Is monkeypox a new, emerging sexually transmitted disease? A rapid review of the literature
Abstract
Monkeypox, a milder disease compared to smallpox, is caused by a virus initially discovered and described in 1958 by the prominent Danish virologist von Magnus, who was investigating an infectious outbreak affecting monkey colonies. Currently, officially starting from May 2022, an outbreak of monkeypox is ongoing, with 51 000 cases being notified as of September 1, 2022—51 408 confirmed, 28 suspected, and 12 fatalities, for a grand total of 51 448 cases. More than 100 countries and territories are affected, from all the six World Health Organization regions. There are some striking features, that make this outbreak rather unusual when compared with previous outbreaks, including a shift on average age and the most affected age group, affected sex/gender, risk factors, clinical course, presentation, and the transmission route. Initially predominantly zoonotic, with an animal-to-human transmission, throughout the last decades, human-to-human transmission has become more and more sustained and effective. In particular, clusters of monkeypox have been described among men having sex with men, some of which have been epidemiologically linked to international travel to nonendemic countries and participation in mass gathering events/festivals, like the “Maspalomas (Gran Canaria) 2022 pride.” This review will specifically focus on the “emerging” transmission route of the monkeypox virus, that is to say, the sexual transmission route, which, although not confirmed yet, seems highly likely in the diffusion of the infectious agent.
1 POXVIRUSES AND THE MONKEYPOX VIRUS
Poxviruses comprise a vast family (Poxviridae) of several members, which are large, brick-shaped, enveloped, and double-stranded DNA viruses that can infect a variety of hosts and replicate entirely within the cytoplasm of invertebrate and vertebrate cells, including human ones.1-4 Variola virus, of the Orthopoxvirus genus, is undoubtedly the most notorious member of this family and has been the infectious agent responsible for one of the most, if not the most, deadly diseases of human history (smallpox) until it was declared fully eradicated by means of immunization with the closely related vaccinia virus in 1980.5
Members of the Chordopoxvirus subfamily have rather large and complex genomes ranging from approximately 130 000−150 000 to 230 000−300 000 base pairs and encoding approximately a few hundred (~200) proteins. About half of these proteins are highly conserved across the different viral members and are involved in a series of essential functions including cell attachment and binding/fusion, cell entry, uncoating, genome replication, transcription, and virion maturation and assembly6; the remainder exhibit a higher degree of structural variability, with many of them engaging host defense mechanisms,6, 7 including intracellular signal transduction pathways regulating the apoptotic and the inflammatory response.8
Monkeypox, a milder disease compared to smallpox, is caused by a virus initially discovered and described in 1958 by the prominent Danish virologist von Magnus, who was investigating an infectious outbreak affecting monkey colonies. The first human case was reported on September 1, 1970, affecting a 9-year-old child in the Democratic Republic of the Congo. The disease is endemic in 11 African countries. No cases outside of Africa have been reported until 2003 when a cluster of 47 cases related to infected pet prairie dogs was described in the United States, after the importation of contaminated rodents and other exotic animals from Ghana. Subsequently, further outbreaks have been reported in the United Kingdom, Israel, and Singapore.9, 10
Currently, officially starting from May 2022, an outbreak of monkeypox is ongoing, with more than 51 000 cases being notified as of September 1, 2022—51 408 confirmed, 28 suspected, and 12 fatalities, for a grand total of 51 448 cases. More than 100 countries and territories are affected, from all the six World Health Organization regions. There are some striking features, that make this outbreak rather unusual when compared with previous outbreaks, including a shift on average age and the most affected age group, affected sex/gender, risk factors, clinical course, presentation, and the transmission route.11
Similar epidemiological shifts have been observed and documented for another poxvirus, responsible for a disease known as “molluscum contagiosum” (water warts),12 which initially commonly affected children (aged 1−10 years old), but has become increasingly more frequent among young adults, generally sexually active, some of whom can be immunocompromised.
Initially predominantly zoonotic, with an animal-to-human transmission, throughout the last decades, human-to-human transmission has become more and more sustained and effective. In particular, clusters of monkeypox have been described among men having sex with men (MSM), some of which have been epidemiologically linked to international travel to nonendemic countries and participation in mass gathering events/festivals, like the large-scale “Maspalomas (Gran Canaria) 2022 pride” (Spain) and the Darklands fetish festival in the port city of Antwerp (Belgium).11, 13
This review specifically focuses on the “emerging” transmission route of the monkeypox virus, that is to say, the sexual transmission route, which, although not confirmed yet, seems highly likely in the diffusion of the infectious agent. Given the timely nature of the topic and time/resource constraints, we opted for a rapid review, which represents an emerging form of literature appraisal in the ecosystem of evidence synthesis techniques.14
2 MATERIAL AND METHODS
We performed a comprehensive literature search by mining the major scholarly electronic database PubMed/MEDLINE. We searched for “poxvirus,” “poxviruses,” “orthopoxvirus,” “orthopoxviruses,” “smallpox,” “smallpox virus,” “variola virus,” “vaccinia virus,” “monkeypox,” “monkeypox virus,” “sperm,” “seminal,” and “semen.” We used, when appropriate, “Medical Subject Headings” terms and wild-card options (truncated words). We restricted our search to studies conducted on humans, discarding investigations of veterinary interest. However, we did not apply any time or language filters.
3 RESULTS
3.1 Viruses and the male reproductive tract
The male reproductive tract consists of (i) testes (or testicles, two oval-shaped organs contained in a thick-skinned sac or scrotum, consisting of seminiferous tubules or the spermatogenetic region of the testis, where the process of spermatogenesis occurs, and of the interstitium, where testosterone is produced), (ii) epididymis (a part of the paratesticular region, along with the rete testis and other stuctures of the testicular collecting system, the testicular tunics, and the spermatic cord, where sperm cells move from the testes, finish maturing, and are stored), (iii) vas deferens (ductus deferens, or sperm duct, a long muscular tube running from the epididymis into the pelvic cavity behind the bladder and connecting to the urethra), (iv) ejaculatory ducts (a paired excurrent duct system for sperm transport, maturation, and storage, which is the union of the duct of the seminal vesicles with the ampulla of the vas deferens), (v) accessory sex glandular organs (seminal vesicles, prostate, and bulbourethral glands or Cowper's Glands, which produce and release the seminal fluid, secreting complex substances and molecules into the final ejaculate), and (vi) the penis (an erectile organ for the penetration and delivery of the ejaculate).15, 16
Several viruses can be detected in the human semen and in the male reproductive tract. Salam and Horby17 performed a literature review and found that at least 27 viruses, belonging to a broad range of virus families (from adenoviruses to anelloviruses, arenaviruses, bunyaviruses, filoviruses, flaviviruses, hepadnaviruses, herpesviruses, paramyxoviruses, parvoviruses, polyomaviruses, retroviruses, and togaviruses), can be found in human semen, even though for most of these viruses clear-cut data concerning sexual transmission are lacking.
Epidemiological, molecular/phylogenetical evidence of sexual transmission and/or semen isolation have been unambiguously demonstrated only for 12 viruses (44.4%): namely, Ebola virus,18-22 Marburg virus,23, 24 human pegivirus (also known as GB virus C),25 hepatitis B (HBV) and C (HCV) viruses,26-28 Zika virus,29, 30 cytomegalovirus,31-33 Epstein-Barr virus,34 herpesviruses types 1 (HHV-1), 2 (HHV-2), and 8 (HHV-8, known as Kaposi's sarcoma-associated herpesvirus, KHV),35-37 and human T-cell lymphoma virus type 1.38 Survival in the testis and in sperm of these viruses may be related to their structural biology and stability, specific viral epitopes, immune evasion, and other factors. Many viruses have been found to interact directly with semen, impair its quality, or impact/disrupt spermatogenesis by causing local inflammation.17, 39, 40
Besides these viruses, other viral agents could be potentially isolated from semen, including influenza virus, lymphocytic choriomeningitis virus, phlebotomus fever virus, coxsackie B virus, echovirus, dengue virus, “Severe Acute Respiratory Syndrome” (SARS) coronaviruses type 1 (SARS-CoV) and 2 (SARS-CoV-2), rubella virus, Lassa virus, Crimean-Congo hemorrhagic fever virus, and poxviruses (such as smallpox virus, and vaccinia virus).41-53 However, for SARS-CoV-2 evidence related to sexual transmission is particularly ambiguous and contrasting. Evidence about vaccine-strain vaccinia viruses as sexually transmitted pathogens is scarce and anecdotal, with a few cases of genital lesions, including vaginal, vulvar, and, less frequently, perianal vaccinia infections, in women apparently healthy or with underlying conditions (like atopic dermatitis), reporting having sex with male civilian41-44 or military smallpox vaccinees,45-51, 53 or cases of genital and perianal rash in men having sex with male civilian smallpox vaccinees.52 It can be hypothesized that “sexual intercourse provides the intimate contact required for heteroinoculation.”41 Of note, the cases reported in the MSM community include the first described instance of tertiary vaccinia transmission through sexual contact.52 Interestingly, the secondary vaccinia case reported a history of psoriasis and a possible history of eczema (atopic dermatitis), whilst the tertiary vaccinia case recalled a history of eczema: both exhibited systemic symptoms, requiring hospitalization and vaccinia immune globulin intravenous administration.52
Recently, also the monkeypox virus has been detected in human male semen. In Italy, Antinori et al.54 described four monkeypox cases in MSM in their thirties, with a past history of sexually transmitted diseases (including HAV, HBV, HCV, and syphilis), two were HIV-positive and the other two HIV-negative on HIV pre-exposure prophylaxis (PrEP). Interestingly, one of the four cases was a sex worker. The quantification of the viral load in the seminal fluid by means of the quantification cycle (Cq) technique yielded values comparable to those obtained for nasopharyngeal swabs (ranging from 27 to 30). Of note, the seminal sample was collected 5−7 days after symptoms onset. While, on the one hand, Cq values seem to exclude the hypothesis of biological sample contamination, on the other hand, they may be not enough to allow the virus to be isolated and cultured. However, virus infectivity cannot be ruled out and warrants further research.
In another recent study, Thornhill et al.55 analyzed an extensive series of monkeypox infection cases from 16 countries, suggesting a substantial likelihood of its sexual transmission, supported by findings of primary genital, anal, and oral mucosal lesions, which could represent the inoculation site. Still, according to these authors, virus DNA was PCR detectable in seminal fluid in 29 of the 32 tested cases (90.6%); however, it has to be determined yet if those viruses are competent for replication and transmission.
As previously said, the finding of a relatively high number of viruses across a span of such diverse viral families, including poxviruses and, in particular, monkeypox virus, seems to suggest that the presence of a virus in human male semen may depend on an array of parameters, including biochemical/biophysical features in terms of specific/conserved epitopes, the ability of a virus to replicate within the male reproductive tract and/or to exert direct cytopathic effects, and its structural stability, even though all this does not necessarily mean that the virus can be sexually transmitted.
Some factors can explain the presence of a virus in semen: for instance, (i) some mechanisms of immune evasion/escape, especially at the level of the male reproductive tract, (ii) high levels of viremia, (iii) some local/systemic inflammatory mediators that can alter the blood-testis barrier (or to be more precise, the blood-testis-deferens-epididymis barrier) permeability, (iv) systemic immunosuppression, and, finally, (v) a past history or the coexistence of sexually transmitted diseases, such as hepatitis or syphilis. Viruses could have also reached the testes, coming from accessory glands.56, 57
Finally, viral persistence in human male semen does not imply its replicating capacity: viruses can persist even if they are unable to replicate since the testis is immunologically privileged (the so-called “sanctuary site theory”). Mammalian testes represent, indeed, a unique immunological milieu, which is restricted to enable the various steps and processes of spermatogenesis.56, 57
3.2 Poxviruses and direct cytopathic effects at the level of the male reproductive tract
Smallpox can be subdivided into the following five clinical types: (i) ordinary, which has represented the most commonly observed type, characterized by fever and several vesiculopustular skin lesions, and affecting unvaccinated individuals, (ii) modified, without fever and few vesiculopustular skin lesions, affecting vaccinated individuals, (iii) variola sine eruptione, with fever, but without skin lesions, affecting vaccinated individuals, and two rare, but severe forms, namely, (iv) the flat form (with erythemas, and flat vesicles with little fluid), and (v) the hemorrhagic one (with erythemas, petechiae, and ecchymoses), both affecting unvaccinated individuals.58 Smallpox, especially the hemorrhagic clinical form,59 is a rare cause of acute inflammation and infection affecting the testicular interstitium (“multifocal interstitial orchitis”), with or without central necrosis, as well as of other smallpox-associated testicular and male reproductive lesions, including inflammation of the prostate gland (“prostatitis”), and of the epididymis. The latter inflammation is generally characterized by lymphohistiocytic infiltrates (“lymphohistiocytic epididymitis”).
A temporal correlation, even though loose, between the development of these lesions and the progression of the cutaneous ones has been described. According to Martin60 and previous research,61-65 the papular stage on the skin corresponded to hyperemia and infiltrates of large lymphocytes at the testicular interstitium level. During the pustulation stage, necrosis of the walls of the seminiferous tubules and the interstitium could be described. The picture may be age-specific, with a more ischemic picture predominating in children, because of their less-developed testicular vascularization.
From a histopathological perspective, the inflammatory infiltrate is generally histiocytic and lymphocytic, and less commonly eosinophilic, along with a seminiferous tubule degeneration.63, 66 Particularly advanced forms of smallpox can exhibit azoospermia,67 as well as arteritis (including mononuclear endarteritis with endothelial hypertrophy), thrombosis, perivascular hemorrhage, and rather discrete infarcts.63, 68
According to a report by Chowdhury et al.69 azoospermia is quite a common finding in people with a history of smallpox. Out of 31 subjects with a past history of smallpox, 15 (48.4%) displayed complete azoospermia, while 1 (3.2%) showed sperm density of less than 10 million/ml.
In a case-control study carried out in Bombay, India, Phadke et al.70 found that, while the incidence rates of severe and moderate oligospermia were, respectively, practically comparable in the smallpox and control series, the incidence of azoospermia was, however, higher in the smallpox series (42.57% vs. 17.87% in the control series). Similarly, the rate of normospermia was lower in the smallpox series (30.17% vs. 52.52% in the control series). Of note, the incidence rate of obstructive azoospermia was higher in the smallpox series (79.36% vs. 46.23% in the control series). On the other hand, the rates of testicular lesions and partial or complete arrest of spermatogenesis at various levels, as well as germinal cell aplasia, severe tubular atrophy, and hyalinization of the tubules were similar in both series.
3.3 Monkeypox virus and its atypical clinical presentation during the “2022 monkeypox epidemic”
According to a recent review of the literature,11 anogenital lesions would be present in 31.4% of the monkeypox cases. Two recent publications describe a “pure” genital presentation of monkeypox, which is highly unusual for this disease, as it is classically known in endemic countries. In France, Davido et al.71 described a 37-year-old MSM on PrEP, with a past history of treated syphilis. The patient presented sudden fever, headache, mild diarrhea, and genital macular lesions without itching. Of note, face, trunk, and extremities (limbs, palms, and soles) were spared. Another 37-year-old self-defining MSM, with a past history of vitiligo, presented an initial series of itching, and vesicular lesions affecting the penis. Subsequently, the patient became febrile, with macular lesions and pubic erythema. Perianal lesions and inguinal adenopathy were also described. In Portugal, Patrocinio-Jesus and Peruzzu72 described the case of a 31-year-old MSM, HIV-positive on medications, with excellent virologic control, that presented with fever, sore throat, and painless genital rash. Ulcerated lesions and umbilicated pustules affecting his penis were noted, along with vasicopustular lesions affecting his face and hands. In the United Kingdom, Heskin et al.73 have described monkeypox infection in a serodiscordant couple. The two subjects reported perioral white spots, perianal blistering lesions, anogenital, and pubic lesions, along with lymphadenopathy, fever, headache, and diarrhea. Interestingly, the authors noted a close correlation between the lesion site and the type of sexual practice, pointing to sexual transmission as a novel route of monkeypox infection. Finally, anogenital signs/symptoms had been rarely described in previous outbreaks, such as those occurring in the United Kingdom and in Israel.11
All these findings have been confirmed by a recently published systematic review of the literature and meta-analysis, which synthesized data related to 4222 confirmed cases of monkeypox, of which 3876 (91.8%) were potentially deemed due to sexual transmission.74 The most commonly reported clinical manifestations were painful perianal and genital lesions, together with fever, lymphadenopathy, headache, and malaise.74
Furthermore, the origin of the present monkeypox epidemics, as previously said, has been associated with large sex-related parties among MSM, such as those at Antwerp (Belgium) and at Maspalomas (Spain), explaining the high prevalence of monkeypox infection in this group.11, 74 This is interesting since it could have impacted viral fitness, affecting endemic and epidemic circulation, as well as virulence, and facilitating transmission. Mass gatherings may have resulted in the geographic introductions of human-amplified monkeypox viruses and led to the emergence of human disease. Translating to infectious diseases a concept from the field of evolutionary genetics, this is known as the “founder effect.”75
3.4 Poxviruses, the “imperfect nature” of the blood-testis barrier, and the testes as an immunologically privileged site
The blood-testis barrier is a unique, extremely complex, biologically, and physiologically fascinating ultrastructure in the mammalian testis.76, 77 It consists of Sertoli cells—the epithelial cells of the seminiferous epithelium—and, in rodents, also of peritubular myoid cells. It is located near the basement membrane of the seminiferous tubule segregating the seminiferous epithelium into the basal and the adluminal/apical compartments. Different types of junctions make the blood-test barrier particularly tight and sealed, restricting trans and paracellular flux of several biomolecules (from hormones to other endocrine and paracrine factors), electrolytes, water, and drugs/toxicants (Figure 1).
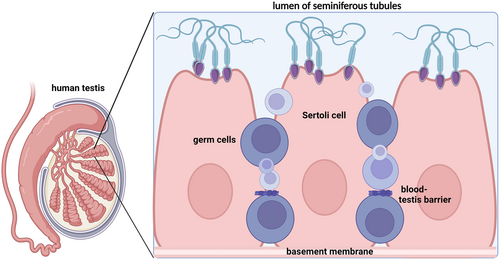
These various families of junctions include actin-based tight junctions, gap junctions, intermediate filament-based desmosomes, and basal ectoplasmic specializations. The latter represent a testis-specific, atypical adherens junction. Besides regulating the passage of molecular compounds, the blood-test barrier enhances and supports/promotes the various phases of the spermatogenesis process.78-80
The Leydig cells are responsible for testosterone production and release and are located in the testicular interstitium, together with fibroblasts, macrophages, blood, and lymphatic vessels. The Sertoli cells along with the Leydig cells can secrete an array of multiple immunosuppressive molecules, including activin A, Transforming Growth Factor beta, and other growth factors, multifunctional cytokines, Programmed Death-Ligand 1 (PD-L1, also known as cluster of differentiation or CD-274 or B7 homolog 1), Growth arrest-specific protein type 6, and testosterone,81-83 among others, which exert either direct or indirect effects, inhibiting/regulating immune cell activation. Since semen is highly immunogenic, these molecules enable its survival and maturation.84
Moreover, the Sertoli cells are able to synthesize two membrane-associated proteoglycans containing, as glycosaminoglycan moieties, either chondroitin sulfate only or chondroitin sulfate and heparan sulfate; the latter proteoglycan is, therefore, known as the mixed proteoglycan.79 Poxviruses can potentially exploit the binding to these molecules to effectively enter host cells. Other molecules are integrin β1 and CD98/CD98 receptor85, 86: interestingly, these are also expressed at the level of Sertoli cells and are involved in various molecular events, such as testicular development and spermatogenesis.80
4 CONCLUSIONS
Poxviruses can lead to interstitial infiltration and necrosis of seminiferous tubules, temporally and morphologically correlating with the lesions affecting the skin and mucous membranes. They can exert direct cytopathic cellular effects.87 Moreover, the monkeypox virus has been isolated from seminal samples.
However, data are scarce and sometimes contrasting, and, despite scholarly advancements, there remain significant gaps in our knowledge and understanding of viral persistence, in particular of monkeypox virus, in human male semen, which merits further investigation. More specifically, future research should be able to provide a convincing explanation to some still unanswered questions like the quantification of monkeypox viral load, shedding and viability in human male semen, its persistence and stability, and at what concentrations it can be sexually transmitted if it can be sexually transmitted. It is important, indeed, to differentiate between “sexual transmission” and “transmission associated with sexual contact.” In the first scenario, transmission could be occurring through body fluids exchanged during sexual intercourse (even though it is difficult to understand why we are not seeing heterosexual transmissions if seminal fluid plays a relevant role in virus transmission), whilst in the second scenario, transmission could occur via close contact with mucosal surfaces, scarification, mucosal/skin lesions, or even respiratory exposures (through expiratory droplets) or contact with contaminated clothes.
Finally, from currently available evidence, if sexual contact plays a key role, monkeypox does not seem to be an exclusively sexually transmitted disease, since infection in children88 is also being reported, talking in favor of other transmission routes, including the household transmission or other “complex” routes of monkeypox exposures that have not yet been demonstrated in humans or in animal models.89 As such, further research is urgently needed.
AUTHOR CONTRIBUTIONS
Nicola Luigi Bragazzi conceived the manuscript. All authors (Nicola Luigi Bragazzi, Jude Dzevela Kong, and Jianhong Wu) contibuted to the present manuscript.
ACKNOWLEDGMENT
N. L. B. and J. D. K. acknowledge support from IDRC (Grant No. 109981). J. D. K. acknowledges support from New Frontier in Research Fund-Exploratory (Grant No. NFRFE-2021- 00879) and NSERC Discovery Grant (Grant No. RGPIN-2022-04559).
CONFLICT OF INTEREST
The author declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.




