A seroepidemiological study across age groups before and after the 2010–2011 mumps epidemic in Japan
Abstract
In Japan, large-scale mumps epidemics recur every 4–6 years because of low vaccination coverage. This study aimed to describe the seroprevalence of mumps in the Japanese population and identify the age groups most affected. The prevalence of anti-mumps antibodies was evaluated based on 1000 serum samples obtained from the Japanese National Serum Reference Bank. These samples consisted of 50 sera for each of 10 different age groups, collected during 2007–2008 (pre-epidemic period) and 2012–2013 (post-epidemic period). Seropositivity was lowest in the 6–11 months subgroup (3% and 0% in pre- and post-epidemic periods, respectively) and highest in the 10–14 years group (66% and 72% in pre- and post-epidemic periods, respectively). A comparison of anti-mumps antibody prevalence throughout the two periods considered revealed a large rise in seropositivity among the 2004–2008 birth cohort, using that of the 1–4 years group as representative in the pre-epidemic period (from 22% in pre- to 58% in post-epidemic periods; p = 0.0002). These results indicate that most people likely gain antibodies to the mumps virus during their childhood, especially during the first epidemic that they experience after their second year of life. Therefore, children should be vaccinated against mumps soon after their first birthday for effective prevention.
1 INTRODUCTION
In Japan, although live attenuated mumps vaccines have been provided under the voluntary vaccination schedule since 1981, they are not included in the national routine immunization program. The mumps vaccination coverage rate remains low. A questionnaire study conducted in Japan throughout 2005 reported that local mumps vaccination coverage was only 23.2%1; another Japanese study, based on a hospital admission survey conducted in 2012, reported it as 27.6%.2 The measles–mumps–rubella combined vaccine was temporarily provided as an alternative to the measles vaccine and as part of the national routine childhood vaccination schedule from 1989; however, this program was stopped in 1993 because of an increased incidence of aseptic meningitis that was likely related to vaccination.3 The epidemiology of mumps in Japan is monitored by pediatric sentinel site surveillance based on the National Epidemiological Surveillance of Infectious Diseases (NESID) system.4 The number of clinically diagnosed mumps cases is reported weekly from approximately 3000 pediatric sentinel sites, which are distributed all over Japan according to the regional population size. Despite mumps being a vaccine-preventable disease, low vaccination coverage has led to recurrent large-scale epidemics every 4–6 years in Japan, as the available NESID data since 1982 shows. During the last two decades, large-scale mumps epidemics have occurred four times—in 2000–2001, 2005–2006, 2010–2011, and 2015–2016.3 Circulating genotypes of the mumps virus have changed with time. In Japan, the most predominant circulating genotypes reported were genotype B in the 1980s, genotype J and B in the 1990s, and genotype G in the 2000s.5 In recent outbreaks among vaccinated populations, genotype G was described as the most widely circulated genotype globally.6 Mumps can also be a sizable public health problem owing to its potentially severe complications such as irreversible deafness (0.01%–0.5% of cases), aseptic meningitis (1%–10% of cases), encephalitis (0.02%–0.3% of cases), orchitis, oophoritis, mastitis, and pancreatitis. These complications are more common among adults than children.7
There are no seroepidemiological data for mumps that span all age groups in Japan because mumps is not included in the National Epidemiological Surveillance of Vaccine-Preventable Diseases (NESVPD) program, which is the national seroepidemiological surveillance program in Japan.8 Seroepidemiological assessment indicates the population at risk with potential susceptibility to infection and facilitates the assessment of the optimal age for immunization. This study aimed to describe the seroprevalence of mumps among all age groups of the Japanese population by simultaneously evaluating the increase in seroprevalence through an epidemic in three birth-year cohorts, thereby identifying the most affected age groups in a mumps cyclic epidemic as fundamental data for immunization programs and policy improvement.
2 MATERIALS AND METHODS
2.1 Specimen collection and evaluation
Serum samples were obtained from the National Serum Reference Bank at the National Institute of Infectious Diseases, which stores serum specimens with information about the sample providers’ age and sex as well as the serum collection year and prefecture. Based on the nature and limitation of the National Serum Reference Bank, neither the histories of natural mumps infection nor mumps vaccination in these serum providers were available. The serum specimens are routinely collected from a nationally representative sample of randomly selected healthy individuals from all age groups.
NESID data showed that a recent mumps epidemic occurred during 2010–2011. To compare seroprevalence before and after this epidemic, the pre-epidemic period was defined as 2007–2008 and the post-epidemic period as 2012–2013 (a 5-year interval) in this study. For the present study, 50 samples for each of 10 age groups (<1 year, 1–4, 5–9, 10–14, 15–19, 20–29, 30–39, 40–49, 50–59, and ≥60 years) during 2007–2008 and 2012–2013 (500 samples for each period) were evaluated. The <1 year group was subdivided into subgroups of 0–5 months and 6–11 months to account for the significant influence of maternal antibodies during the first 6 months after birth. The <1 year group in 2007–2008 included 10 infants aged 0–5 months, 38 infants aged 6–11 months, and 2 infants whose exact ages were unknown. Furthermore, there were 15 infants aged 0–5 months and 35 infants aged 6–11 months in the <1 year group in 2012–2013.
A fixed 1:1 ratio for men and women was maintained for each age group. Samples were selected to reflect a distribution similar to that of the actual population of the studied regions. This weighting was performed according to the population of three separate regions based on 2010 data from the Japanese Census Bureau. Thus, numbers of samples for both periods were selected in a ratio of 11:9:5 for eastern (Hokkaido, Tohoku, and Kanto), central (Chubu and Kinki), and western (Chugoku, Shikoku, and Kyusyu) regions, respectively.
2.2 Laboratory methods and categorization
All serological tests were performed at the National Institute of Infectious Diseases using enzyme immunoassays (EIAs) in duplicate. Anti-mumps immunoglobulin G (IgG) levels were determined according to the manufacturer's instructions of a commercially available kit (SEIKEN mumps IgG; Denka Seiken Co. Ltd.). The results were reported as quantitative EIA values based on each sample's optical density. Samples were considered seronegative for EIA if values were <2.0, equivocal for those between 2.0 and <4.0, and seropositive for those ≥4.0.
2.3 Statistical analysis
Seroprevalence rates are summarized as percentages according to categorization based on EIA values (seropositive, equivocal, and seronegative), and the antibody titers of seropositive samples were calculated as geometric mean titers (GMTs) with 95% confidence intervals (CIs) for every age group during each period. Additionally, changes in seroprevalence and GMT results in the same birth cohort from before to after the 2010–2011 mumps epidemic were evaluated to determine the impact of the epidemic on distributions of seroprevalence by age group. Due to the 5-year interval between the two study periods, three birth cohorts could be observed in this study. The children in the <1 and 1–4 years groups during 2007–2008 were assumed to be in the 5–9 years group during 2012–2013. They were considered a part of the 2004–2008 birth-year cohort in this study. Similarly, persons born during 1999–2003 and 1994–1998 were considered part of the 5–9 years group and 10–14 years groups during 2007–2008 and also part of the 10–14 years group and 15–19 years group during 2012–2013, respectively. Concerning the 2004–2008 birth-year cohort, during 2007–2008, 100 samples were collected from two age groups (<1 year and 1–4 years). Infants might have been affected by maternal immunity and/or might have had a lower likelihood of exposure to the mumps virus. Therefore, to avoid overestimation due to reduced levels of exposure, when evaluating the change in seroprevalence during the interval between the two study periods, only seroprevalence in the 1–4 years group during 2007–2008 was used as an estimate for the 2004–2008 birth-year cohort in the pre-epidemic period for the primary comparison. Additionally, a comparison that included the <1 year group data was performed as a supplementary sensitivity analysis.
The chi-square test was used to compare seroprevalence values of each birth-year cohort between 2007–2008 and 2012–2013; it was also used to compare seroprevalence rates in the same age groups between the two periods. p Values < 0.05 were considered statistically significant. The data were analyzed using JMP® software, version 11.2.0 (SAS Institute Inc., 1989–2021).
2.4 Ethical considerations
This study was conducted in compliance with relevant laws and institutional guidelines and in accordance with the ethical standards of the Declaration of Helsinki. The stored serum specimens for the National Serum Reference Bank are collected from a nationally representative sample of randomly selected healthy individuals of all age groups every year. The local public health officials obtain informed consent before collecting serum from healthy individuals who participate in the annual seroepidemiological survey based on the NESVPD program. Among those in the pediatric age group, the parents or guardians are approached during routine health check-ups to obtain consent before participation. Ethical approval was obtained as part of the work performed under the function of the National Serum Reference Bank, which has received ethical approval for the release of up to 1000 anonymized specimens for a single public health study.
3 RESULTS
3.1 Seroprevalence rates before and after the 2010–2011 epidemic
Figure 1 shows the seroprevalence of IgG antibodies during 2007–2008 and 2012–2013. Mumps IgG seroprevalence patterns of both study periods were similar. No statistically significant difference between proportions of seropositive samples in the same age group during the two periods was observed, except for the 40–49 years group (p = 0.02).
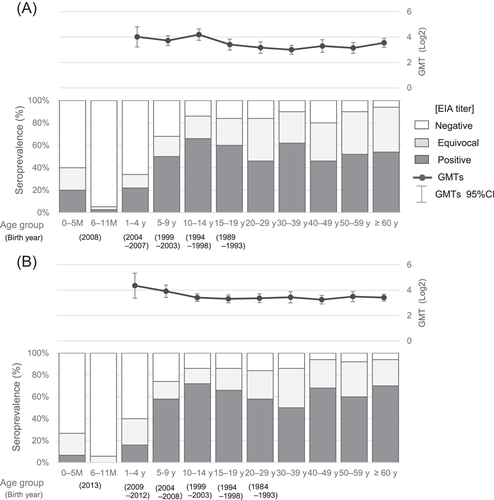
The 6- to 11-month infants subgroup had the lowest level of seropositivity, namely 3% and 0% during 2007–2008 and 2012–2013, respectively. Both infants of unknown ages in the 2007–2008 period were seronegative. The seropositivity proportions gradually increased with age in childhood, that is, up to the teens, and peaked in the 10–14 years group (66% in 2007–2008 and 72% in 2012–2013; Figure 1, Supporting Information: Table). Among adults (≥20 years old), the rate of mumps seropositivity ranged from 46% to 62% in 2007–2008 and 50% to 70% in 2012–2013. In other words, adults had higher proportions of equivocal EIA results (24%–40%) than children aged 1–19 years (12%–24%). Additionally, seronegative adults remained in the range of 6%–20% in 2007–2008 and 6%–16% in 2012–2013.
3.2 GMTs of seropositive samples
Figure 1 shows the GMT values of antibody-positive cases during each period. The highest GMT values were observed in childhood, that is, in the 10–14 years group in 2007–2008 and in the 1–4 years group in 2012–2013. GMT values of children (1–19 years) were slightly higher than those of adults (≥20 years), although there was a statistically significant difference only in the pre-epidemic period (p < 0.0001). Similarly, GMT values among antibody-positive plus equivocal categories were also greater among children than adults.
3.3 Change in mumps seroprevalence and GMTs through the epidemic in the three birth cohorts
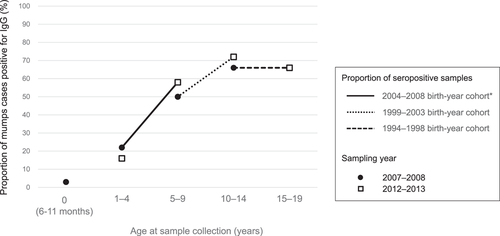
In this study, we aimed to assess changes in seroprevalence observed throughout two study periods among the following three 5-year birth cohorts: 2004–2008, 1999–2003, and 1994–1998. Among children who were born during 2004–2008, the prevalence of anti-mumps IgG increased from 22% (1–4 year age group in 2007–2008) to 58% (5–9 year age group in 2012–2013; p = 0.0002; Figure 2, Table 1). If data of the <1 year group during 2007–2008 are considered, a larger rise in the seroprevalence of the birth cohort after the epidemic was observed (from 14% to 58%; p ≤ 0.0001). Also among children who were born during 1999–2003, the prevalence of anti-mumps IgG increased from 50% (5–9 year age group in 2007–2008) to 72% (10–14 year age group in 2012–2013; p = 0.024). On the other hand, for the 1994–1998 birth cohort, which included the 10–14 years group during 2007–2008 and the 15–19 years group during 2012–2013, no change was observed in the prevalence of anti-mumps antibodies (66% were seropositive during both periods), with a decrease in the GMT observed (Table 1).
| Seroprevalence | GMT (log2) | ||||||
|---|---|---|---|---|---|---|---|
| Birth year | 2007–2008 (95% CI) | 2012–2013 (95% CI) | Difference (95% CI) | p Value | 2007–2008 (95% CI) | 2012–2013 (95% CI) | p Value |
| 2004–2008a | 22.0% (12.8–35.2%) | 58.0%(44.2–70.6%) | +36.0% (18.1%–53.9%) | 0.0002 | 4.0 (3.2–4.8) | 3.9 (3.4–4.4) | 0.65 |
| 1999–2003 | 50.0% (36.7–63.4%) | 72.0% (58.3–82.5%) | +22.0% (3.4%–40.6%) | 0.02 | 3.7 (3.3–4.1) | 3.4 (3.1–3.7) | 0.16 |
| 1994–1998 | 66.0% (52.2–77.6%) | 66.0% (52.2–77.6%) | 0% (−18.6% to 18.6%) | >0.99 | 4.2 (3.7–4.6) | 3.3 (3.0–3.6) | 0.006 |
- Abbreviations: CI, confidence interval; GMT, geometric mean titer.
- a The prevalence of this birth-year cohort during 2007–2008 was plotted using the 1–4 years group data to avoid overestimation due to lower exposure in the <1 year group.
From a different perspective, when comparing these birth cohorts, seropositivity in 1- to 4-year old of the 2004–2008 birth cohort (16.0%) was lower than that of the 1999–2003 birth cohort (22.0%; Figure 2), whereas the proportion of seropositive plus equivocal in that age group of the 2004–2008 birth cohort was higher than that of the 1999–2003 birth cohort (Supporting Information: Table).
Additionally, as a sub-analysis, a similar comparison was performed that included samples from adult age groups at 5-year intervals (Supporting Information: Figure), though the number of samples was not uniform. There was no significant difference between the proportion of seropositive samples in each birth cohort when values were compared across the 5-year interval, except among individuals from the 1964–1968 birth-year cohort who were 40–44 years old in 2007–2008 (p = 0.005).
4 DISCUSSION
This study described seroprevalence rates of two periods (before and after a mumps epidemic in Japan). Results indicated that most people gained IgG antibodies against the mumps virus during childhood but not before 1 year of age. Additionally, changes in seroprevalence from the pre-epidemic period to after the epidemic in each birth cohort suggested that the mumps epidemic most likely affected children aged approximately 5 years.
This result aligns with age distributions reported using pediatric sentinel site surveillance data based on the NESID program.4 NESID data show that 90%–93% of the annually reported mumps cases occurred in children below the age of 10 years. Further, 60% of cases were among individuals under the age of 6 years while 0.3%–0.6% were among infants aged <1 year throughout 2007–2013.3 Although it has been said that higher rates of subclinical mumps infections occur among infants and children younger than 3 years, this serological study revealed that only a few infants aged <1 year were infected.9 The seropositivity rate among infants aged <6 months, which likely reflected the presence of maternal antibodies, was lower than the reported prevalence of seropositivity among pregnant Japanese women.10 Alternatively, the difference may be explained by attenuation of maternal antibodies in infants.
Seropositive individuals are those that have either been previously naturally infected or vaccinated. Vaccine-induced immunity tends to result in lower levels of antibodies than those detected subsequent to natural infection and, in vaccinated individuals, antibody levels may further decline owing to vaccine failure.11 This means that the estimated proportion of naturally infected people might be higher than the difference between seroprevalence and vaccination coverage rates. Vaccination histories of study participants were unavailable due to the nature of the National Serum Bank data; as a proxy to that, NESVPD data pertaining to mumps vaccine coverage rates by age group in 2008 and 2013 were available for use. Those respective values were as follows: 15.8% and 22.1% in 1- to 4-year old, 22.9% and 28.6% in 5- to 9-year old, 16.5% and 20.1% in 10- to 14-year old, and 25.2% and 19.8% in 15- to 19-year old.8 These data indicate that vaccination coverage rates in each age group were lower than those of seropositive individuals, except in the 1–4 years group during 2012–2013. There was a roughly 35%–50% difference between seropositivity and coverage rates in individuals aged ≥10 years. Considering the possibility of lower titers in vaccinated people and also the potentiality that they were included among an equivocal category, findings suggested that the proportion of teenagers with acquired immunity increased due to mumps virus infection in childhood and assumed more than the difference between previous seropositivity and vaccination coverage rates, as mentioned above.
From another perspective, reduced levels of seropositivity versus vaccine coverage rates in the 1- to 4-year-old age group throughout 2012–2013 might be partially explained by the lower titer of antibodies present in vaccinated individuals.11 Mumps vaccination coverage in all groups under 15 years of age increased from one period to the next, according to the NESVPD data described above. This finding seems to be supported by data showing that, conversely, higher proportions of seropositive plus equivocal samples in the 1–4 years-of-age group were observed during 2012–2013 versus 2007–2008, despite lower reported rates of seropositivity (Supporting Information: Table).
To propose a vaccination strategy that helps avoid large outbreaks of mumps infection, we tried to determine which age groups were most affected by cyclic mumps epidemics. Considering that seroprevalence in the 2004–2008 birth cohort aged <5 years in the pre-endemic period increased greatly during the mumps epidemic in 2010–2011, this age group was considered most likely to be infected during the epidemic (Figure 2). Most individuals of this birth cohort were aged less than 2 years or were not yet born at the time of the previous 2005–2006 mumps epidemic. This result suggests that children who experience their first epidemic especially during early childhood are primarily affected by it.
Considering that the reported estimated basic reproductive numbers for mumps ranged from 4 to 712 to 11–14,7 it is necessary for herd immunity to be achieved in more than 75%–93% of the population to suppress mumps endemics. Another study estimated it as 90%–92%.13 In our study, at least 66% and 60% of 1- to 4-year old were seronegative during 2007–2008 and 2012–2013, respectively. In addition, 12% during 2007–2008 and 24% during 2012–2013 were within the equivocal category with low IgG titers. The accumulation of susceptible people, such as those who were born after the last major mumps epidemic, may reduce population-level herd immunity and contribute to epidemic recurrence. This scenario indicates that a mumps vaccination in early childhood as part of the routine vaccination schedule would protect vaccinated individuals before another epidemic and prevent the accumulation of a population susceptible to mumps.
This study also revealed the unignorable existence of adult susceptible individuals; 6%–20% of Japanese adults tested negative for anti-mumps IgG. Further, 24%–40% of adults were categorized as equivocal with a low titer. Mumps infection in adults tends to occur with more severe symptoms and consequences. For example. Takagi et al.14 demonstrated that the incidence of mumps deafness per 10 000 patients was 73.6 in adolescence and adulthood, 8.4 times higher than that of childhood. When 0- to 64-year old were considered, Ohfuji et al.15 also reported the incidence of meningitis and orchitis as common mumps-related complications in 26- to 35-year old. These facts may suggest that vaccines should be administered to high-risk persons with no history of mumps infection or mumps vaccination who are exposed to mumps patients, for example, health-care workers and child caregivers.
This study has several limitations. First, the vaccination status and disease history data of the participants were not available, and we referred to NESVPD vaccination coverage data when considering mumps disease burden. Second, the number of samples was limited by a regulation of the National Serum Reference Bank. To ensure better representation, we performed population and geographical matching across Japan. Additionally, seroprevalence rates among adolescents and young adults were similar to those reported previously in these age groups in the Japanese population.10, 16 Finally, because the evaluation rates of seroprevalence change between two study periods were conducted using serial 5-year birth cohorts, except infants aged <1 year old, we were only able to specify the 5-year age range in children most affected by the mumps epidemic.
The findings of the current study indicate that most individuals developed anti-mumps IgG between the ages of 1 and 10 years. Further, in areas of Japan with low mumps vaccine coverage, they were likely to have been infected during the first epidemic that they experienced after their second year of life. Therefore, prompt vaccination in early childhood, such as at the age of 1 year, is appropriate timing for initiating vaccination for preventing infection before exposure. To avoid the next large epidemic in Japan, universal mumps vaccination programs targeting 1-year-old children are desired to be implemented as quickly as possible. More detailed continuous birth-year cohort investigations may be useful for clarifying the impact of mumps epidemics on the seroprevalence of anti-mumps IgG and the optimal timing for mumps vaccination.
AUTHOR CONTRIBUTIONS
Keiko Tanaka-Taya, Saeko Morino, and Hiroshi Satoh conceived the study. Saeko Morino and Hiroshi Satoh contributed to the study design. Saeko Morino implemented the EIA measurement. Saeko Morino and Hiroshi Satoh analyzed the data. Saeko Morino, Hiroshi Satoh, and Motoi Suzuki interpreted the data and contributed to the discussion. Saeko Morino wrote the first draft of the manuscript. Satoru Arai and Keiko Tanaka-Taya supervised the draft. All authors agreed with the results and conclusion of the study and approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors appreciate Yuzo Arima for reviewing the manuscript and for proving critical comments on the analysis and interpretation of data. This study was supported by a Grant-in-Aid Vaccine Demand Forecasting Project in Japan and the Japan Agency for Medical Research and Development (AMED; Grant numbers: JP21fk0108612 and JP22fk0108612).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Research data are not shared.