A soluble DR5-Fc chimeric protein attenuates inflammatory responses induced by coronavirus MHV-A59 and SARS-CoV-2
Hong Peng, Birong Zheng, and Sidi Yang contributed equally to this study.
Abstract
Mortality in coronavirus disease 2019 (COVID-19) patients has been linked to the presence of a “cytokine storm” induced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, which involves elevated levels of circulating cytokines and immune-cell hyperactivation. Targeting cytokines during the management of COVID-19 patients has the potential to improve survival rates and reduce mortality. Although cytokine blockers and immune-host modulators are currently being tested in severely ill COVID-19 patients to cope with the overwhelming systemic inflammation, there is not too many successful cases, thus finding new cytokine blockers to attenuate the cytokine storm syndrome is meaningful. In this paper, we significantly attenuated the inflammatory responses induced by mouse hepatitis viruses A59 and SARS-CoV-2 through a soluble DR5-Fc (sDR5-Fc) chimeric protein that blocked the TNF-related apoptosis-inducing ligand–death receptor 5 (TRAIL–DR5) interaction. Our findings indicates that blocking the TRAIL–DR5 pathway through the sDR5-Fc chimeric protein is a promising strategy to treat COVID-19 severe patients requiring intensive care unit admission or with chronic metabolic diseases.
1 INTRODUCTION
A novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was identified in early 2020 as the cause of the global epidemic coronavirus disease 2019 (COVID-19).1 During SARS-CoV-2 infection, patients present systemic symptoms of varying severity that are associated with an aggressive inflammatory response.2 Immune system activation and production of inflammatory cytokines are essential for the host to execute an antiviral reaction. However, hyperactivation of the immune system promotes an acute increase in circulating levels of proinflammatory cytokines, which may result in a “cytokine storm.” COVID-19 patients requiring intensive care unit(ICU) admission displayed higher blood concentrations of interleukin 6, CXCL10, CCL2, and tumor necrosis factor (TNF)-α as compared to patients with moderate symptoms.3 Since its outbreak until now, many studies have been performed to find efficient cytokine blockers and immune-host modulators to cope with the overwhelming systemic inflammation, for example, CD24-Fc had been showed promising efficacy in a clinical trial of hospitalized COVID-19 patients receiving oxygen support,4 thus finding new cytokine blockers to attenuate the cytokine storm syndrome is meaningful.5
TNF-related apoptosis-inducing ligand (TRAIL) belongs to the TNF family and is encoded by the TNFSF10 gene. Death receptor 5 (DR5) is a cell surface receptor that binds to TRAIL, and their interaction not only triggers apoptosis but also certain inflammatory responses.6 In patients with chronic obstructive pulmonary disease (COPD), systemic inflammation was observed, and circulating TRAIL and DR5 increased and were negatively correlated with lung function.7 Individuals with COPD infected with SARS-CoV-2 are associated with higher mortality compared to individuals without COPD.8 These results imply that TRAIL and DR5 play an important role in inflammation. Here, we employed a soluble DR5-Fc (sDR5-Fc) chimeric protein to attenuate the inflammatory responses induced by coronavirus mouse hepatitis virus A59 (MHV-A59) and SARS-CoV-2 infection, indicating that blocking the TRAIL–DR5 pathway through the sDR5-Fc chimeric protein could be a promising strategy to treat with COVID-19 severe patients requiring ICU admission or with chronic metabolic diseases.
2 MATERIALS AND METHODS
2.1 Production of sDR5-Fc
sDR5-Fc was designed to contain the extracellular fragment of human DR5 (amino acids 1–182) and the human immunoglobulin G1 (IgG1) Fc fragment. It was expressed in CHO-K1 cells by stable transfection and purified from culture supernatant using protein-A affinity column. The quality of the purified protein was assessed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and high-performance liquid chromatography (Shimadzu). Endotoxins were measured using a limulus amebocyte lysate assay (Xiamen Bioendo Technology Co., Ltd.).
2.2 Cells lines, viruses, and mice
Mouse neuroblastoma Neuro-2a cells (Neuro-2a) and Murine L2 cells were obtained from American Type Culture Collection. Neuro-2a cells and L2 cells were cultured in Gibco modified Eagle's medium containing 10% heat-inactivated fetal bovine serum (Gibco), 100 units/ml of penicillin, and 100 µg/ml of streptomycin and maintained in 5% CO2 at 37°C.
MHV-A59 strain was kindly provided by Rong Ye (Shanghai Medical School of Fudan University). African green monkey kidney Vero E6 cell line (Vero E6) was kindly provided by Dr. Hui Zhang (Sun Yat-sen University [SYSU]). MHV-A59 was propagated according to the method described previously.9 Briefly, the virus was propagated in Neuro-2a cells followed, the large debris was spun down and the supernatant was used as a stock solution. The titer of the virus was determined by plaque assay in L2 cells exactly as described.10
C57BL/6 mice were purchased from Guangdong Laboratory Animal Research Center. K18-hACE2 transgenic C57BL/6 mice were purchased from Jackson Laboratory. All the mice were maintained in animal facilities under specific pathogen-free conditions. The handling of mice and experimental procedures were approved by the Animal Welfare and Research Ethics Committee of the Institute of Biophysics, SYSU.
SARS-CoV-2 (B.1.617.2, GDPCC 2.00096) was isolated from a patient infected with the SARS-CoV-2 Delta variant admitted to the Guangzhou Eighth People's Hospital by the Center for Disease Control and Prevention of Guangdong Province.11
SARS-CoV-2 infection experiments were performed in the BSL-3 laboratory of SYSU or Guangzhou Customs District Technology Center.
2.3 MHV-A59 infection in vivo
Four-week-old female C57BL/6 mice were lightly anesthetized with isoflurane and then inoculated in the orthotopic liver with 100 µl of MHV-A59 virus at 1 × 106 or 2 × 105 plaque-forming unit (PFU), and control mice were subjected to orthotopic liver infection with 100 μl of phosphate-buffered saline (PBS). At intervals of 3 days postinfection (dpi), mice from each group were killed, liver from infected and noninfected control mice were rinsed in ice-cold PBS, all tissues were separated for reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and for histological analysis.
Six-week-old female C57BL/6 mice were lightly anesthetized with isoflurane and then inoculated in the orthotopic liver with 100 μl of MHV-A59 virus at 1 × 106 PFU, and control mice were subjected to orthotopic liver infection with 100 μl of PBS. The mice were monitored daily for health conditions and weight. According to the results in Figure 2B, the weight reduction in the MHV-A59-infected group was no more than 20% during the experiment, the survival depicted here was real survival.
2.4 SARS-CoV-2 infection in vivo
Six-week-old K18-hACE2 transgenic C57BL/6 mice were mock-infected (PBS), or infected with 1 × 105 PFU of SARS-CoV-2 B.1.617.2 (Delta) variant. The mice were monitored daily for health condition and weight. At intervals of 3 dpi, mice from each group were killed, and lungs from infected and noninfected control mice were rinsed in ice-cold PBS, and all tissues were separated for RT-qPCR and for histological analysis.
2.5 Liver function assay (alanine transaminase)
Alanine transaminase (ALT) activity was determined in ethylenediaminetetraacetic acid-treated serum using the Alanine Transaminase Activity Assay Kit (Nanjing Jiancheng Bioengineering Institute), which was were performed following the manufacturer's recommendations. Samples were analyzed in triplicate wells per mouse sample, and the mean value of these technical replicates was used for subsequent analysis.
2.6 Histopathology evaluation
Liver tissues were removed from the mice at 3 dpi, half of the liver were used for histological analysis, perfused with 10% buffered formalin, paraffin-embedded, and stained with hematoxylin and eosin. The pathology of the liver was evaluated under a microscope (Shunyu; ICX41).
2.7 Cytokines profiles
For the detection of proinflammatory cytokines, the total RNA was isolated from tissue samples with TRIzol reagent under the instruction of the manufacturer. The messenger RNAs (mRNAs) were reverse transcribed into complementary DNA(cDNA) by PrimeScript RT reagent Kit (Takara). The cDNA was amplified by a fast two-step amplification program using ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd.). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used to normalize the input samples via the ΔΔCT method. The relative mRNA expression level of each gene was normalized to GAPDH housekeeping gene expression in the untreated condition, and fold induction was calculated by the ΔΔCT method relative to those in untreated samples. Primers for different genes were listed in Table 1.
| Gene | Forward 5′−3’ | Reverse 5′−3’ |
|---|---|---|
| SARS-COV-2 sgN | CCAGGTAACAAACCAACAA | TGAGTGAGAGCGGTGAACCAA |
| Tnfsf10 | ATGGTGATTTGCATAGTGCTCC | GCAAGCAGGGTCTGTTCAAGA |
| Tnf | CCCTCCAGAAAAGACACCATG | GCCACAAGCAGGAATGAGAAG |
| ll6 | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| Ifng | TGTTACTGCCACGGCACAGT | CTGGCTCTGCAGGATTTTCAT |
| l1-1b | CCACCTTTTGACAGTGATGA | GAGATTTGAAGCTGGATGCT |
| Ccl2 | TCATAGCAGCCACCTTCATTC | CTCTGCACTGAGATCTTCCTATTG |
| Ccl3 | TGTACCATGACACTCTGCAAC | CAACGATGAATTGGCGTGGAA |
| Ccl4 | TTCCTGCTGTTTCTCTTACACCT | CTGTCTGCCTCTTTTGGTCAG |
| Ccl5 | GCTGCTTTGCCTACCTCTCC | TCGAGTGACAAACACGACTGC |
| Cxcl1 | CTGGGATTCACCTCAAGAACATC | CAGGGTCAAGGCAAGCCTC |
| Cxcl10 | CCTGCCCACGTGTTGAGAT | TGATGGTCTTAGATTCCGGATTC |
| lfit1 | CAAGGCAGGTTTCTGAGGAG | GACCTGGTCACCATCAGCAT |
| Gapdh | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
2.8 Enzyme-linked immunosorbent assay
Serum was analyzed for mouse TNF-α level by Enzyme-Linked Immunosorbent Assay Kit (R&D System; MTA00B), according to the manufacturer's protocol.
2.9 Data analysis and statistical comparisons
The unpaired two-tails Student's t-test and one-way analysis of variance were used to determine the statistical significance. The values were presented as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; and ns, not significant.
3 RESULTS AND DISCUSSION
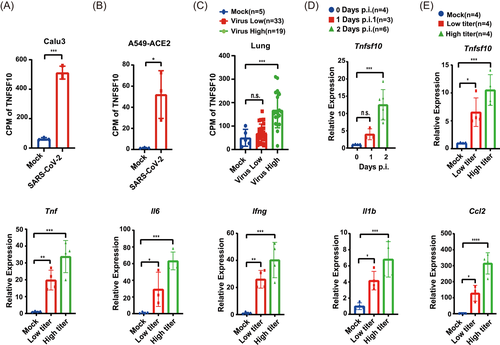
To assess whether TRAIL is involved in SARS-CoV-2 infection, we reanalyzed an RNA-sequencing database of several cell lines infected with SARS-CoV-2 (GSE147507) and human autopsy specimens from patients who succumbed to SARS-CoV-2 infection (GSE152075) and observed that the transcription level of TNFSF10 significantly increased both in SARS-CoV-2-infected cells and lung specimens from patients with COVID-19 (Figure 1A–C).12
We designed an sDR5-Fc chimeric protein containing the extracellular domain of human DR5 and the Fc domain of human IgG1, with high affinities with TRAIL broadly in different species.13 We first used an MHV-A59 infection model to test the anti-inflammation effect of sDR5-Fc. Like SARS-CoV-2, MHV-A59 is a member of beta-coronaviruses and leads to severe inflammatory injury in mouse liver, analogous to severe lung inflammatory injury in patients with severe COVID-19.14
First, we found the mRNA level of tnfsf10 increased in time and dose-dependent manner, implying TRAIL is a potential participant in MHV-A59-induced fulminant hepatitis (Figure 1D). Furthermore, the transcription level of proinflammatory cytokines increased in a dose-dependent pattern, suggesting the involvement of inflammatory cells, such as macrophages and NK cells, in liver injury (Figure 1E).
Next, we checked whether sDR5-Fc could attenuate the immune cytokine response. An orthotopic liver injection was performed in 6-week-old mice. One group of mice infected with MHV-A59 was injected intraperitoneally with sDR5-Fc (20 mg/kg), then mice were monitored over a 7-day time course. One day after infection, one group was reinjected with sDR5-Fc (20 mg/kg) (Figure 2A). The body weight of mice lost significantly 3 dpi, but the group that received sDR5-Fc showed weight enhancement 4 days postvirus infection (Figure 2B). All mice succumbed to infection at 7 dpi. Mock-infected mice or infected mice treated with sDR5-Fc appeared healthy, maintained weight, and survived MHV-A59 infection for the duration of this study, except two mice of the sDR5-Fc injected group died at 6 and 7 dpi (Figure 2C). Notably, as a hepatic virus, MHV-A59 caused fulminant hepatitis and displayed hepatocyte necrosis and hemorrhage, but injection of sDR5-Fc significantly relieved the hepatitis symptom (Figure 2D). Histological results of liver slices also proved the remission effect of sDR5-Fc (Figure 2D). Next, we checked serum ALT level, which served as a marker for MHV-induced acute hepatitis. Serum ALT levels dramatically increased in MHV-A59-infected mice, which decreased by about 60% in sDR5-Fc treated mice, further confirming the effect of sDR5-Fc (Figure 2E). Interestingly, we also observed decreased virus titer of MHV-A59 in the sDR5-Fc treated group (Figure 2F), which was probably mediated by the Fc fragment. As engagement of the IgG1 Fc domain with members of the FcγR family is responsible for triggering the effector cell responses critical for host protection against infection.15 Recently, a group reveals that monocytes take up antibody-opsonized SARS-CoV-2 through an FcγR but viral replication is aborted.16 Fc binds to an Fc receptor on the phagocyte, facilitating phagocytosis. Fc LALAPG mutant showed eliminated binding to FcγRI, II, III, and C1q, which could be used as a negative control to explore the exact mechanism in the future.
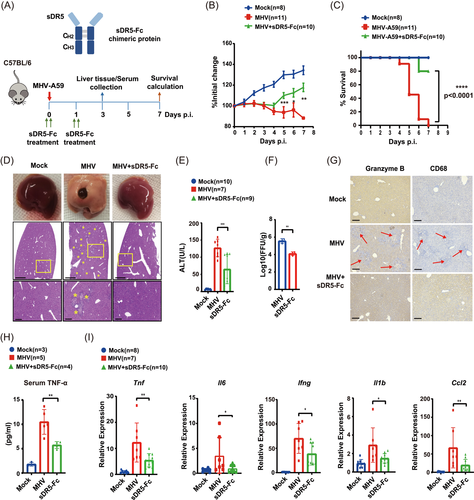
Liver histopathological changes induced by MHV-A59 infection would associate with inflammatory leukocyte infiltration, and we then evaluated the localized infiltration of innate and adaptive immune cell populations in liver slice samples. Immunoreactive macrophages (CD68+) and cytotoxic lymphocyte-derived protease granzyme B were present in the MHV-A59 inoculated controls at 3 dpi, but the mice treated with sDR5-Fc showed decreased inflammatory leukocyte infiltration (Figure 2G). Proinflammatory cytokines are a group of cytokines that were originally identified as mediators of inflammation, which could be induced by MHV-A59 infection. Compared with MHV-A59-infected mice, the sDR5-Fc treatment decreased about 50% TNF-α level in serum (Figure 2H), as well as the transcription level of indicated proinflammatory cytokines (tnf, ll6, lfng, ll1b, and Ccl2) (Figure 2I).
Next, we investigated the anti-inflammatory effect of sDR5-Fc in the SARS-CoV-2 infection mouse model. K18-hACE2 transgenic C57BL/6 mice were intranasally inoculated with SARS-CoV-2 and were injected intraperitoneally with sDR5-Fc (20 mg/kg) on the first day and 1 dpi separately, and all mice succumbed at 7 dpi (Figure 3A). sDR5-Fc treatment neither rescued the weight lost (Figure 3B) nor prevented the mice from death in this model (data not shown), because K18-hACE2 mice may die from SARS-CoV-2 Delta-induced encephalitis other than lung disease.17 But sDR5-Fc injection could significantly reduce the pathological damage of lung tissue, especially vascular hemorrhage and fibrosis (Figure 3C). Besides that, sDR5-Fc also decreased inflammatory leukocyte infiltration in the lung (Figure 3D) and the amount of virus in lung tissues (Figure 3E). Meanwhile, we also observed decreased transcription levels of proinflammatory cytokines after sDR5-Fc treatment (Figure 3F). These results indicated that sDR5-Fc is capable of reducing inflammation induced by SARS-CoV-2.

Recently, Wang et al.13 found that sDR5-Fc can block TRAIL to improve cardiac function after myocardial infarction (MI). Considering MI is also a typical clinical symptom in COVID-19 severe patients, implying that sDR5-Fc has the potential to attenuate the myocardial injury in COVID-19 severe patients. Our study reveals that blocking the TRAIL-DR5 pathway through sDR5-Fc decreases the cytokine level induced by MHV-A59 and SARS-CoV-2 infection, which may be used as a cytokine blocker to treat COVID-19 severe patients requiring ICU admission or with chronic metabolic diseases.
AUTHOR CONTRIBUTIONS
Deyin Guo and Hailong Zhang conceived and planned the overall structure of this project, and edited the manuscript. Hong Peng, Birong Zheng, and Sidi Yang carried out the experiments and drafted the manuscript. Jie Du, Liu Cao, and Lihong Liu analyzed the data. Zengyi Ma, Junyu Wu, and Chunmei Li discussed and proofread the final manuscript. All authors have read and approved the final paper.
ACKNOWLEDGMENTS
The authors would like to thank the Center for Disease Control and Prevention of Guangdong Province for providing the Delta variant of SARS-CoV-2. This study was supported by grants from the National Natural Science Foundation of China (32041002, 81702724) and the Shenzhen Science and Technology Program (JSGG20200225150431472, KQTD20180411143323605, JCYJ20190807161009621)
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.