Tecovirimat therapy for severe monkeypox infection: Longitudinal assessment of viral titers and clinical response pattern—A first case-series experience
Lennart Hermanussen and Ilka Grewe contributed equally to this study.
Marc Lütgehetmann, Julian Schulze zur Wiesch, and Stefan Schmiedel contributed equally to this study.
1 INTRODUCTION
Tecovirimat is the only drug approved by the European Medicines Agency (EMA) for the treatment of monkeypox (MPX) under exceptional circumstances.1 Tecovirimat inhibits the viral spreading of orthopoxviruses2, 3 and proved to be very effective in animal models.4 A human safety study did not show any severe side effects.5 Data on the efficacy of tecovirimat therapy of MPX in humans have not been established in prospective trials and the therapeutic use has only been documented in single cases6-10—but all these reports are lacking detailed descriptions of treatment responses and viral kinetics during treatment. We and others have recently established methods for serial assessment of MPX viral DNA loads in various materials.11-13 Here, we report the clinical treatment experience with tecovirimat, including longitudinal viral load kinetics in different compartments, in three patients with severe MPX. Tecovirimat was administrated at a daily dosage of 1200 mg together with a meal.10
1.1 Case vignettes
1.1.1 Severe course of MPX in a patient with ulcerative colitis treated with vedolizumab
In July 2022, we admitted a 54-year-old Afro-American male who had tested positive for MPX. The patient reported sexual encounters with several male partners 4 days before the first symptoms. He initially presented with inguinal lymphadenitis in addition to penile, scrotal, and perianal lesions. Especially for the first days, he suffered from fever and malaise. Within a week more than 100 marks scattered across the whole body, including his face, hairy scalp, trunk, and extremities, as well as oral, palmar, and plantar regions (Figure 1A, panel 1). Dark spots appeared on his soles, which were painful and rendered him unable to walk (Figure 1A, panel 2). Suspecting a bacterial superinfection of some lesions, an oral anti-infective therapy with amoxicillin and clavulanic acid was initiated.
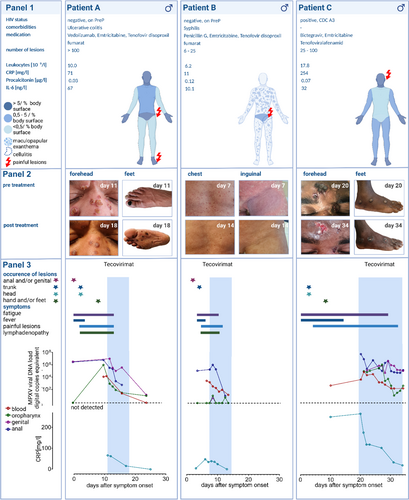
The only known pre-existing condition of this patient was ulcerative colitis (UC) which was in remission under standard therapy with vedolizumab. The patient was tested human immunodeficiency virus (HIV) negative at admission and was stable on HIV pre-exposition prophylaxis with emtricitabine/tenofovirdisoproxil (PrEP). At the time of hospitalization, there were no gastrointestinal symptoms other than pain at defecation. A mild ulcerative proctitis but no active colitis was found endoscopically. Laboratory findings included white blood cell counts within the normal range, but elevated C-reactive protein (CRP) (71 mg/L) and interleukin-6 (IL-6) (67 ng/L) values (Figure 1A, panel 1). Treatment with tecovirimat was initiated at a progressive and prolonged infection, a seriously ill condition, and high viral DNA loads of MPX virus (MPXV) in all specimens. The lesions partly faded, no novel lesions developed, and the patient's subjective condition rapidly improved. Also, viremia and viral DNA loads of oropharyngeal, penile, and anal swabs decreased after treatment onset (Figure 1A, panel 3). Antiviral therapy with tecovirimat was terminated early after 7 days and the patient was discharged in good health.
1.1.2 Severe generalized symptoms in a patient with MPX and syphilis coinfection
A 44-year-old Caucasian male patient, who had tested positive for MPX, presented to our ID clinic. The patient was tested HIV negative, was taking PrEP, and had no known immunosuppressive condition. He reported sexual contact with other men 6–10 days before symptom onset. Initially, he developed two gluteal skin lesions that had already developed crusts 3 days postsymptom onset. When he presented to the hospital, papules with concomitant confluent erythema had occurred on the trunk and extremities. Additionally, the patient showed a macular plantar and palmar exanthema, as well as a most painful asymmetrical inguinal lymphadenitis with regional imbibition and severe muscle ache. Notably, the patient had been vaccinated against MPX with Imvanex 4 days before.
Due to severe generalized symptoms and the suspicion of a highly active infection, early treatment with tecovirimat for 7 days was initiated. Within the following days, the patient's clinical condition rapidly improved, the inguinal lymphadenopathy and muscle aches resolved, and the papular lesions and concomitant erythema faded (Figure 1B). In parallel, the patient was also found to suffer from acute syphilis (TP-Blot: IgG/IgM positive, Treponema pallidum particle agglutination assay 1:5000, venereal disease research laboratory test 1:16; as of June 2022, a prior test had been negative) and treatment with intramuscular penicillin was administered.
1.1.3 Severe MPX in an HIV-positive patient with an unknown mode of transmission
In August 2022, a 31-year-old male Somali presented with severe MPX. Any men who have sex with men was denied by the patient. As a preknown condition, he had an HIV infection with a compromised immune status (CD4+ T cells: 50/µl). Antiretroviral therapy was just started 3 months ago with poor adherence. The initial HIV titer of 1.29 × 106 copies/ml rapidly decreased upon observed antiretroviral therapy in the hospital. Symptoms had originally developed 20 days before admission, at a time when he was isolated for a mild severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. At first, he showed large papular lesions on his nose and forehead. Subsequently, mostly isolated lesions with central necrosis appeared all over the body, but sparing the genital area (Figure 1C, panel 2). Furthermore, he suffered from intermittent fever, malaise, and weakness. Laboratory findings included highly elevated CRP (254 mg/dl) levels and leucocytosis (17.8 x 109/L), but normal procalcitonin levels (Figure 1C, panel 1). Treatment with tecovirimat was initiated as a highly active, uncontrolled infection was suspected. Due to painful tissue damage, comedication with opioids was needed. As his subjective health only slowly improved, treatment was extended to 14 days. CRP and viral DNA load decreased in all compartments within the first week of treatment with tecovirimat (Figure 1C, panel 3).
1.2 Longitudinal assessment of viral DNA loads and laboratory pattern under tecovirimat treatment
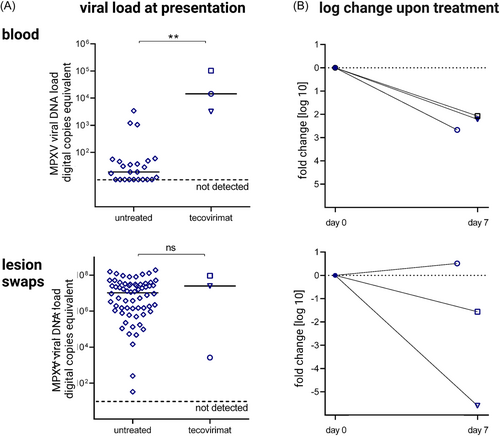
The three patients assessed here were admitted at a range of 3–20 days after symptom onset. At this time, they had significantly higher MPX viremia (1.4 × 104, 3.2 × 103, and 1.0 × 105 copies/ml) when compared to the median of a larger overall local cohort of previous patients with less severe disease courses (n = 21): median 1.7 × 101 copies/ml (range: under limit of detection–3.4 × 103 copies/ml) (Figure 2A). However, baseline viral DNA load obtained from lesion swabs did not significantly differ between our three index patients (2.6 × 103, 2.5 × 107, and 9.2 × 107) and the baseline swabs from the overall cohort (n = 64): median 1 × 107 copies/ml (range: 3.3 × 101–2.0 × 108 copies/ml) (Figure 2A). Upon treatment initiation, viral DNA load in blood decreased by 2.21 log in median (−2.67, −2.21, and −2.07 log) within 1 week (Figure 2B). Overall, viral DNA load from oropharyngeal, anal, penile, and lesion swabs had widely variable baseline levels and decreased far less uniformly at a greater range under therapy (Supporting Information: Figure 1).
Only Patient C showed a transient increase of the γ-glutamyltransferase within the first week of treatment (max. 277 U/L), while the transaminases remained inconspicuous (data not shown).
Even in patients with presumably highly active disease, IL-6 levels were only moderately increased. All patients showed elevated CRP values and normal procalcitonin levels (Figure 1, panel 1). Under treatment with tecovirimat and in parallel to the observed clinical improvement of all three patients, the CRP levels decreased accordingly (Figure 1, panel 3).
2 DISCUSSION
Here, we present detailed clinical and virologic data of the prospective, longitudinal assessment of three individuals with severe MPX infection who were treated with tecovirimat. All three patients had individual potential risk factors for severe disease courses (Figure 1, panel 1). The detection of high viremia seemed to be limited to severe courses—comparable to the virological findings in patients with SARS-CoV-2 infection.14, 15 The assessment of viral titers obtained from swabs of skin lesions is most likely more prone to variations due to difficulties in standardizing the sample acquisition. Therefore, we propose to evaluate MPX viremia as a potential prognostic marker, for risk assessment, to decide when to initiate an antiviral therapy and as a parameter to monitor the treatment response. Although the CRP level is a nonspecific inflammation marker and potentially influenced by bacterial coinfections and superinfections, which did not correlate with the number of lesions in chickenpox patients,16 it could be a suitable additional marker for the assessment of the severity of MPX and to monitor treatment responses.17
The severe MPX course in a UC patient treated with vedolizumab might be coincidental. However, vedolizumab is a recombinant antibody targeting the α4β7-integrin. that aids lymphocytes and especially CD4+ T cell homing to the lamina propria from gut-inductive sites where immune responses are classically first induced (Peyer's patches and mesenteric lymph nodes).18 Antigen-specific α4β7+ CD4+ T cells are also found in genital mucosa19-21 and blockade of this integrin by vedolizumab might impact the local adaptive T-cell response against MPXV.
Patient C and another patient of a recently published case both had uncontrolled HIV viremia, low CD4+ TH cell counts, and a severe course of MPX.10 This suggests a relevant role of the adaptive CD4+ T-cell response in the viral control of MPX infection.
In Patient B as well as described in that same case report,10 clinical and laboratory values might be distorted by the presence of concomitant sexually transmitted infections (STI) like acute syphilis (Figure 1B). Providers are advised to diligently rule out any additional STI when assessing a possible MPX patient.
Overall, the antiviral treatment with tecovirimat in this small case series was well tolerated with no significant side effects. Clinical improvement was observed, which coincided with a >2 log drop in the level of MPX viremia within 7 days. As MPX is a self-limiting disease, the observed course upon tecovirimat cannot be solely attributed to the therapy. Prospective, placebo-controlled studies supplemented by cohort studies need to evaluate the safety, tolerability, and efficacy of tecovirimat in early and later treatment initiation, the length of therapy (7–14 days or even longer), or response-guided therapy algorithms. Additionally, virological and laboratory tools to assess treatment response, infectivity, or escape mutations are required.
AUTHOR CONTRIBUTIONS
Conceptualization: Julian Schulze zur Wiesch, Marc Lütgehetmann, Stefan Schmiedel, Lennart Hermanussen, and Ilka Grewe. Formal analysis: Julian Schulze zur Wiesch, Marc Lütgehetmann, Hui Ting Tang, Dominik Nörz, Lennart Hermanussen, and Ilka Grewe. Project administration: Julian Schulze zur Wiesch, Stefan Schmiedel, Lennart Hermanussen, and Ilka Grewe. Resources: Martin Aepfelbacher, Ansgar W. Lohse, Marylyn M. Addo, Julian Schulze zur Wiesch, Stefan Schmiedel, Marc Lütgehetmann, Stefan Unger, Christian Hoffmann, and Dirk Berzow. Writing—original draft: Lennart Hermanussen, Julian Schulze zur Wiesch, and Ilka Grewe. Supervision: Julian Schulze zur Wiesch, and Stefan Schmiedel. Writing—review and editing: All authors.
ACKNOWLEDGMENTS
We thank the patients for granting permission to publish their medical history.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Raw data were generated at the University Medical Center Hamburg-Eppendorf. Derived data supporting the findings of this study are available from the corresponding author on request.




