery from COVID-19.38 The underlying pathophysiology could be attributed to sustained endothelial cell activation, which increases the release of adhesion molecules, promotes leukocyte recruitment, and further enhances the pro-inflammatory state.39 Sustained release of excess thrombogenic mediators into the circulation leads to a persistent hypercoagulability and thrombogenic state.40
The close Interplay between inflammation, endothelial dysfunction, and thrombosis warrants discussing the role of extended prophylactic anticoagulation and statin. The ongoing HElping Alleviate the Longer-term Consequences of COVID-19 (HEAL-COVID) trial aims to investigate the utility of apixaban and atorvastatin in mitigating symptoms of long COVID (NCT04801940). Although heparin effectively reduces mortality among hospitalized patients with COVID-19, there was a significantly increased risk of bleeding with a full dose of heparin.41 Direct oral anticoagulants are an attractive alternative, with the advantages being the convenience of oral administration and lower bleeding risk compared to warfarin and other anticoagulants. Statins have been shown to have pleiotropic effects on the endothelium. Endothelial dysfunction may be reversed via the anti-inflammatory properties, improved vasodilation by increasing nitric oxide production, and reduced oxidative stress.42 The endothelium-dependent effects of statins can be observed as soon as 2 weeks after initiation.43 Given the biological plausibility of anticoagulation and statins in the treatment of long COVID, the HEAL-COVID trial has selected low-dose apixaban to be administered over 14 days and atorvastatin 40 mg daily over 12 months in the postdischarge setting. The role of atorvastatin is also being explored in a randomized, single-blinded trial involving 400 participants with ongoing neurological symptoms attributed to COVID-19 (NCT04904536). The primary outcome is neurological processing speed.
Traditionally used for treating hypertriglyceridemia and reducing residual cardiovascular risk, omega-3 fish oil has several benefits that could potentially be harnessed for managing long COVID. These include immunomodulatory effects, which can enhance innate immunity, anti-inflammatory benefits, inhibiting platelet aggregation and activation of the coagulation cascade, reducing oxidative stress, and promoting vasodilation by increasing angiotensin (1–7) and reducing angiotensin II.44 An ongoing trial (NCT05121766) is currently evaluating the role of omega-3 fish oil on post-COVID fatigue and anosmia.
3.1.3 Chronic inflammation and pulmonary fibrosis
Chronic inflammation and hypoxia may induce pulmonary vascular damage by altering the morphology of the capillary plexus and promoting the formation of microthrombi.45 These changes, in turn, activate the release of transforming growth factor-beta (TGF-β), which mediate the fibroproliferative changes observed in the lungs of COVID-19 patients.46 The common sequelae of pulmonary involvement are unresolved fibrosis, leading to pulmonary hypertension and impaired ventilatory function.47 Respiratory dysfunction could explain some common symptoms of long COVID, such as reduced effort tolerance and fatigue.
Therapeutic agents such as pirfenidone, corticosteroids, and montelukast could be potential treatments targeting the respiratory system in long COVID. In the completed High-dose versus low-dose prednisolone in symptomatic patients with post-COVID-19 diffuse parenchymal lung abnormalities: an open-label randomized trial (COLDSTER trial), an open-label study evaluating the utility of prednisolone in patients with post COVID-19 parenchymal lung abnormalities, the administration of prednisolone 10 mg daily for 6 weeks to individuals with persistent dyspnea or hypoxemia 3 to 8 weeks from the onset of acute COVID-19 was associated with an improvement in dyspnea and respiratory function.27 This finding was corroborated by the Methylprednisolone as Adjunctive Therapy for Patients Hospitalized with Coronavirus Disease 2019: A Randomized, Double-blind, Phase IIb, Placebo-controlled trial (Metcovid trial), a randomized, double-blind, placebo-controlled study, which reported that a short course of methylprednisolone in the acute phase of COVID-19 could lead to significant attenuation of inflammation in the postacute period and hasten the recovery of respiratory function.48 Ongoing trials are evaluating the effects of other agents such as colchicine, pirfenidone, a novel synthetic lung surfactant, and human serum albumin on respiratory symptoms and function.
3.1.4 Long COVID and the neurological and olfactory systems
Neurocognitive complaints are common and can persist for at least 1 year after recovery from acute COVID-19 infection.49 Multiple mechanisms have been postulated, including alteration of the blood-brain barrier, thereby facilitating the transfer of cytokines and exaggerating neuroinflammation,50 aberrant immune responses,51 and encephalopathy from sepsis or hypoxia. The SARS-CoV-2 virus may also invade the olfactory nerves.52 The neurological manifestations can be diverse, ranging from fatigue to “brain fog” to olfactory dysfunction. Various pharmacological targets have been repurposed and being tested for suitability in treating the neurocognitive dysfunction in long COVID. These include atorvastatin, vortioxetine (antidepressant), fampridine (used for multiple sclerosis), RUCONEST (C1-esterase inhibitor for treating hereditary angioedema), and intravenous gammaglobulin. PEA-LUT, a nutritional supplement, has been shown to reduce olfactory dysfunction and improvement in individuals with persistent post-COVID anosmia.28 Several nasal sprays containing ivermectin, and retinoic acid, are also being evaluated for the treatment of post-COVID olfactory dysfunction.
3.1.5 Repurposing nutraceuticals and supplements
Increased oxidative stress and immune dysregulation have been hypothesized to contribute to long COVID.53, 54 This has led to a re-emergence of interest in repurposing nutraceuticals and vitamin supplements as potential therapeutic strategies for long COVID.
Vitamin C plays a vital role in the phagocytosis of neutrophils, without which could lead to the formation of neutrophil traps and lead to microcirculation dysfunction.55, 56 High-dose intravenous vitamin C has been shown to reduce mortality in severe COVID-19 disease via improving oxygenation, attenuating the cytokine storm and oxidative stress.57 Vitamin C could also alleviate fatigue in patients with persistent post-COVID symptoms.58
Vitamin D may play an integral role in the treatment of long COVID. In a small pilot trial evaluating the impact of vitamin D3 supplementation in patients with mild to moderate COVID-19 infection, 5000 IU of vitamin D supplementation daily for 2 weeks reduced the time to recovery for cough and dysgeusia.29 Several postulated benefits of vitamin D include augmentation of innate immunity, attenuation of inflammation, regulation of vasodilatation by promoting nitric oxide synthesis, and defending against oxidative stress.59 Moreover, vitamin D deficiency may be associated with reduced exercise tolerance and fatigue in COVID-19 patients.60 Incorporating vitamin D replacement in long COVID could be beneficial via the mechanisms described.
Other supplements such as soy61 and taurine62 could be tapped for their roles in regulating TGF-β action directly and indirectly. Lowering TGF-β activity could slow down fibrosis in the lungs or myocardium. l-arginine, the precursor of nitric oxide, could be administered as a supplement with antioxidant effects and vasodilatory properties and potentially improves endothelial function.63
3.1.6 Gut dysbiosis and the potential role of probiotics in long COVID
Emerging data have demonstrated the impact of COVID-19 infection on the gut microbiome, disrupting the homeostasis between gut commensals and promoting opportunistic pathogens' growth.64 Consequently, alterations in the intestinal environment could affect short-chain fatty acid production by specific gut commensals. This change could have crucial immunomodulatory implications, as certain short-chain fatty acids, such as butyrate, are involved in regulating phagocytosis and inflammatory responses.65 The gut dysbiosis has been shown to persist after recovering from the acute infection and may be associated with symptoms of long COVID.65
Probiotics could be a promising treatment strategy in relieving the symptoms of long COVID. A randomized controlled trial incorporating the use of probiotics showed greater reduction in fatigue compared to placebo.30 Probiotics increase the availability of gut microbe-derived short-chain fatty acids, which would, in turn, enhance the immune responses and inhibit inflammation.66 Several clinical trials (NCT05080244, NCT04813718, NCT05227170) are evaluating the efficacy of probiotics in the prevention and management of long COVID.
3.1.7 Novel therapies
Cell-based therapeutic targets using mesenchymal stem cells (MSCs) as immunomodulators could regulate immune responses by suppressing cytokine release and enhancing anti-inflammatory pathways.67 Moreover, the regenerative capacity of MSCs may be tapped upon to repair damaged endothelial tissues in blood vessels and epithelial linings of the lungs.68 Pilot phase I to II studies are currently underway to evaluate the safety and efficacy of mesenchymal stem cells on functional capacity and symptom improvement in long COVID.
4 DISCUSSION
We have provided an update on the published and ongoing clinical trials on potential therapeutic agents that can be used to manage long COVID syndrome. The six published trials were conducted when there was growing awareness but limited information about the long COVID syndrome. While these trials offered early insights into the role of repurposing specific therapeutic agents, the data is not robust due to the small sample sizes. With advancing understanding of long COVID, a parallel increase in the number of trials evaluating interventions targeting the various mechanisms of the long COVID syndrome have emerged. However, we observed two main limitations in these trials.
First, there was significant heterogeneity in the definition of long COVID and the assessment of treatment outcomes. While major organizations have published diagnostic criteria,8, 9 these have not been adequately incorporated into the patient selection for clinical trials. Greater adoption of standardized long COVID syndrome diagnostic criteria will be essential to facilitate the selection of trial participants to minimize baseline differences and improve the external validity of the results. Furthermore, there was paucity of information about the trial participants including their vaccination status and the SARS-CoV-2 variant they were infected with. In addition, the assessment of treatment outcomes needs to be rigorous and standardized to ensure the internal validity of the results. Changes in biomarkers, endothelial, vascular function, structural organ changes, and gut microbiome composition can be evaluated accurately using established methods pre- and postintervention. While some of the symptoms of long COVID, for example, shortness of breath and effort tolerance, can be objectively assessed using spirometry and cardiopulmonary exercise testing to correlate with respiratory and exercise capacity, other symptoms of the long COVID syndrome, such as fatigue, could be highly subjective and prone to reporting bias. Concurrent development and validation of symptom scores and patient-reported outcome measures specific for long COVID may be necessary to overcome this challenge. Furthermore, given the high volume of patients affected by long COVID, cost-effective analyses need to be undertaken to determine the long-term effectiveness and outcomes of long COVID interventions.
The second limitation of the ongoing trials is the exclusion of patients with known cardiovascular risk factors. There is growing evidence that long COVID could potentially be a “cardiovascular-centric” condition,33, 34, 38 with persistent endothelial dysfunction and thrombosis.36, 39 Excluding individuals with underlying cardiovascular risk factors could undermine the true impact of long COVID in the long term. Moreover, incorporating patients' comorbidities, such as diabetes mellitus, hyperlipidemia, and hypertension, is instrumental in cardiovascular risk stratification. For instance, long COVID syndrome could be a “second hit” for individuals with diabetes mellitus. These individuals have higher baseline cardiovascular risks, are more likely to develop autonomic dysfunction69 and potentially suffer from more severe orthostatic hypotension and chronic fatigue. In addition, pre-existing diabetes-related complications, such as peripheral neuropathy and musculoskeletal issues could make rehabilitation more challenging. There is an unmet need to implement clinical trials targeting specific subsets of the populations to identify and prioritize treatment strategies to mitigate long-term complications.
Although we have covered pharmacological strategies for the long COVID syndrome, this is only one aspect of the overall management. Nonpharmacological options are currently being evaluated in clinical trials. These interventions include pulmonary rehabilitation, exercise, cognitive training, and repurposing hyperbaric oxygen therapy for chronic fatigue. A multidisciplinary and holistic approach will be crucial in improving outcomes of patients with the long COVID syndrome.
There are limitations in this review. While we have thoroughly searched for published clinical trials and scoped clinical trial registries for ongoing trials, we did not include all available trial registries and literature databases. As such, we could have missed clinical trials or conference proceedings. Moreover, we only searched for publications in English and could have missed trials published in other languages. Given the varied methodology, clinical heterogeneity of the trial participants, and variations in the characterization of the long COVID syndrome, we were unable to assess for bias in these trials thoroughly.
In conclusion, this review provides a summary of pharmacological interventions that could be used to treat long COVID and identifies gaps in the ongoing trials. Future randomized controlled trials need to incorporate exposure method (laboratory confirmed COVID-19); COVID-19 variants and vaccination status in the stratification. They need to adopt standardized diagnostic criteria for long COVID, include participants with comorbidities, and implement rigorous assessment of trial outcomes. The symptoms assessments tools need to be validated and standardized and must assess key mental, physical and social outcomes. For generalizability, data needs to be presented across the range of ethnic and social determinants.
In addition, we echo the need for concerted efforts in basic and translational research on long COVID. Basic research provides the foundation and improves our understanding of the pathophysiology and mechanisms of long COVID. Translational research promotes the development of intervention strategies. Completing this process is to test the interventions in well-designed clinical trials. Upholding the tripartite relationship between basic, translational, and clinical research will be vital in advancing our understanding and the management of the long COVID syndrome.
AUTHOR CONTRIBUTIONS
Ying Jie Chee collected, analyzed the data and wrote the first draft of the manuscript. Bingwen Eugene Fan conceived, designed the study, collected, analyzed the data and critically revised the manuscript. Barnaby Edward Young conceived, designed the study and critically revised the manuscript. Rinkoo Dalan conceived, designed the study and critically revised the manuscript. David Chien Boon Lye conceived, designed the study and critically revised the manuscript. All authors agreed to be accountable for all aspects of the work and approved the final version to be published.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created in this study.




