Murine diabetic models for dengue virus infection
Xuling Liu, Yingfang Liu, Hao Wu, and Zihan He contributed equally to this study.
Abstract
Dengue virus (DENV) is a critical public health concern in tropical and subtropical regions worldwide. Thus, immunocompetent murine models of DENV infection with robust viremia are required for vaccine studies. Diabetes is highly prevalent worldwide, making it frequent comorbidity in patients with dengue fever. Therefore, murine models are needed to understand viral pathogenesis and disease progression. Acquired-induced and inherently diabetic C57BL/6 and db/db mice were inoculated with DENV-3 via the tail vein. After infection, both the diabetic C57BL/6 and db/db mice showed obvious weight loss with clinical manifestations. Quantitative reverse-transcription polymerase chain reaction revealed robust and replicable viremia in the two types of diabetic mice. Immunohistochemical detection showed persistent DENV-3 infection in the liver. Enzyme-linked immunosorbent assay for cytokine detection revealed that diabetic mice showed more severe inflammatory responses than did nondiabetic mice, and significant histological alterations were observed in diabetic mice. Thus, the diabetic mice were more susceptible to DENV infection than the nondiabetic mice. Taken together, we established two types of immunocompetent diabetic mice for DENV infection, which can be used to further study the mechanisms of dengue pathogenesis in diabetes and to develop antiviral pharmaceuticals and treatments.
1 INTRODUCTION
Dengue is the most common mosquito-borne viral disease caused by one of the four serotypes of dengue virus (DENV-1 to DENV-4), which affects approximately 390 million people in over 128 countries annually, including Brazil, Singapore, and China.1, 2 Dengue infections cause varying degrees of dengue, with major clinical manifestations ranging from a self-limiting illness called dengue fever to life-threatening forms of serious dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS).3 To date, there are no effective pharmaceuticals or completely safe and widely available vaccines capable of preventing viral infection when used in endemic regions, and dengue has become a serious public health concern worldwide.4, 5
Murine models of DENV are beneficial for understanding viral pathogenesis and disease progression, which are fundamental for preclinical testing of drugs and vaccines.6 However, dengue mouse models have been limited owing to the deficiency in or complete lack of interferon (IFN) receptors. This is because rodents are not natural hosts for DENV.7, 8 IFNs are activated effectively and stop viral replication early when wild-type (WT) mice are infected with DENV, resulting in a very limited infection that does not cause disease.8, 9 Thus, mice deficient in IFN-α/β and γ-receptors (AG129 mice) on the 129/SV background are regarded as the most popular DENV mouse models for evaluating therapeutics.10, 11 After infection, viral load, cytokine storm, massive organ damage, and critical vascular leakage have been detected in AG129 mice.12-14 Similarly, signal transducer and activator of transcription 1 and 2 proteins, the downstream components of the IFN signaling pathway, are regarded as the targets for deficiency. However, because these immunocompromised mice lack IFN-signal-dependent components of the antiviral immune response, they are not ideal models for vaccine studies,8 and an immunocompetent mouse model for DENV infection is needed.
There are some important factors for the progression to severe dengue, including antibody-dependent enhancement (ADE),15-17 viral serotype,18, 19 age,20 host genetics,21, 22 and several comorbidities, such as hypertension, bronchial asthma, and diabetes.23-26 Focusing on these factors when establishing animal models of DENV infection will be useful for a better understanding of disease progression and pathogenesis. For example, ADE in animal models has been reported in research on rhesus macaques, in which viremia was enhanced by secondary heterotypic infection,27-29 providing implications for DENV pathogenesis. Among these factors, 2.8% of the world population suffers from diabetes, which is expected to increase to more than 5.4% by 2025.30 The high global prevalence of diabetes makes it frequent comorbidity in patients with dengue.31 Thus, it is important to elucidate the pathogenic mechanism of DENV infection in patients with diabetes, and a murine diabetes model for DENV infection is required.
C57BL/6 mice were fed a high-fat diet (60% saturated fat) to construct murine diabetes models32-34 as acquired-induced diabetic mice. A high-fat diet results in increased weight gain and, over time, stable hyperglycemia with progressively increasing hyperinsulinemia.35 With inherent diabetes, the mutation in db/db mice were traced to the db gene, which encodes for leptin receptors and is characterized by obesity, hyperglycemia, and insulin resistance.36-38 Both mouse strains have been extensively used for the investigation of diabetes.
In the present study, two types of diabetic mice were inoculated with DENV-3 to establish DENV infection immunocompetent mouse models characterized by weight loss, robust viremia, severe inflammatory response, and histological alterations.
2 METHODS
2.1 Reagents
Mouse tumor necrosis factor(TNF)-α, Interleukin 6 (IL-6), IL-1β, and IFN-γ Enzyme-Linked Immunosorbent Assay (ELISA) Kits were obtained from Neobioscience Technology Co., Ltd. Fetal bovine serum (FBS) and Roswell Park Memorial Institute (RPMI)-1640 serum were purchased from Gibco. NucleoSpin® RNA virus was obtained from Macherey-Nagel. Evo M-MLV RT Premix for quantitative polymerase chain reaction (qPCR) and Pro Taq HS Premix Probe qPCR Kit was obtained from Accurate Biotechnology Co., Ltd.
2.2 Cell lines and virus preparation
C6/36 cells, a mosquito cell line from Aedes albopictus, were grown in RPMI-1640 supplemented with 10% FBS at 28°C. DENV-3 strain H87 was grown in C6/36 cells for 2–3 days.
2.3 Mouse strains and DENV infection
BKS-Cg-Dock7m+/+ Leprdb (db/db) and its WT mice were obtained from Nanjing Junke Bioengineering Co., Ltd., as one of the murine diabetes models. C57BL/6 mice were obtained from the Guangdong Medical Laboratory Animal Center. To establish an acquired-induced diabetes model, the C57BL/6 mice were fed D12492 (rodent diet with 60 kcal% fat) until the blood glucose stabilized above 11.1 mmol/L.39 Age-matched male mice 16–17 weeks of age were used, and each experimental and control group contained more than three animals. Both db/db and diabetic C57BL/6 mice were inoculated with 1 × 107 plaque-forming unit (PFU) DENV-3 via tail vein injection, whereas the control group was inoculated with RPMI-1640 medium. After inoculation, all mice were monitored at least once a day for symptoms and signs of illness, and their weights were recorded daily, until 10 days postinfection (dpi).40
2.4 Histology and immunohistochemical detection
During an autopsy, the brains, spleens, liver, and kidneys were harvested and immediately fixed for 16–24 h in 10% paraformaldehyde. Tissues for paraffin embedding were submitted to Wuhan Servicebio Technology Co., Ltd., for double-blind pathological diagnosis. The organs were processed and sectioned before staining with hematoxylin and eosin (H&E) and examined microscopically for histopathological changes.12 These organs were also harvested for immunohistochemical analyses using 4G2 antibody (tagged E protein of DENV-3).41
2.5 Cytokine determinations
Serum was collected daily after infection. Mouse TNF-α, IL-6, IL-1β, and IFN-γ ELISA kits were used to establish a standard curve to determine the levels of TNF-α, IL-6, IL-1β, and IFN-γ, respectively, according to the manufacturer's instructions.12
2.6 Viral detection kinetics in blood
Blood samples were collected daily postinfection for viremia detection.6 Viral RNA was extracted using the NucleoSpin® RNA Virus and quantified by quantitative reverse-transcription PCR (qRT-PCR) using an ABI StepOnePlusTM real-time PCR system. Primers DENV-3/F (5′-TTTGGACGTGGACTTGCACC-3′), DENV-3/R (5′-CTGCCAGGGACACATTTGCT-3′), and the probe (5′-FAM-AGCATCAGCCTGGACATTGTACGCCGTG-BHQ1-3′) were designed in our laboratory.
2.7 Statistical analysis
Differences were analyzed using the Student's t-test. Statistical significance was set at p < 0.05.
3 RESULTS
3.1 Clinical manifestation
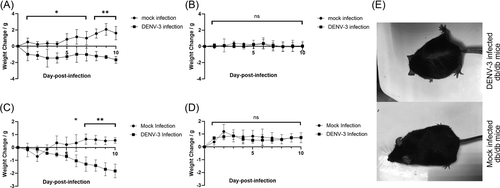
Mice from all groups survived the DENV-3 infection; however, db/db mice developed more severe symptoms than did control mice, including visibly ruffled fur (Figure 1E) and body weight loss (Figure 1A). Diabetic C57BL/6 mice also lost weight at 6 dpi (Figure 1C). In contrast, WT and normal C57BL/6 mice showed no apparent symptoms or weight loss after DENV-3 infection (Figure 1B,D).
3.2 Viremia
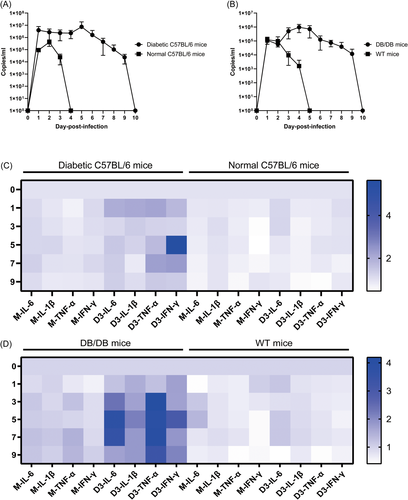
After DENV-3 infection, viremia levels varied in db/db and diabetic C57BL/6 mice up to 9 dpi, with higher titers and longer durations than those in WT and normal C57BL/6 mice, respectively (Figure 2A,B). In diabetic C57BL/6 mice, plasma viral RNA levels peaked at 1 and 5 dpi and decreased thereafter (Figure 2A). In db/db mice, plasma viral RNA levels peaked at 4 dpi and decreased until 9 dpi (Figure 2B).
3.3 Inflammatory response
Neither WT nor normal C57BL/6 mice exhibited significant changes in TNF-α and IL-1β secretion after DENV-3 infection. Two types of nondiabetic mice exhibited mild upregulation of IL-6 secretion after viral infection. The secretion of IFN-γ in normal C57BL/6 mice increased slightly at 1 dpi, and then gradually returned to normal levels (Figure 2C,D). In contrast, in db/db and diabetic C57BL/6 mice, DENV-3 infection-induced significant temporal changes in cytokines, such as IL-1β, IL-6, IFN-γ, and TNF-α, were increased compared to those in mock-infected db/db and diabetic C57BL/6 mice (Figure 2C,D). Serum from infected db/db mice exhibited significantly increased production of IL-6 compared with that in control mice, where IL-6 levels peaked at 5 dpi and decreased thereafter (Figure 2D). In diabetic C57BL/6 mice, the apex of IL-6 production was observed at 1 dpi. IL-6 levels fluctuated between 3 and 9 dpi but increased from 3 to 7 dpi (Figure 2C). Similarly, IL-1β levels peaked at 1 dpi and then declined gradually in diabetic C57BL/6 mice (Figure 2C), whereas the production of IL-1β was at a constant high level after DENV-3 infection in db/db mice (Figure 2D). In diabetic C57BL/6 mice, TNF-α levels peaked at 1 dpi, whereas IFN-γ levels peaked at 5 dpi, with persistently high levels in other periods after infection (Figure 2C). TNF-α was expressed at more than four times the baseline level, while IFN-γ expression exceeded three times the baseline level by 5 dpi in infected db/db mice (Figure 2D).
3.4 Viral space–time distribution
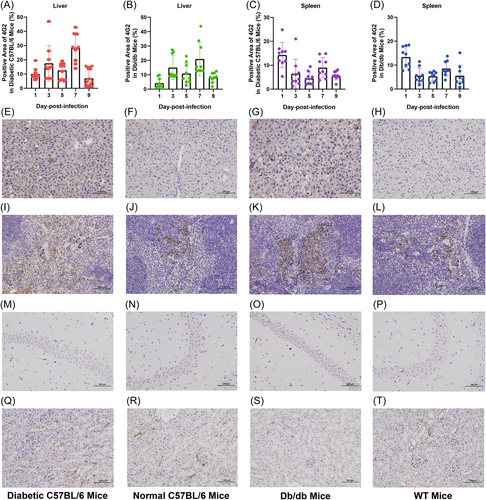
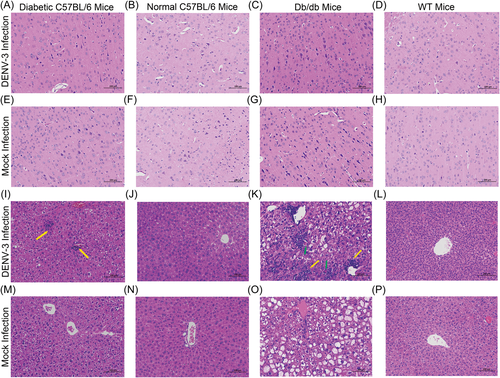
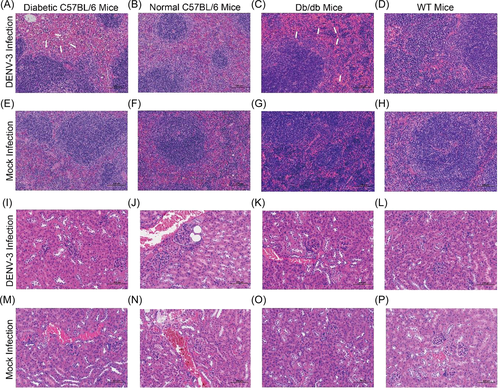
To assess viral infection in more detail, we examined the cellular tropism of DENV-3 in mice through immunostaining for viral envelope protein. In both diabetic C57BL/6 and db/db mice, the livers had a detectable virus at 1–9 dpi, with a peak at 7 dpi (Figure 3A,B). Liver sinusoids and cells were significantly positive for the virus (Figures 3E,G). DENV-3 was only transiently present in the livers and spleens of both WT and normal C57BL/6 mice at 1 dpi (Figure 3F,H,J,L). In the spleen of diabetic C57BL/6 and db/db mice, the E protein of DENV-3 was observed at 1 dpi, after which the positive signal was weak (Figure 3C,D,I,K). In the four types of mice, no infected cells were detected in the brain (Figure 3M–P), and very weak positive signals were detected in the kidneys at only 1 dpi (Figure 3Q–T).
3.5 Histological alterations
In diabetic C57BL/6 and db/db mice, examination of H&E-stained semithin sections revealed significant lesions in the liver and spleen, whereas the brains and kidneys did not show overt tissue damage associated with DENV-3 infection.
Even though diabetic C57BL/6 and db/db mice were infected with DENV-3, the lesions in the brain were mild (Figure 4A,C,E,G). Both normal C57BL/6 and WT mice had no significant pathological changes in the brain, whether or not they were infected with DENV-3 (Figure 4B,D,F,H). The liver showed the most severe histological alterations in diabetic mice. Severe hepatocyte swelling and steatosis were observed in both diabetic C57BL/6 mice and db/db mice (Figure 4I,K,M,O). During DENV-3 infection, inflammatory cell lesions were often observed in both mouse strains (Figures 4I,K). In addition, the livers of DENV-3-infected db/db mice exhibited remarkable punctate necrosis and eosinophil bodies (Figure 4K). In mock- and DENV-3-infected nondiabetic mice, hepatocytes were closely arranged, with clear boundaries, normal size, and no obvious abnormality (Figure 4J,L,N,P). In the two types of diabetic mice, after DENV-3 infection, the splenic sinusoid exhibited mild dilatation with splenic sinus congestion and numerous red blood cells in the red pulp (Figure 5A,C,E,G). However, both mock- and DENV-3-infected control mice had no significant lesions in the spleen, the red pulp and white pulp were clearly demarcated, and lymphocytes were closely arranged in normal shape (Figure 5B,D,F,H). No unusual pathological changes were observed in the kidney. The cortex and medulla were clearly demarcated, and the renal tubules were closely arranged, maintaining normal structures (Figure 5I–P).
4 DISCUSSION
Diabetes is considered a possible risk factor or predictor of worse outcomes in patients with DENV infection.23-26, 31, 42 Given the high global prevalence of diabetes and dengue, we sought to establish a model that recapitulates significant aspects of human dengue in diabetic mice to illustrate the pathogenesis in this uniquely large population. In our study, the diabetic mice were more susceptible to DENV than mice without diabetes, similar to previous clinical studies.23-26
When mice with diabetes were infected, visible weight loss was observed, contributing to the clinical signs of disease for model establishment. Weight loss is a significant sign of illness in murine models of DENV infection.13, 43, 44 Because DENV can be inhibited by IFN signaling during infection, immunocompetent mice rarely show weight loss after viral infection where highly virulent strains or intracranial injection is not employed,45-47 which is consistent with the lack of weight loss in WT and normal C57BL/6 mice in this study. Nevertheless, marked weight loss was observed in DENV-infected AG129 mice, which was used as an important indicator of model validity.12, 44, 48
Viremia in the two types of diabetic mice persisted for 9 days, which was much longer than that in other immunocompetent mice reported previously.49 Virus levels are usually associated with dengue disease severity,50 and viremia is one of the most important indicators of dengue infection in murine models. Mice are not natural hosts of DENV; therefore, viremia was transient and low in immunocompetent mice compared with that in IFN-deficient AG129 or SCID mice.8 In infected AG129 mice, the virus persisted until 9 dpi in the serum.13, 51 In contrast, even if each mouse was inoculated with 1 × 108 PFU DENV-2 intravenously, the viral genome in the serum could not be detected by qRT-PCR in BALB/c and C57BL/6 mice.49 Apparently, DENV strains of low virulence have difficulty in causing viremia in immunocompetent mice,49, 52 but long-term viremia was detected in our models, which represented a way to study experimental dengue in the mouse. Overall, in our study, the robust DENV replication observed in both mouse strains will enable convenient testing of potential drugs against DENV in these in vivo systems.
Generally, immunohistochemical characterization is based on the cellular tropism of DENV.53, 54 In these diabetic murine models, DENV antigens were detected in the liver cells, as previously reported in patients.55-57 Significant positive signals were detected in the liver sinusoids and cells, which was postulated to have persistent effects on the liver, reminding us to be concerned about the corresponding liver damage.58 In diabetic mice, a strong signal was detected in the spleen, which indicated a high copy number and supportive evidence of infection and viral replication.57 This finding is supported by in vitro studies that have demonstrated DENV replication in human B lymphoblastoid cells.59 The absence of the virus in the brain suggested that DENV-3 failed to pass through the blood–brain barrier in these animal models, which resembled that in several immunocompetent mice reported previously.6
Pathological events are a likely consequence of virus-induced cell death and massive inflammatory reactions.14, 60 In the present study, histological alterations associated with DENV-3 infection were detected in the liver and spleen, similar to those in AG129 mice.48 In the DENV-3-infected diabetic mice, inflammatory cell lesions, remarkable punctate necrosis, and eosinophilic bodies were found in the liver with long-term DENV-3 infection, which is in agreement with the liver damage in virus-infected AG129 mice12, 14 and were previously detected in DENV-infected patients.56 Splenic sinus congestion and numerous red blood cells in the red pulp were found in the spleen, indicating a relationship between DENV-3 infection and increased vascular permeability in the spleen.
Severe leakage of fluid and proteins in DHF/DSS is related to the cytokine cascade that targets vascular endothelial cells.61 IL-6, IL-1β, IFN-γ, and TNF-α, which are secreted in high concentrations mostly by T cells, endothelial cells, and monocytes/macrophages, were detected in the serum of patients with DHF/DSS.60, 62-66 We detected high proinflammatory cytokine levels from 1 dpi, indicating that the infected diabetic mice had systemic inflammation, and both the innate and adaptive immune systems were activated. The overproduction of IL-6, IL-1β, IFN-γ, and TNF-α, which are known to activate the immune system, was the most likely cause of the reduction in viremia observed at 4 or 5 dpi. IFN-γ production is essential for host resistance to DENV infections.67 In a human challenge model for DENV infection, sustained IFN-γ production was associated with protection against fever and viremia during the acute phase of the illness.68 The overproduction of IFN-γ in the present study provides indirect evidence that the virus persists in infected diabetic mice. Upregulation of IL-6 might play a crucial role in the enhanced production of antiplatelet or antiendothelial cell autoantibodies, elevated levels of tissue plasminogen activator, and a deficiency in coagulation, thereby increasing the likelihood of severe hemorrhage in DHF/DSS.69-71 IL-1β secretion might affect vascular permeability, leading to plasma leakage and hemoconcentration, and contribute to proinflammatory outcomes that might play a role in dengue immunopathogenesis.65, 72 Fever, one of the most typical symptoms in all dengue patients, is induced by IL-1β and TNF-α, which are two of the most important cytokines in endogenous pyrogens.73 TNF-α also induces apoptosis in dengue and mediates endothelial damage during the disease.66, 74 Our diabetic murine models for DENV-3 infection showed several inflammatory response characteristics that were consistent with patients with dengue fever.
Epidemiological evidence supported that dengue fever and diabetes are possible risk factors or predictors of worse outcomes,23-26, 42 but the pathogenic mechanism has not been elucidated due to the lack of systematic research. It is unclear whether dengue infection exacerbates diabetes or whether diabetes contributes to dengue severity. Only an autopsy report has indicated that the pathophysiological features and increased risk of severe dengue in patients with diabetes might be related to cytokine overproduction.75 Some basic studies have shown that IL-6, IL-1β, and TNF-α might promote the progression of diabetes in different ways,76, 78, 79 similar to the strong inflammatory response in the DENV-3-infected diabetic murine model established herein. In addition, hepatosteatosis is frequently associated with the development of diabetes,80 which is consistent with the severe hepatocyte swelling and steatosis in the liver of both diabetic C57BL/6 and db/db mice. Previous in vitro studies suggested that host lipid metabolism could be hijacked by DENV for its replication81 and that exogenous fatty acids enhanced the effects of DENV infection.82, 83 In our study, the diabetic mice had a longer duration of viremia than did the control mice, and thus, more in vivo experiments are needed to demonstrate the role of diabetic lipogenesis in DENV replication. Therefore, our mouse model can lay the foundation for studying the biological mechanism of diabetes complicated by DENV infection.
Overall, we established two types of diabetic mice for DENV infection. The replicable weight loss, viral space–time distribution, long-period viremia, strong inflammatory response, and histological alterations suggest the feasibility and practicality of these animal models for developing antiviral pharmaceuticals and treatments. For dengue vaccine testing, despite the reproducible viremia and immunocompetence in our diabetic murine model, viremia for all four serotypes needs to be explored. We should also be cognizant of the limitation that the pathogenic mechanism in diabetes with DENV infection has not yet been elucidated, warranting further research. Further, systematic studies on the mechanisms underlying the worse prognosis of diabetes complicated with DENV infection are still lacking. Since these DENV-3-infected diabetic murine models have similarities with previous models, they may be applicable as in vivo systems for studying the biological basis.
AUTHOR CONTRIBUTIONS
Xuling Liu: Conceptualization, methodology, writing, validation, and data curation. Yingfang Liu: Methodology, validation, and data curation. Hao Wu: Methodology, validation, and data curation. Zihan He: Methodology, validation, and data curation. Zhuoyun Li: Formal analysis. Zhiran Qin: Formal analysis. Jianhai Yu: Visualization. Li Zhu: Visualization. Qinghua Wu: Visualization. Weiwei Xiao: Visualization. Chenguang Shen: Supervision. Chengsong Wan: Supervision. Bao Zhang: Conceptualization, methodology, and supervision. Wei Zhao: Writing, supervision, project administration, and funding acquisition.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (Grant Nos. 81730110 and 31470271), Yangjiang Science and Technology Program key projects (Grant No. 2019010), Basic Research Project of Key Laboratory of Guangzhou (Grant No. 202102100001), Guangzhou Science and Technology Program key projects (Grant No. 201803040006), and Guangdong Science and Technology Program key projects (Grant Nos. 2021A1515220017 and 2021B1212030014).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
All animal protocols were approved by the Experimental Animal Ethics Committee of the Guangdong Medical Laboratory Animal Center (approval number: C202110-2; approval date: October 30, 2021).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.