Real-life impact of tenofovir disoproxil fumarate and entecavir therapy on lipid profile, glucose, and uric acid in chronic hepatitis B patients
Qi Zhang, Jinlin Liang, and Junhua Yin contributed equally to this study as the first authors.
Abstract
The impact of long-term nucleos(t)ide analogs treatment on host metabolism is a concern. Hence, we conducted this study to compare the effect of entecavir (ETV) and tenofovir disoproxil fumarate (TDF) on metabolic parameters among chronic hepatitis B (CHB) patients. In this real-life retrospective study, 2030 CHB outpatients treated with ETV or TDF at Nanfang Hospital, China, were included. For treatment-naïve patients, pretreatment and semiannual metabolic parameters were collected. For treatment-experienced patients, metabolic parameters were collected at the first visit. Propensity score matching (PSM) was used to balance the effects of potential confounding factors. Among 122 treatment-naïve patients and 1908 treatment-experienced patients, ETV-treated patients were older with a higher percentage of metabolic syndrome. After PSM, the characteristics were comparable between the two groups. For treatment-naïve patients, four lipid parameters, including total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein, and triglyceride levels showed a decreasing trend during the 42-month TDF treatment, while they remained relatively stable or increased during ETV treatment. At Month 30, the levels of TC and LDL among TDF-treated patients were significantly lower than those among ETV-treated patients (TC: 4.7 mmol/L vs. 3.9 mmol/L, p = 0.004; LDL: 3.0 mmol/L vs. 2.4 mmol/L, p = 0.009). For treatment-experienced patients, we also observed lower levels of lipid parameters in patients with different durations of TDF treatment. The levels of glucose and uric acid were similar among ETV- and TDF-treated patients. TDF has a lipid-lowering effect in CHB patients, which provides a basis for the selection of antiviral drugs for aging CHB patients.
1 INTRODUCTION
Worldwide, there are an estimated 292 million people living with hepatitis B virus (HBV), putting them at risk of liver diseases such as cirrhosis, hepatic failure, and hepatocellular carcinoma (HCC), which makes chronic hepatitis B (CHB) virus infection a major global medical problem.1, 2
Metabolic disorders are the main characteristic of metabolic syndrome (MetS), which is a clustering of obesity (particularly central adiposity), raised blood pressure, dysglycemia, elevated triglyceride levels, and low high-density lipoprotein (HDL) cholesterol levels.3 The prevalence of MetS has been consistently increasing due to the global increase in the consumption of high caloric food and sedentary habits, especially in Asian populations where the standardized prevalence of MetS is as high as 31.1%.2, 4, 5 MetS is considered a risk factor for cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM). Moreover, several studies also demonstrated that MetS was associated with liver fibrosis progression and liver cancer among patients with or without CHB.3, 6, 7
The impact of long-term treatment with nucleos(t)ide analogs (NAs) on host metabolism in CHB patients has always been a concern. Several studies have shown that NAs can induce adverse effects related to lipid metabolism in patients infected with human immunodeficiency virus (HIV).8 Nevertheless, it was reported that coformulated tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) had a lipid-lowering effect not only on HIV-infected subjects but also on subjects with preexposure prophylaxis (PrEP) for HIV, which was likely attributable to TDF.9, 10 Moreover, Shaheen et al. found a greater decline in total cholesterol (TC), low-density lipoprotein (LDL) cholesterol, and HDL cholesterol levels in CHB carriers receiving TDF than in those receiving entecavir (ETV).11 In short, various NAs may exert different effects on lipid metabolism, although they have similar potent antiviral efficacy.12
However, few studies have reported the effects of NAs on other metabolic parameters such as triglyceride (TG), glucose (GLU), and uric acid (UA) levels in patients with CHB, and related study with Chinese patients are limited. Thus, we conducted this study, to compare the effects of ETV and TDF, as the recommended first-line anti-HBV drugs, on various metabolic parameters among patients with CHB.
2 METHODS
2.1. Patients
Adult outpatients with CHB receiving ETV or TDF who were seen in the Department of Infectious Disease, Nanfang Hospital, Southern Medical University, Guangdong, China, in 2017 were included in this study. All the patients were divided into treatment-naïve and treatment-experienced patients according to whether they had initiated treatment with ETV or TDF at the time of enrollment in the study. The enrolled treatment-naïve patients were those who had both a pretreatment assessment and at least one posttreatment metabolic parameter assessment. We also enrolled treatment-experienced patients who had received stable ETV or TDF therapy for at least 1 year at the time the metabolic parameters were assessed in 2017. The exclusion criteria for the treatment-naïve and treatment-experienced patients were as follows: (1) patients with HCC or other malignant tumors; (2) patients coinfected with hepatitis C virus, hepatitis D virus, or HIV; (3) patients with other liver diseases (alcoholic liver disease, autoimmune liver disease, hepatic failure, etc.); (4) patients who were pregnant; and (5) patients who were using hormones or immunosuppressants.
2.2. Data collection
Data collected included demographic information such as age and sex, date of antiviral therapy initiation, alcohol history, the use of concomitant medications, and comorbidities (i.e., cirrhosis, fatty liver, coronary artery disease, diabetes mellitus, hypertension, and hyperuricemia). For treatment-naïve patients, pretreatment and semiannual (±3 months) fasting metabolic parameters including TG (mmol/L), TC (mmol/L), LDL (mmol/L), HDL (mmol/L), GLU (mmol/L), and UA (μmol/L) levels, were collected, and the data for the analysis were as of January 2021. For treatment-experienced patients, fasting metabolic parameters were collected at the first test in 2017. All data were obtained from outpatient electronic medical records.
2.3. Statistical analysis
For the clinical characteristics, continuous variables are expressed as medians with interquartile ranges and were compared by Mann–Whitney U tests, while categorical variables are expressed as numbers and percentages and were compared by the χ2 test or Fisher's exact test as appropriate. To compare the effects of ETV and TDF on metabolic parameters, 1:1 propensity score matching (PSM) was performed to balance the effects of potential confounding factors. Propensity scores were computed by logistic regression with age, sex, cirrhosis status, metabolic disease, pretreatment metabolic parameters (only for treatment-naïve patients), and months of treatment (only for treatment-experienced patients). Multivariate logistic regression was performed to identify the factors related to the lipid-lowering effect. All p-values were two-tailed, and the level of significance was set at p < 0.05. Statistical analyses were performed using Statistical Package for the Social Sciences (version 26.0) and R (version 4.1.2).
3 RESULTS
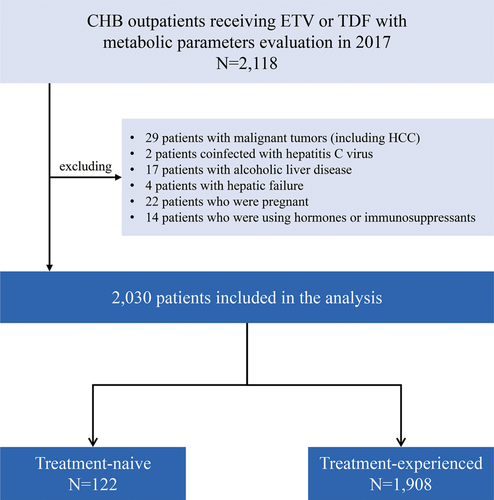
A total of 2030 CHB patients were enrolled in this study, including 122 treatment-naïve and 1908 treatment-experienced patients, after excluding 29 patients with malignant tumors, 2 patients infected with chronic hepatitis C, 17 patients with alcoholic liver disease, 4 patients with hepatic failure, 22 pregnant patients, and 14 patients who were receiving hormones or immunosuppressants (Figure 1).
3.1. Clinical characteristics of the treatment-naïve patients
Table 1 shows the clinical characteristics of the treatment-naïve patients at pretreatment. A total of 85 ETV-treated and 37 TDF-treated patients were included in the analysis. The ETV-treated patients were older than the TDF-treated patients (45.0 years vs. 33.0 years, p < 0.001), with a higher percentage of patients with MetS (49.4% vs. 27.0%, p = 0.022). The ETV-treated patients also had a higher rate of cirrhosis (37.6% vs. 10.8%, p = 0.003). There were no significant differences in metabolic parameters between the two groups, except for HDL levels (1.2 [ETV] vs. 1.4 [TDF] mmol/L, p = 0.035). After PSM, the clinical characteristics of the patients at pretreatment were well balanced between the groups (Table 1).
| Overall | Propensity-score matched | |||||
|---|---|---|---|---|---|---|
| ETV | TDF | p-value | ETV | TDF | p-value | |
| No. | 85 | 37 | - | 37 | 37 | - |
| Male, n (%) | 65 (76.5) | 27 (73.0) | 0.680 | 29 (78.4) | 27 (73.0) | 0.588 |
| Age, years | 45.0 (36.0–53.5) | 33.0 (28.0–38.5) | <0.001 | 35.0 (29.5–42.0) | 33.0 (28.0–38.5) | 0.272 |
| HBV DNA (log10IU/ml) | 5.3 (3.0–6.5) | 6.0 (4.5–7.8) | 0.040 | 5.8 (4.6–7.2) | 6.0 (4.5–7.8) | 0.770 |
| HBeAg positive, n (%) | 36 (42.4) | 25 (67.6) | 0.010 | 25 (67.6) | 25 (67.6) | 1.000 |
| Cirrhosis, n (%) | 32 (37.6) | 4 (10.8) | 0.003 | 5 (13.5) | 4 (10.8) | 1.000 |
| Metabolic syndrome, n (%) | 42 (49.4) | 10 (27.0) | 0.022 | 14 (37.8) | 10 (27.0) | 0.321 |
| TC (mmol/L) | 4.8 (4.2–5.3) | 4.9 (4.3–5.5) | 0.459 | 4.9 (4.4–5.3) | 4.9 (4.3–5.5) | 0.689 |
| LDL (mmol/L) | 3.0 (2.6–3.3) | 3.0 (2.5–3.3) | 0.660 | 2.9 (2.6–3.2) | 3.0 (2.5–3.3) | 0.570 |
| HDL (mmol/L) | 1.2 (1.0–1.5) | 1.4 (1.1–1.7) | 0.035 | 1.3 (1.1–1.6) | 1.4 (1.1–1.7) | 0.390 |
| TG (mmol/L) | 1.1 (0.9–1.4) | 0.9 (0.8–1.5) | 0.300 | 1.1 (0.8–1.6) | 0.9 (0.8–1.5) | 0.304 |
| GLU (mmol/L) | 5.1 (4.8–5.8) | 5.0 (4.8–5.5) | 0.516 | 5.1 (4.7–5.6) | 5.0 (4.8–5.5) | 0.742 |
| UA (μmol/L) | 373.0 (275.5–442.0) | 361.0 (310.5–422.0) | 0.742 | 387.0 (304.0–471.0) | 361.0 (310.5–422.0) | 0.210 |
- Note: Metabolic syndrome including diabetes mellitus, hypertension, and hyperuricemia.
- Abbreviations: ETV, entecavir; GLU, glucose; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TC, total cholesterol; TDF, tenofovir disoproxil fumarate; TG, triglyceride; UA, uric acid.
3.2. Kinetics of metabolic parameters during ETV or TDF antiviral treatment
During up to 42 months treatment of TDF treatment, the four lipid parameters, TC, LDL, HDL, and TG levels, showed a decreasing trend, while they remained relatively stable or showed an increasing trend among ETV-treated patients (Figure 2A–D). The levels of each lipid parameter were significantly or numerically lower in the TDF group than in the ETV group at each timepoint. Especially for the levels of TC and LDL, the differences between the two groups were apparent. At Month 30 of treatment, the levels of TC and LDL among the TDF-treated patients were significantly lower than those among the ETV-treated patients (TC: 4.7 mmol/L vs. 3.9 mmol/L, p = 0.004; LDL: 3.0 mmol/L vs. 2.4 mmol/L, p = 0.009). However, we observed that the levels of GLU and UA showed no obvious changes during ETV or TDF treatment (Figure 2E,F). There were similar results among the patients before PSM (Supporting Information: Figure 1).

3.3. Changes in metabolic parameter categories
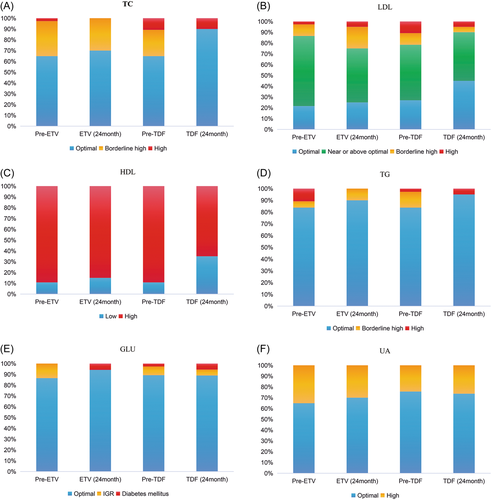
We classified the metabolic parameters into different categories by the National Cholesterol Education Program (NCEP) criteria and related clinical guidelines13, 14 (Supporting Information: Table 1) and analyzed the change in the proportion of patients with different categories before and after ETV and TDF treatment. As shown in Figure 3, the rate of borderline high and high lipid levels tended to decrease in the TDF-treated patients, while it remained stable or tended to increase in ETV-treated patients. Especially for TC levels, the rate of borderline high and high TC levels decreased from 35.1% at pretreatment to 10.0% at Year 2 in the TDF group (p = 0.050). Regarding GLU and UA levels, there were no obvious changes in the proportion of patients with different categories after 2 years of treatment with TDF or ETV.
A shift table was also used to show the percentage of patients with different changes (an increase, maintenance, or a decrease) during ETV or TDF treatment. After 2 years of treatment, the percentage of patients with a significant lipid decrease (≥ a 1-level decrease) was higher in the TDF group, while the percentage of patients with lipid increase (≥ a 1-level increase) was higher in the ETV group (Supporting Information: Figure 2).
3.4. Pretreatment factors related to the lipid-lowering effect
To further identify the association between pretreatment factors and the lipid-lowering effect, a multivariate logistic regression analysis was conducted with the inclusion of group, sex, age, HBV DNA, HBeAg, cirrhosis, MetS, and the lipid test levels at pretreatment in the model. The regression analysis showed that TDF treatment was an independent factor associated with the lipid-lowering effect (OR = 3.87, 95% CI: 1.07 −13.93, p = 0.039, Table 2).
| Univariate analysis | ||||
|---|---|---|---|---|
| OR (95% CI) | p-value | Multivariate analysis | ||
| Group (TDF vs. ETV) | 3.18 (1.01–9.97) | 0.048 | 3.87 (1.07–13.93) | 0.039 |
| Sex (male vs. female) | 3.13 (0.77–12.79) | 0.112 | - | - |
| Age (≥ vs. <35 years) | 1.67 (0.52–5.34) | 0.389 | - | - |
| HBV DNA (log10IU/ml) | 1.20 (0.85–1.68) | 0.305 | - | - |
| HBeAg positive (yes vs. no) | 1.31 (0.44–3.86) | 0.629 | - | - |
| Cirrhosis (yes vs. no) | 1.06 (0.29–3.78) | 0.935 | - | - |
| Metabolic syndrome (yes vs. no) | 0.73 (0.24–2.20) | 0.577 | - | - |
| TC (≥ vs. <5.2 mmol/L) | 3.69 (1.01–13.46) | 0.048 | 2.92 (0.69–12.42) | 0.147 |
| LDL (≥ vs. <2.6 mmol/L) | 6.33 (1.27–31.57) | 0.024 | 4.78 (0.85–26.90) | 0.076 |
| HDL (≥ vs. <1 mmol/L) | 1.24 (0.28–5.59) | 0.778 | - | - |
| TG (≥ vs. <1.7 mmol/L) | 3.88 (0.65–23.38) | 0.139 | - | - |
| GLU (≥ vs. <6.1 mmol/L) | 1.74 (0.23–13.35) | 0.596 | - | - |
| UA (> vs. ≤420 μmol/L) | 2.51 (0.70–8.98) | 0.156 | - | - |
- Note: Enter method was used for the multivariate logistic regression analysis. Lipid-lowering effect was defined as ≥ a 1-level decrease of any lipid parameters at Year 2 compared with pretreatment. Metabolic syndrome including diabetes mellitus, hypertension, and hyperuricemia.
- Abbreviations: ETV, entecavir; GLU, glucose; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TC, total cholesterol; TDF, tenofovir disoproxil fumarate; TG, triglyceride; UA, uric acid.
3.5. Clinical characteristics of the treatment-experienced patients
Table 3 shows the clinical characteristics of the treatment-experienced patients. Patients were divided into three subgroups based on the number of years of treatment (1–2 years, 2–3 years, 3–5 years). In each subgroup, the ETV-treated patients were older than the TDF-treated patients. In addition, in the 1–2 years subgroup, 67 (11.6%) and 9 (5.0%) patients had MetS in the ETV and TDF groups, respectively (p = 0.010). After PSM, the clinical characteristics of the treatment-experienced patients were well matched.
| Overall | Propensity-score matched | |||||
|---|---|---|---|---|---|---|
| ETV | TDF | p-value | ETV | TDF | p-value | |
| Years for treatment | 1–2 years | |||||
| No. | 576 | 180 | - | 180 | 180 | - |
| Male, n (%) | 471 (81.8) | 136 (75.6) | 0.067 | 140 (77.8) | 136 (75.6) | 0.618 |
| Age, years | 41.0 (35.0–49.8) | 37.0 (32.0–42.8) | <0.001 | 37.0 (32.0–42.8) | 37.0 (32.0–42.8) | 0.836 |
| Months for treatment | 18.0 (14.0–21.0) | 17.0 (14.0–20.0) | 0.130 | 17.0 (14.0–20.8) | 17.0 (14.0–20.0) | 0.618 |
| Cirrhosis, n (%) | 143 (24.8) | 17 (9.4) | <0.001 | 13 (7.2) | 17 (9.4) | 0.446 |
| Metabolic syndrome, n (%) | 67 (11.6) | 9 (5.0) | 0.010 | 7 (3.9) | 9 (5.0) | 0.609 |
| Years for treatment | 2–3 years | |||||
| No. | 476 | 86 | - | 86 | 86 | - |
| Male, n (%) | 387 (81.3) | 66 (76.7) | 0.325 | 70 (81.4) | 66 (76.7) | 0.453 |
| Age, years | 44.0 (37.0–50.0) | 38.0 (32.0–45.3) | <0.001 | 38.0 (34.0–45.0) | 38.0 (32.0–45.3) | 0.915 |
| Months for treatment | 30.0 (27.0–33.0) | 29.0 (27.0–32.0) | 0.019 | 29.0 (27.0–32.0) | 29.0 (27.0–32.0) | 0.505 |
| Cirrhosis, n (%) | 135 (28.4) | 10 (11.6) | 0.001 | 9 (10.5) | 10 (11.6) | 0.808 |
| Metabolic syndrome, n (%) | 58 (12.2) | 9 (10.5) | 0.651 | 10 (11.6) | 9 (10.5) | 0.808 |
| Years for treatment | 3–5 years | |||||
| No. | 520 | 70 | - | 70 | 70 | - |
| Male, n (%) | 424 (81.5) | 50 (71.4) | 0.046 | 51 (72.9) | 50 (71.4) | 0.850 |
| Age, years | 43.0 (37.0–51.8) | 41.0 (36.0–46.0) | 0.004 | 39.5 (35.0–46.0) | 41.0 (36.0–46.0) | 0.568 |
| Months for treatment | 46.0 (41.0–52.0) | 47.5 (40.0–52.0) | 0.814 | 47.5 (41.0–53.0) | 47.5 (40.0–52.0) | 0.376 |
| Cirrhosis, n (%) | 134 (25.8) | 5 (7.1) | 0.001 | 4 (5.7) | 5 (7.1) | 1.000 |
| Metabolic syndrome, n (%) | 63 (12.1) | 5 (7.1) | 0.221 | 6 (8.6) | 5 (7.1) | 0.753 |
- Note: Metabolic syndrome including diabetes mellitus, hypertension, and hyperuricemia.
- Abbreviations: ETV, entecavir; TDF, tenofovir disoproxil fumarate.
3.6. Comparison of metabolic parameters among treatment-experienced patients
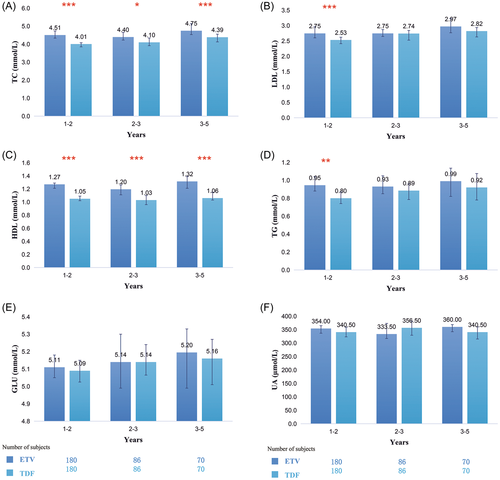
We compared the differences in each metabolic parameter between TDF- and ETV-treated patients in the three subgroups. The results showed that in the three subgroups, the levels of lipid parameters were lower in the TDF-treated patients than in the ETV-treated patients before and after PSM. Especially in the 1–2 years subgroup, significant differences were found in the four lipid parameters (TC: 4.5 mmol/L vs. 4.0 mmol/L, p < 0.001; LDL: 2.8 mmol/L vs. 2.5 mmol/L, p < 0.001; HDL: 1.3 mmol/L vs. 1.1 mmol/L, p < 0.001; TG: 1.0 mmol/L vs. 0.8 mmol/L, p = 0.002, Figure 4A–D). Although the levels of GLU were significantly lower in the TDF group before PSM, they were comparable between the two groups after PSM (Figure 4E). The levels of UA were also not significantly different between the two groups before and after PSM (Figure 4F).
4 DISCUSSION
In this real-life retrospective cohort study, we compared the impact of treatment with ETV and TDF on metabolic parameters among CHB patients. In both treatment-naïve and treatment-experienced patients, we found that the lipid parameter levels tended to decrease during TDF treatment, while they remained relatively stable or tended to increase slightly during ETV treatment. However, the levels of GLU and UA showed almost no change during TDF or ETV treatment. Our study provided more valuable information on the effect of long-term NAs treatment on various metabolic parameters for CHB patients, which is important for an aging population with a greater likelihood of comorbidities.
Although a lipid-lowering effect of TDF treatment was reported in previous studies, most of them were performed in patients with HIV.15-17 Furthermore, few studies have focused on the impact of NAs on glucose and uric acid levels. The effect of NAs on host metabolism is not entirely clear, which is one of the concerns for CHB patients due to long-term treatment duration and the increasing prevalence of MetS. Thus, we obtained more evidence on this topic using treatment-naïve and treatment-experienced CHB cohorts receiving ETV or TDF.
In the current study, among treatment-naïve patients, we found that the levels of the lipid parameters had a decreasing trend in TDF-treated patients during 42 months of treatment. Furthermore, the proportion of patients with borderline high and high lipid levels was decreased in the TDF group. Multivariate logistic regression also showed that TDF (vs. ETV) was an independent factor related to lipid-lowering effect. Moreover, numerically or significantly lower levels of lipid parameters in TDF-treated patients than in the ETV-treated patients were also demonstrated among treatment-experienced patients with various years of treatment. All the above evidence suggested a lipid-lowering effect of TDF, which was consistent with previously reported studies. Suzuki et al. found that the levels of TC, LDL, and non-HDL were significantly reduced in TDF-treated CHB patients after 6–12 months of treatment, while there was no significant change in ETV-treated patients,18 and the levels of TC, HDL, LDL, and oxidized LDL were increased significantly after switching from TDF to tenofovir alafenamide (TAF).19 Squillace et al. came to a similar conclusion that TC, HDL, and LDL levels increased in patients with HIV after switching from TDF to TAF.20 The above conclusions not only support the lipid-lowering effect of TDF but also demonstrate the opposite effect of TAF, which are both the prodrug of tenofovir, on lipid profile. In addition, among the treatment-experienced patients, the lower levels of LDL and TG in TDF-treated patients than in ETV-treated patients without statistically significant differences among the subgroups of 2–3 years and 3–5 years may be due to the small number of patients in each group, which warrants further studies with larger sample sizes. We also found that the levels of TC, LDL, and TG increased when the years of treatment were prolonged, which may be due to the increasing age or the different levels of lipid parameters at pretreatment in the three subgroups, which did not contradict the lipid-lowering effect of TDF.
Few studies have explored the mechanism of the lipid-lowering effect of TDF. TDF is postulated to have a lower direct effect on mitochondrial fatty acid metabolism.11, 21 Suzuki et al. reported that TDF modulated lipid metabolism by upregulating CD36 via PPAR-α activation. CD36 can bind LDL and long-chain fatty acids and transport them into cells, resulting in lower LDL levels.18 These findings may partly explain the lipid-lowering effect of TDF.
Except for lipid parameters, we also analyzed the effects of ETV and TDF on two other metabolic parameters and found that the levels of GLU and UA showed no obvious change during treatment and were comparable between the ETV and TDF groups. Considering the limited sample size and retrospective analysis, more studies are needed to investigate the effect of TDF on GLU and UA levels.
MetS can increase the risk of developing T2DM and cardiovascular disease.3 A high level of LDL and a low level of HDL are considered to be independent risk factors for cardiovascular disease.22 Delaney et al. observed an inverse association between TDF and common carotid intima-media thickness in patients with HIV.23 Furthermore, Chen et al. found that TDF was strongly associated with a lower risk of incident heart failure in HIV-infected patients.24 More interestingly, several recent studies indicated that TDF treatment was associated with a significantly lower risk of HCC compared with ETV treatment in CHB patients.25, 26 We hypothesized that the lipid-lowering effect of TDF may explain the favorable endpoints in terms of cancer and noncancer events. However, it is worth emphasizing that we found that both LDL and HDL levels were decreased during TDF treatment. Therefore, whether TDF reduces the risks of cardiovascular events and HCC by improving MetS is still an unsolved issue that needs further investigation.
Nonetheless, this study also had a few limitations. First, the sample size of treatment-naïve patients enrolled in this study was small because the majority of patients without a series of lipid tests during treatment were excluded from the analysis. Second, factors related to metabolic parameters such as body mass index, smoking history, exercise and diet were not available. Third, metabolic parameters may not fully characterize the complex changes in metabolism induced by antiviral therapy. Insulin resistance and the Framingham risk score, which are associated with T2DM and CVD, were not evaluated in the current study.27, 28 Fourth, the patients in this study were from an Asian population with CHB, and the effect of TDF in patients of other ethnicities (e.g., Caucasian, African) still needs further investigation.
In conclusion, TDF, as the first-line anti-HBV drug, has a lipid-lowering effect in CHB patients, which provides a basis for the selection of antiviral drugs for aging CHB patients. The impact of TDF on cardiovascular events during long-term treatment still needs further investigation.
AUTHOR CONTRIBUTIONS
Concept and design: Rong Fan. Data collection: Qi Zhang, Jinlin Liang, Yiyue Jiang, Ning Yu, Xingmei Liao, Siru Zhao, Leyuan Wu. Data analysis: Qi Zhang, Jinlin Liang. Drafting of the manuscript: Qi Zhang, Jinlin Liang. Critical revision of the manuscript: Rong Fan, Junhua Yin. Supervision: Rong Fan.
ACKNOWLEDGMENT
This study was supported by the National Natural Science Foundation of China (82170610 to Rong Fan).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.