Efficacy of an accelerated vaccination schedule against hepatitis E virus infection in pregnant rabbits
Abstract
An important goal of the Hepatitis E virus (HEV) vaccine is to prevent adverse pregnancy outcomes caused by different HEV genotypes during pregnancy, but studies directly evaluating maternal vaccination for HEV are lacking. Here we report maternal vaccination using HEV 239 vaccine in a pregnant rabbit model. Two dose of accelerated vaccination schedule (0, 7 days) induced high titers of anti-HEV protective antibodies in a short period of time in pregnant rabbits, which could protect the pregnant rabbits from HEV infection and adverse pregnancy outcomes. In addition, the immunized rabbits transfer maternal antibodies to pups through the placenta and breast milk, which protect neonates against HEV infection. Our results suggest that, besides vaccinating nonpregnant individuals, HEV 239 vaccine may also be discreetly considered for maternal vaccination.
Abbreviations
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- ELISA
-
- Enzyme-linked immunosorbent assay
-
- GMT
-
- geometric mean titer
-
- HEV
-
- hepatitis E virus
-
- RT-nPCR
-
- reverse transcription-nested PCR
1 INTRODUCTION
In most people, Hepatitis E virus (HEV) often causes acute self-limited disease with a low case fatality rate of 0.2%–1%.1 However, pregnant women are particularly vulnerable to HEV with a high rate of maternal mortality (up to 30%), miscarriage, premature delivery, and stillbirth.2 There are currently four major HEV genotypes (HEV1-4) closely related to human hepatitis. In countries prevalent with HEV1 and HEV2, hepatitis E-induced high mortality in pregnant women still exists and remains a serious and severe public health problem.3 In developed countries and China where HEV3 and HEV4 are prevalent, high mortality in HEV-infected pregnant women is rarely seen4-6 but relative high rate of premature delivery was reported.7, 8 There is currently no approved drug against HEV. Although the broad-spectrum antiviral ribavirin has been proved efficient in treating chronic HEV infection, it is unsuitable for use in pregnancy due to the risk of teratogenicity.9 Before the discovery of an efficient and safe anti-HEV drug, active vaccination against HEV may be an alternative to protect pregnant women from HEV infection and its associated severe adverse outcomes. Several vaccines, including influenza vaccine, pertussis vaccine and SARS-CoV-2 vaccine are currently recommended or under human clinical trial for pregnant women.10, 11 The safety and efficacy of any vaccine when inoculates during pregnancy warrants urgent elucidation especially for people living in settings of endemic HEV or settings of high risk of outbreak.
A hepatitis E vaccine (HEV 239 vaccine, Hecolin®) has been licensed in China since 2012 and proved to be highly effective in protecting against both HEV1 and HEV4 infections in the general population in Phase 3 trial.12 A WHO position paper stated that HEV 239 vaccine should be used to prevent outbreaks and to reduce the high risk of infection for people in ongoing hepatitis E outbreak settings.13 Under these circumstances, the routine hepatitis E vaccination strategy (0, 1, and 6 months) might not provide timely protection for people in need. Especially, during the early phase of an outbreak, women who are already pregnant are in urgent need of immediate protection. A recent Phase 4 trial showed that an accelerated schedule of hepatitis E vaccination (0, 7, and 21 days) demonstrated desirable immunogenicity and safety profile.14 This accelerated schedule provides a potential opportunity for protection during pregnancy. During the Phase 3 trial, 37 pregnant women were inadvertently vaccinated with HEV 239 vaccine and no adverse infant or maternal outcomes were observed.15 However, the trial is not designed to study the efficacy of vaccination during pregnancy. Therefore, a suitable animal model that can recapitulate the HEV-induced adverse pregnancy outcomes is needed for the evaluation of the safety and efficacy of HEV 239 vaccine vaccination during pregnancy. We previously established a HEV infection pregnant rabbit model that can manifest with high mortality, miscarriage, and vertical transmission of HEV16 and complete vaccination before mating can induce sufficient immunity against pregnancy HEV infection.17 In this study, we developed an accelerated hepatitis E vaccination strategy in pregnant rabbits adapted from a Phase 4 trial conducted in human volunteers and evaluated the efficacy of this strategy.
2 MATERIALS AND METHODS
2.1 Animals
Seven-month-old female Japanese white female rabbits weighing between 3.0 and 5.0 kg were obtained from the Beijing Jinmuyang Experimental Animal Breeding Co. Ltd. Serum and feces specimens from selected rabbits were collected once a week for 2 weeks to test ALT and AST levels and to establish a baseline. Enzyme-linked immunosorbent assay (ELISA) and reverse transcription-nested PCR (RT-nPCR) were performed to ensure that all specimens were negative for HEV RNA and antibodies against HEV.
For pregnant rabbits, 7-month-old female rabbits were paired with 12-month-old male rabbits. Once they mated successfully, the rabbits were defined at starting embryotic stage (Day 0). The successful rate of mating pregnancy of female rabbits in rut is about 1/3.
The animal experiments were approved by the Committee of Laboratory Animal Welfare and Ethics, Peking University Health Science Centre.
2.2 Vaccine and viruses
The HEV 239 vaccine (Hecolin®; Xiamen Innovax Biotech) is a 26 kDa recombinant polypeptide expressed by the Escherichia coli system derived from the 368–606 aa segment of the HEV1 (GenBank D11092) ORF2 protein. It is currently the only commercially available HEV vaccine globally.
The rabbit-derived HEV-3ra (CHN-BJ-R14) and human-derived HEV4 (hHEV 4d) strains were all made from feces (GenBank accession No. JX109834 and MT993748). The HEV-positive fecal samples were diluted in sterile phosphate buffer saline respectively to make 10% (wt/vol) suspensions and then centrifuged at 4000g at 4°C for 30 min. The clarified suspension was filtered through 0.45 and 0.22 μm filters and titrated subsequently. All HEV-positive inocula were adjusted to approximately 5 × 106 copies/ml. Rabbits were inoculated intravenously via ear veins or abdominal cavity.
2.3 The experiment of HEV 239 vaccine accelerated vaccination schedule for pregnant rabbits
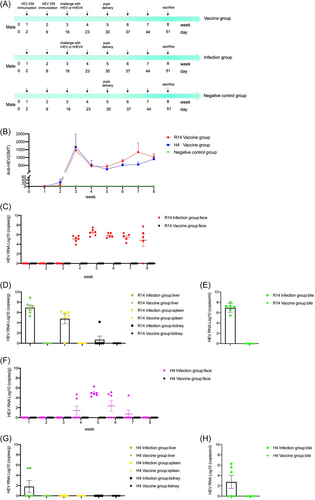
Experimental scheme: HEV 239 vaccine immunized at Day 2 and 9. Seven-month-old pregnant rabbits were randomly divided into five groups: (1) R14 vaccine group (n = 6), pregnant rabbits were inoculated with 10 μg of HEV 239 vaccine intramuscularly on the 2nd and 9th day after mating, and were challenged intravenously with 5 × 106 copies/ml rHEV at Day 16; (2) R14 infection group (n = 6), pregnant rabbits were challenged with rHEV only at Day 16; (3) H4 vaccine group (n = 6), pregnant rabbits were inoculated with 10 μg of HEV 239 vaccine intramuscularly on the 2nd and 9th day after mating, and were challenged intravenously with 5 × 106 copies/ml hHEV4 at Day 16; (4) H4 infection group (n = 6), pregnant rabbits were challenged with hHEV4 only at Day 16; (5) negative control group (n = 6), the rabbits were normally mated and produced, and no vaccine or HEV strains was inoculated. The pups were delivered after a full-term pregnancy at Day 30. They were divided into two groups. Group 1 pups were euthanized and collected for fetal blood immediately and measured for anti-HEV antibody titers. Group 2 pups were euthanized and collected for fetal blood and measured for anti-HEV antibody titers 2 weeks later (at Day 44). As well, Maternal milk were harvested on the day of delivery and 2 weeks later for measuring anti-HEV antibody titers. Serum and fecal specimens from pregnant rabbits were collected weekly for measuring anti-HEV antibody titers and fecal HEV RNA. Maternal rabbits were euthanized at Week 8, their liver, spleen, kidney and bile were collected for measuring HEV RNA.
2.4 The acquisition of anti-HEV antibodies serum with geometric mean titer (GMT) of 1024
At Week 8 when rabbits were euthanized, maternal blood of R14 vaccine group of HEV 239 vaccine immunized at Day 2 and 9 was obtained by superior vena cava puncture. The Blood samples were collected in Corning 50 ml RNase-free centrifuge tubes, centrifuged at 4°C, 4000 rpm for 10 min, and the serum was dispensed into 1.5 ml EP tubes and 50 ml centrifuge tubes, respectively. The serum in the 1.5 ml EP tube was immediately subjected to quantitative detection of anti-HEV antibody, and the serum anti-HEV antibody GMT was measured and labeled on the corresponding 50 ml centrifuge tube and stored in a −80℃ refrigerator for later use.
2.5 The experiment of maternal vaccination on offspring protection
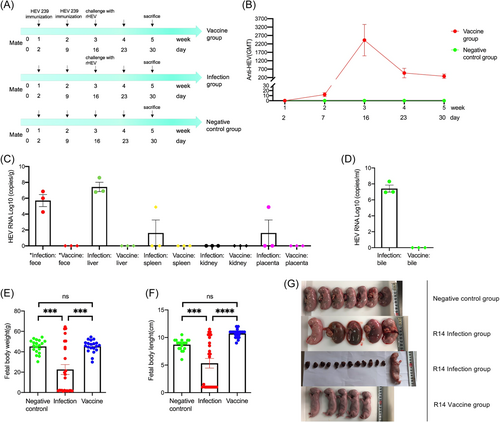
Experimental Scheme 1: 7-month-old pregnant rabbits were randomly divided into three groups: (1) R14 vaccine group (n = 3), pregnant rabbits were inoculated with 10 μg of HEV 239 vaccine intramuscularly on the 2nd and 9th day after mating, and were challenged intravenously with 5= × 106 copies/ml rHEV at Day 16; (2) R14 infection group (n = 3), pregnant rabbits were challenged with rHEV only at Day 16; (3) negative control group (n = 3), the rabbits were normally mated and produced, and no vaccine or HEV strains was inoculated. Serum of the pregnant rabbits were collected weekly for measuring anti-HEV antibody titers. The pups were Cesarean delivered before a full-term pregnancy at Day 30. Maternal feces, liver, spleen, kidney, placenta, and bile were collected for measuring HEV RNA. Fetal body weight and length were measured and representative images of fetuses were collected.
Experimental Scheme 2: Nine newborn rabbits were randomly selected from the negative control group and R14 vaccine group of HEV 239 vaccine immunized at Day 2 and 9 respectively and challenged with rHEV of 1/10 maternal rabbits' dose, observing the pups' survival rate.
2.6 The experiment of antibody prevention and treatment
Seven-month-old pregnant rabbits were randomly divided into two groups: (1) Antibody prevention group (n = 4), pregnant rabbits were daily inoculated intravenously with 10 ml anti-HEV antibodies with GMT of 1024 from the 11th to 15th day after mating for 5 consecutive days, which totaled 50 ml; and were challenged intravenously with 5 × 106 copies/ml rHEV at Day 16; (2) Antibody treatment group (n = 4), pregnant rabbits were challenged intravenously with 5 × 106 copies/ml rHEV at the 16th day after mating, and were daily inoculated intravenously with 10 ml anti-HEV antibodies with GMT of 1024 from the 23rd day after mating when fecal virus shedding began to be observed to the 27th day for five consecutive days, which totaled 50 ml. Serum and fecal specimens from pregnant rabbits were collected weekly for measuring anti-HEV antibody titres and fecal HEV RNA load. Maternal liver, spleen, kidney, and bile were collected for measuring HEV RNA load at Day 51.
2.7 Quantification of viral load in feces, organs, and bile from the rabbits
Total RNA was extracted from 200 μl fecal suspensions, 100 mg tissues and 200 μl bile, using TRIzol reagent (Invitrogen, Burlington) or EasyPure viral RNA/DNA kit (TransGen Biotech). Reverse transcription was performed using the SuperScript IV kit (Invitrogen) according to manufacturer's instructions and cDNA was then amplified by nested-PCR for partial ORF2 genome on an automatic PCR system (Applied Biosystems ProFlex PCR). HEV viral load was determined using a commercial one-step real-time quantitative PCR assay (GoTaq® Probe 1-step RT-qPCR System Kit, Promega) as previously described.17, 18
2.8 Detection or quantification of anti-HEV
Anti-HEV antibody were detected by commercial ELISA kits (Wantai) according to manufacturer's instructions. Signal-to-cutoff (S/CO) values were calculated and values >1 were considered positive. Quantification of total anti-HEV was measured as previous descripted.17 Titres were measured by two-fold serial dilutions.
2.9 Statistical analysis
Prism 9 software (Graphic software) was used for charts and statistical analyses. The analysis of Variance was conducted to compare the difference in each group. Asterisks denote statistical significance (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001). The data are reported as the mean ± SEM.
3 RESULTS
3.1 Efficacy of HEV 239 vaccine accelerated vaccination schedule for pregnant rabbits
We tested the efficacy of HEV 239 vaccine in maternal vaccination in pregnant rabbits. Six pregnant rabbits of R14 vaccine group were intramuscularly immunized with two doses of 10 μg HEV 239 vaccine at Day 2 and 9, respectively (Figure 1A). High anti-HEV antibody titers of 1451 were detected at Day 16 (Figure 1B). After intravenously challenged with 5 × 106 copies/ml CHN-BJ-R14 (rHEV) at Day 16, all of the six pregnant rabbits in R14 infection group had persistent fecal virus shedding, whereas no fecal virus shedding was detected from the R14 vaccine group (Figure 1C). As shown in Table 1, the overall incidence of adverse pregnancy outcomes in R14 infection group was 33.3% (2/6), with 16.7% (1/6) maternal death (happened at Day 29), and 16.7% (1/6) miscarriage. In R14 vaccine group, we also observed 16.7% (1/6) stillbirths, there was no adverse pregnancy outcome in negative control group. At Day 51, including the rabbit of maternal death, HEV RNA was detected in maternal liver 100% (6/6), spleen 83.3% (5/6), kidney 16.7% (1/6), and bile 100% (6/6) from the R14 infection group; whereas no virus was detected in any organs from the HEV 239-vaccinated animals (Figure 1D,E). No obvious abnormal reactions were found in the rabbits of negative control and vaccine group.
| Group | Adverse pregnancy outcomes (%) | Total (%) | ||
|---|---|---|---|---|
| Death | Miscarriage | Stillbirth | ||
| R14 infection | 1/6 (16.7) | 1/6 (16.7) | 0/6 (0) | 2/6 (33.3) |
| R14 vaccine | 0/6 (0) | 0/6 (0) | 1/6 (16.7) | 1/6 (16.7) |
| H4 infection | 0/6 (0) | 0/6 (0) | 1/6 (16.7) | 1/6 (16.7) |
| H4 vaccine | 0/6 (0) | 0/6 (0) | 0/6 (0) | 0/6 (0) |
| Negative control | 0/6 (0) | 0/6 (0) | 0/6 (0) | 0/6 (0) |
In addition to protect against the rHEV strain, we also tested the HEV 239 vaccine for protection against hHEV4 strain. Like the protection effect against the rHEV strain, the HEV 239 vaccine also has a protection effect against the hHEV4 strain (Figure 1F–H). The overall incidence of adverse pregnancy outcomes in H4 infection group was 16.7% (1/6), it was stillbirth. All rabbits in H4 vaccine group had normal delivery and no adverse pregnancy outcomes occurred (Table 1).
3.2 The effect of maternal vaccination on offspring protection
The internal environment of uterus of the pregnant rabbit before giving birth is complex. It is difficult to obtain the placenta and observe the appearance of all neonates after the pregnant rabbit giving birth. To obtain the placenta to study the vertical transmission of HEV virus and visually observe the status of the fetuses before birth, we performed cesarean delivery before a full-term pregnancy at Day 30 in R14 vaccine group (n = 3), R14 infection group (n = 3) and negative control group (n = 3) (Figure 2A). High anti-HEV antibody titers of 2423 were detected at Day 16 (Figure 2B). At Day 30, HEV RNA was detected in maternal feces 100% (3/3), liver 100% (3/3), spleen 33.3% (1/3), kidney 0% (0/3), placenta 33.3% (1/3), and bile 100% (3/3) from the R14 infection group; whereas no virus was detected in anywhere from the HEV 239-vaccinated animals (Figure 2C,D). In addition, there was no significant difference in the average fetal body weight and length of pregnant rabbits between the negative control group and R14 vaccine group; compared with the negative control group and R14 vaccine group, the average fetal body weight and length of the pregnant rabbits in R14 infection group were significantly lighter and shorter (p < 0.001); moreover, the offspring of the pregnant rabbits in R14 infection group were severely absorbed (Figure 2E–G).
Previous studies have showed that the antibodies could transfer from the mothers with vaccination during pregnancy through placenta to the fetuses, and that neutralizing antibodies were found in the maternal milk during lactation period.19 In this part of the study, we aimed to explore the change of antibodies in neonatal serum and maternal milk, and whether they can clear HEV.
As shown in Figure 3A, IN R14 vaccine group immunized at Day 2 and 9, the GMT of anti-HEV antibodies in maternal serum was 427 and 1339 at Week 5 (pregnant rabbits giving birth) and Week 7 (2 weeks after giving birth), and maternal milk was 99 and 435 at Week 5 and 7, both of which showed an increasing trend. However, the GMT of anti-HEV antibodies in neonatal serum was 100 and 6 at Week 5 and 7, showing a decreasing trend. Similarly, the GMT of anti-HEV antibodies in maternal serum, maternal milk and neonatal serum in H4 vaccine group showed the same changing trend with those of R14 vaccine group (Figure 3B).
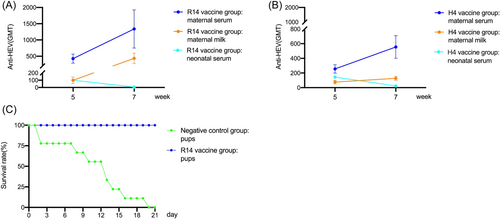
Nine newborn rabbits were randomly selected from the negative control group and R14 vaccine group respectively and challenged with rHEV strains of 1/10 maternal rabbits' dose. After 21 days, the pups of the R14 vaccine group all survived, and those of negative control group all died (Figure 3C).
3.3 Antibody prevention and treatment
To explore the critical GMT value of anti-HEV antibodies that can prevent HEV infection during pregnancy, we performed passive transfer experiment of serum antibodies (Figure 4A). After the antibody transferred and before virus inoculation, the GMT of serum anti-HEV antibodies of the four pregnant rabbits in antibody prevention group was 64, 256, 64, and 128, respectively, which averaged 128, and decreased down to 1, 1, 0, and 1 in the 8th week after mating (Figure 4B). None of the four pregnant rabbits in antibody prevention group had fecal virus shedding before birth, four rabbits had fecal virus shedding in the 1st week after birth (Week 6), and two had fecal virus shedding in the following 2 weeks (Week 7 and 8) (Figure 4C). HEV RNA was detected in maternal liver 50% (2/4), spleen 0% (0/4), kidney 0% (0/4), and bile 75% (3/4), respectively.
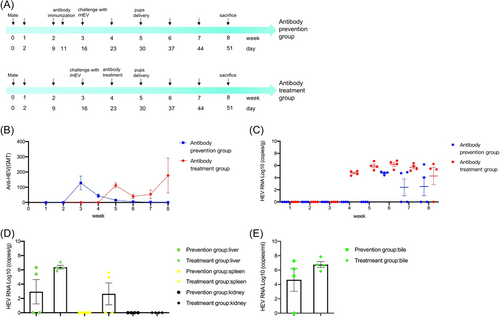
HEV infection can clear spontaneously in most immunocompetent patients without antiviral treatment.3 At present, there is no specific drug against HEV infection, separate or combined use of RBV and IFNα are currently the main treatment of HEV infection, but they are teratogenic and therefore contraindicated in pregnant women.20-23 This part of the study was exploratory and aimed at figuring out whether the serum with neutralizing antibodies could clear HEV infection (Figure 4A). After 2 weeks of virus inoculation when antibody was transferred, the GMT of serum anti-HEV antibodies of the four pregnant rabbits in antibody treatment group was 128, 128, 64, and 128, respectively, which averaged 112, decreased first and then increased to 64, 4, 512, and 128 in the 8th week after mating (Figure 4B). The four pregnant rabbits in antibody treatment group had continuous fecal virus shedding all the time after virus inoculation (Figure 4C). HEV RNA was detected in maternal liver 100% (4/4), spleen 50% (2/4), kidney 0% (0/4), and bile 100% (4/4) in the 8th week after mating (Figure 4D,E).
4 DISCUSSION
In the study, we aimed to explore the effect of vaccination on preventing HEV-related adverse pregnancy outcomes in pregnant rabbits through animal experiments, so as to provide experimental evidence for widely carrying out HEV vaccination in pregnant women and for determining appropriate vaccination strategies. However, the gestation period of the rabbits was only about 1 month, which was 10 months for human. In this case, the routine HEV vaccination schedule (0, 1, and 6 months) for human was not applicable and should be adjusted for rabbits accordingly. Besides, HEV outbreaks frequently occur in some deprived countries and regions in Asia and Africa.24 In recent years, HEV outbreaks have also happened in the regions with local armed conflicts and requiring humanitarian assistance.25 For the emergency responders and the people who have to go to outbreak areas or those who are in the outbreak areas, the vaccination should be completed in a shorter time.
In our previous studies, two doses of 10 μg HEV 239 vaccine on Week 0 and 4 in child-bearing female rabbits prevented the rabbits from HEV infection in later pregnancy, and prevented HEV-related adverse pregnancy outcomes.17 But so far, there is no study proving the safety and efficacy of HEV 239 vaccine inoculated during pregnancy. Therefore, we did further exploration into the safety and efficacy of HEV 239 vaccine inoculated during pregnancy. The results showed that two doses of 10 μg HEV 239 vaccine at different periods of pregnancy could all induce anti-HEV antibodies and protect the pregnant rabbits from being infected with HEV. Similar to the effect of preventing HEV1 and HEV4 infections in general population,12 HEV 239 vaccine could prevent the infections of rHEV and HEV4 in pregnant rabbits. The results of this part of the study suggested for the first time that HEV 239 vaccine inoculated during pregnancy can prevent HEV infection and HEV-related adverse pregnancy outcomes in pregnant rabbits.
Vaccinating women during pregnancy has two distinct potential benefits. First, it protects the woman from infections that she may be particularly susceptible to during pregnancy, which in turn, protects the fetus from congenital infection and other harmful effects of maternal infection. Second, maternal vaccination may be used for the primary intention of protecting the developing fetus and infant from infection during the first months of life through the placental transfer of neutralizing immunoglobulin G (IgG) antibodies and/or secretory immunoglobulin A (IgA) antibodies in the mother's breast milk.10 In this study, anti-HEV antibodies were continuously detected in the serum of newborn rabbits and the mother rabbit's breast milk. Compared with the continuous downward trend of anti-HEV antibodies in the serum of newborn rabbits, the anti-HEV antibodies in mother rabbit's breast milk continued to increase. It is similar with a study conducted by Schlaudecker et al.26 in which sustained high levels of influenza-specific IgA antibodies were found in the breast milk of women vaccinated against influenza during pregnancy for up to 6 months after birth. Constrained by conditions, our study did not monitor the anti-HEV antibodies in neonatal serum and maternal milk for a long time. All of the results suggested that HEV 239 vaccine could not only protect pregnant rabbits from HEV infections and HEV-related adverse pregnancy outcomes but also neonates, which on the one hand protect foetuses against HEV infection from intrauterine, on the other hand against external HEV infections, which was also proved in subsequent infection experiment in neonatal rabbits.
In the accelerated vaccination group of pregnant rabbits, HEV 239 vaccine induced high titre anti-HEV antibodies with an average GMT of 1558. High titre antibodies protected them from HEV infections and prevented adverse pregnancy outcomes. To explore the critical GMT value of preventing HEV infections during pregnancy, we performed passive transfer experiments of serum antibodies. It was found that half of the pregnant rabbits could be protected from HEV infections when the average GMT of serum anti-HEV antibodies reached 128. In the Phase 3 trial, pregnant women were initially excluded. However, there were 37 and 31 women getting pregnant during the trial in vaccine group and control group, respectively. There was no difference in the incidence of adverse effects and also in the weight and length of the neonate between the two groups, which might indicate the safety of HEV 239 vaccine in pregnant women.12, 15 In another Phase 4 clinical trial (no one infected and no infection experiment), the immunogenicity and safety of accelerated vaccination schedule of HEV 239 vaccine (0, 7, 21 days) was proved in healthy adults (pregnant women not included).14 To sum up, it is feasible to carry out emergency vaccination of two doses of HEV 239 in pregnant women especially those in high-risk areas.
In the last part of the study, we explored whether the serum with neutralizing anti-HEV antibodies was effective in treating the pregnant rabbits infected with HEV, but it was unfortunately found ineffective in our experimental design. Based on the severity of pregnant women infected with HEV and the situation of no effective treatment available, we should look at the issue of HEV 239 vaccination during pregnancy scientifically, improve women's confidence in vaccination, so as to better protect the health of pregnant women and fetuses.
AUTHOR CONTRIBUTIONS
Ling Wang directed and supervised the research. Fan Zhang and Ling Wang designed the study. Fan Zhang performed the study and wrote the original manuscript. Zhaogeng Yang and Cong Dai analyzed the data, contributed to manuscript preparation and assisted in polishing the manuscript. Qiyu He, Zhaochao Liang and Tianxu Liu participated and assisted in the animal experiments. Weijin Huang, Youchun Wang and Lin Wang directed the research. Ling Wang reviewed and edited the manuscript. Ling Wang is the corresponding author of this manuscript.
ACKNOWLEDGMENTS
We thank Xiamen Innovax Biotech, Xiamen, China for providing the vaccines. This work was supported by the Nation Nature Science Foundation of China under grant (81772175, Ling Wang).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




