Antiviral treatment of COVID-19 is associated with lack of immune response
To the Editor,
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was discovered in 2019 with first oral antivirals Nirmatrelvir/Ritonavir (Paxlovid) and Molnupiravir (Lagevrio) in 2021. One of the main concerns surrounding COVID-19 is the sequelae, termed Long Covid affecting almost 20% of patients, with current Government Accountability Office estimates of up to 23 million patients before the BA.4/5 wave of Summer 2022. The current Long Covid hypothesis is viral persistence in reservoirs, mainly in the brain and the GI system.1, 2 This is akin to HIV, and SARS-CoV-2 can be CD4 dependent and infect T helper cells independent of the ACE2-TMPRSS2 pathway.3
It is common for infections to be treated with multiple drugs (e.g., HIV or mycobacteria) to minimize resistance and sequelae. Drugs chosen target different pathways, to ensure no resistance, which may be due to selective use (e.g., oseltamivir) or patient-associated factors. Thus the development of a Paxlovid-resistant SARS-CoV-2 VOC is a matter of when not if.4 It makes theoretical sense that combination of Paxlovid and Lagevrio would lead to better outcomes, less resistance, and prevent and treat Long Covid. In vitro studies have shown a synergistic effect, a drug interactions, although the hypothetical mutagenic properties of Lagevrio remain a concern.5, 6
Paxlovid use has been associated with “rebound,” with some high-profile cases like Dr. Anthony Fauci taking a second course.7, 8 This raised concerns of whether a 5-day course is sufficient and that early suppression of the virus may lead to blunted immune responses causing rebound.
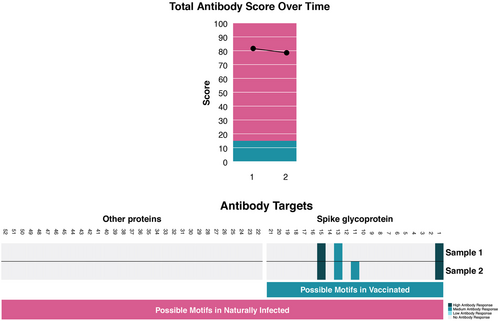
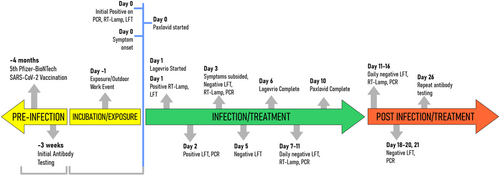
A 37-year-old female with Hashimoto's thyroiditis and insulin resistance on 100 mcg of l-thyroxin and 1000 mg of metformin daily, tested positive for SARS-CoV-2 on a BD Veritor lateral flow test (LFT) as she had been exposed to SARS-CoV-2 36 h prior at an outdoor event. She was vaccinated, boosted and SARS-CoV-2 naïve. She subsequently tested positive on nasal RT-PCR as well as RT-LAMP within hours and developed a sore throat. Owing to a BMI of 43 was Paxlovid started. Next morning, the sore throat continued and the patient was also started on Lagevrio due to a family history and concerns of Long Covid. Sore throat subsided on Day 3, with positive on RT-PCR, RT-LAMP, and LFT until Day 4. The patient continued Lagevrio for 5 days and Paxlovid for 10 days (Figure 2) tolerating them well except for dysgeusia. The patient was also enrolled in a COVID-19 immunity study performing serum epitope repertoire analysis (SERA) of antibodies against SARS-CoV-2.9 (Figure 1, Sample 1). SERA, 3 weeks postinfection demonstrated decreased antibody levels, and a minor change in motifs, indicating that the antivirals stopped the viral replication and blunted the immune response. (Figure 1, Sample 2) The change in motifs as well as the patient's pre- and postinfection antibody profile are consistent with vaccine-induced antibodies against the spike glycoprotein and no antibodies against the nucleocapsid. While some patients do not mount antibodies against the nucleocapsid postinfection,10 the combination of decreasing titers, as well as no “evolution” of the motifs against the original Wuhan spike, in the background of BA4/5 dominance, effectively indicate that from an immunological perspective the infection was as if it never happened. Furthermore, stopping viral replication and release, antivirals may have led to favoring of apoptosis, with increased innate immune response to cell debris versus adaptive response.11 Furthermore, CDC recommends waiting at least 2 months post-COVID-19 for a booster. However, in light of antivirals possibly suppressing immune responses against COVID-19 a more prudent approach may be to boost patients to ensure appropriate immune response while on Paxlovid. That is, instead of Test and Treat, Pax and Boost; especially that the public and clinicians stick to the old codicil that a COVID-19 infection leads to long-lasting immunity.12
Future studies should look if antivirals have any effect on Long Covid, if combination of Paxlovid and Lagevrio leads to better outcomes, if delayed initiation of antivirals leads to better immunological outcomes, if a 10-day course of Paxlovid minimizes risk of rebound, and if a Pax and Boost strategy would be beneficial. This case is also limited by lack of laboratory measurements as well as further immune response analysis outside of the antibody responses, however, in the absence of symptoms, the value of such data would have been limited. Although this case is anecdotal, in light of rapidly evolving data as well as sheer scale of a “secondary” pandemic of Long Covid, raises important questions that need to be answered by investigators studying the SARS-CoV-2 and its sequelae.
CONFLICT OF INTEREST
The author declares no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




