Neutralization of B.1.617.2 variant through antibodies elicited by ChAdOx1-S, BBIBP-CorV, and Gam-COVID-Vac in an Argentinean cohort
There is global increasing concern about the emergence of SARS-CoV-2 variants along with a decrease of vaccine effectiveness. The role of humoral response in the efficacy of vaccines against variants has focused on antibodies that neutralize SARS-CoV-2, since neutralization strongly correlates with protection from symptomatic infection.1 We report our evaluation of the neutralizing potential of antibodies (NPA) elicited by natural infection and/or vaccination against SARS-CoV-2 B.1.617.2 variant, compared to the ancestral wild-type (WT) virus lineage B.1. We studied 309 plasma samples from individuals sorted into four groups: (1) Unvaccinated and recovered from infection by ancestral WT-B.1 (n = 41); (2) vaccinated with two doses of ChAdOx1-S (n = 78); 21 had SARS-CoV-2 infection prior to vaccination (IPV); (3) vaccinated with two doses of BBIBP-CorV (n = 101); 40 with IPV; (4) vaccinated with two doses of Gam-COVID-Vac (n = 89); 55 with IPV (see Supporting Information). The NPA against ancestral WT-B.1 (EPI_ISL_499083) and B.1.617.2 variant (EPI_ISL_6032417) in plasma was determined as previously described.2
To compare the vaccines, IPV and the two viral variants were considered factors and incorporated in a mixed analysis of variance (ANOVA) analysis (taking sample as subject), using Student Newman Keuls as post-hoc (when ANOVA rejected Ho). NTAbs titers were used as response variables. The reciprocal of NTAb titers were transformed into base 2 LOG and the geometric mean titers (GMT) and antiLOG of GMT were calculated. Titers <1/10 were considered 1 and titers >1/640 were considered 1280. Soft R3 was applied and in all cases, the significance level was 5%.
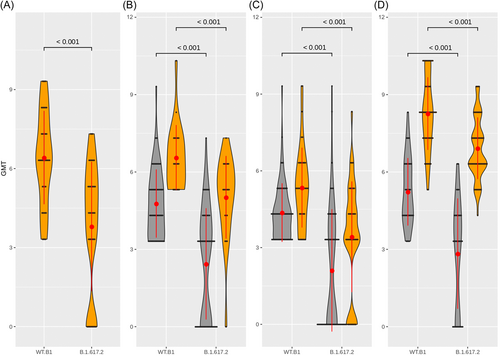
Results showed that considering all the individuals evaluated altogether, a significantly lower NPA was observed against B.1.617.2 compared to ancestral WT-B.1 (GMT 5.8 vs. 3.7, respectively; p < 0.001). When evaluating each group by condition, we detected a significant reduction in the NPA against B.1.617.2 in Group 1 and among individuals vaccinated with two doses of ChAdOx1-S, BBIBP-CorV, or Gam-COVID-Vac, with/without IPV (Figure 1). Group 1 presented a greater reduction of NPA against B.1.617.2 compared to WT-B.1, with a reduction of 2.6 in the GMT. Instead, in individuals vaccinated with BBIBP-CorV, ChAdOx1-S, or Gam-COVID-Vac with IPV, the decrease of GMT against B.1.617.2 compared to WT B.1 was 1.9, 1.5, and 1.4, respectively, while in those individuals without IPV, the reduction of GMT was higher: 2.3, 2.3, and 2.4, respectively (Table 1).
| Vaccine | IPV | SARS-CoV -2 variant | Reciprocal of NTAb titers | LCI- 95% | UCI + 95% | GMT | SD | Group |
|---|---|---|---|---|---|---|---|---|
| BBIBP-CorV | No | B.1.617.2 | 4.32 | 3.57 | 5.22 | 2.11 | 0.14 | A |
| ChAdOx1-S | No | B.1.617.2 | 5.43 | 4.49 | 6.56 | 2.44 | 0.14 | AB |
| Gam-COVID-Vac | No | B.1.617.2 | 7.16 | 5.61 | 9.14 | 2.84 | 0.18 | B |
| BBIBP-CorV | Yes | B.1.617.2 | 10.70 | 8.50 | 13.48 | 3.42 | 0.17 | C |
| UR | Yes | B.1.617.2 | 13.93 | 11.06 | 17.55 | 3.80 | 0.17 | C |
| BBIBP-CorV | No | WT B.1 | 20.68 | 17.10 | 25.01 | 4.37 | 0.14 | D |
| ChAdOx1-S | No | WT B.1 | 27.47 | 22.72 | 33.23 | 4.78 | 0.14 | DE |
| ChAdOx1-S | Yes | B.1.617.2 | 32.45 | 23.42 | 44.95 | 5.02 | 0.24 | E |
| Gam-COVID-Vac | No | WT B.1 | 37.53 | 29.39 | 47.93 | 5.23 | 0.18 | E |
| BBIBP-CorV | Yes | WT B.1 | 40.79 | 32.37 | 51.38 | 5.35 | 0.17 | E |
| UR | Yes | WT B.1 | 85.63 | 67.97 | 107.87 | 6.42 | 0.17 | F |
| ChAdOx1-S | Yes | WT B.1 | 94.35 | 68.10 | 130.73 | 6.56 | 0.24 | F |
| Gam-COVID-Vac | Yes | B.1.617.2 | 121.10 | 98.77 | 148.47 | 6.92 | 0.15 | F |
| Gam-COVID-Vac | Yes | WT B.1 | 308.69 | 251.78 | 378.46 | 8.27 | 0.15 | G |
- Note: Results of the mixed ANOVA (p < 0.001) between the combination of vaccines, confirmed SARS-CoV-2 infection, and SARS-CoV-2 variant. GMTs within a common group are not significantly different (p > 0.05) according to ANOVA and Student Newman Keuls as post hoc.
- Abbreviations: ANOVA, analysis of variance; GMT, geometric mean titers; IPV, SARS-CoV-2 infection prior to vaccination; LCI − 95%, lower confidence interval of reciprocal of NTAb titers; SD, standard deviation; UCI + 95%, upper confidence interval of reciprocal of NTAb titers; UR, unvaccinated and recovered from infection by ancestral WT-B.1; WT, wild type.
The evaluation of vaccinated individuals showed that those immunized with Gam-COVID-Vac and IPV achieved the highest titers of NTAb against B.1.617.2 (Figure 1D). When comparing the GMT against B.1.617.2 between the different groups of vaccinated individuals, there were no significant differences between those immunized with ChAdOx1-S and Gam-COVID-Vac or between ChAdOx1-S and BBIBP-CorV in subjects without IPV; however, we detected significant differences between groups immunized with Gam-COVID-Vac and BBIBP-CorV, respectively. In groups with IPV immunized with BBIBP-CorV or ChAdOx1-S, the GMT were 3.5 and 1.9 times lower than with Gam-COVID-Vac, respectively, yielding significant differences (p < 0.001; Table 1). Although significant differences were observed in NPA from recovered unvaccinated individuals compared to recovered and vaccinated with ChAdOx-1 or Gam-COVID-Vac (p < 0.001), there were no differences between NPA of unvaccinated recovered individuals versus those vaccinated with BBIBP-CorV (p > 0.05).
The comparison between NPA against WT-B.1 and against B.1.617.2 evidenced that the last one decreased more than two GMT in all vaccinated individuals without IPV. The escape of B.1.617.2 variant from neutralization has been previously reported.4-6 Nonetheless, we also show that among individuals with IPV, those immunized with BBIBP-CorV presented NTAbs titers against ancestral WT-B.1 significantly lower than unvaccinated individuals (GMT 5.35 and 6.42, respectively; p < 0.001). Moreover, NTAbs titers against B.1.617.2 were not significantly different between these groups (GMT 3.42 and 3.80, respectively; p > 0.05). Although the lack of cellular response and follow-up for clinical outcome and persistence of NTAb over time are limitations of this study, our findings strongly agree with those reported by Aijaz et al.7 and Badano et al.8 Since NTAbs titers against SARS-CoV-2 have demonstrated to be highly predictive of the host immune protection against infection,9 our results, together with the previously mentioned studies, suggest that BBIBP-CorV would not be the most appropriate vaccine for individuals with IPV, since apparently, it does not contribute significantly to humoral immunity. Evaluation of the cellular response may explain the differences in NTAb response observed among individuals with IPV immunized with BBIBP-CorV.
Our results showed that NTAb against SARS-CoV-2 elicited by natural infection and/or by immunization with two doses of Gam-COVID-Vac, ChAdOx-1 or BBIBP-CorV vaccines were able to neutralize the B.1.617.2 variant. However, there was a significant reduction in the NPA against SARS-CoV-2 B.1.617.2 variant.
AUTHOR CONTRIBUTIONS
Sebastián Blanco, Juan Javier Aguilar, Lorena Spinsanti, Adrián Diaz, Mauricio Beranek, María Elisa Rivarola, Brenda Salomé Konigheim, and Sandra V. Gallego conceived and designed the study. Sebastián Blanco, Juan Javier Aguilar, Lorena Spinsanti, Adrián Diaz, Mauricio Beranek, María Elisa Rivarola, Brenda Salomé Konigheim, and Sandra V. Gallego performed SARS-CoV-2 isolations, plaque reduction neutralization technique, analyzed and interpreted the data and drafted the manuscript. Arnaldo Mangeaud and Elmer Fernández performed the statistical analysis, analyzed and interpreted the data, and revised the final version of the manuscript. All the authors have read and approved the final version of the manuscript.
ACKNOWLEDGMENT
This work was supported by the Fondo para la Investigación Científica y Tecnológica (FONCyT) [PICT IP COVID 19-0464, 2020]. Funding was also provided by the Faculty of Medical Sciences, National University of Córdoba, Argentina, and the Ministry of Health of Córdoba, Argentina.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
This report has been prepared in accordance with specific local regulations (provision n 32/2016, dated September 8, 2016, by the Council for the Ethical Evaluation of Health Research, Ministry of Health of the province of Córdoba, Argentina). The study observed the ethical standards established in the Declaration of Helsinki of 1964 and its subsequent modifications.
Open Research
DATA AVAILABILITY STATEMENT
All data collected for the study will be available with the publication, upon requests directed to the corresponding author. All data collected for the study will be available with the publication, upon requests directed to the corresponding author. The request will be reviewed and approved by the investigators and collaborators based on scientific merit. After approval the request, data will be shared through institutional mails after signing the data access and confidentiality agreement. All data will be available for a minimum of 3 years from the publication of the manuscript.




