Rock1 is a novel host dependency factor of human enterovirus A71: Implication as a drug target
Abstract
Human enterovirus A71 (EV-A71) is the major causative agent of hand-foot-and-mouth disease (HFMD) commonly associated with severe neurological diseases, particularly in children under 5 years of age. Several investigational therapeutic agents and vaccine candidates are being developed. However, no approved drug against EV-A71 infection is available, and no proven drug target has been identified. Since host kinases are key regulators of multiple signaling pathways in response to viral infections, here we screened a kinase inhibitor library and identified potent inhibitors against EV-A71 infection. Among the hits, GSK269962A, a Rho Associated Coiled-Coil Containing Protein Kinase (Rock) inhibitor with potent antiviral activity, was selected for further analysis. We found that this Rock inhibitor not only efficiently suppressed the replication of EV-A71 in RD cells, but also in human intestinal organoids, in a dose-dependent manner. Interestingly, small interfering RNA depletion of Rock1, but not Rock2, significantly restricted viral replication in RD cells, indicating that Rock1 is a novel host dependency factor for EV-A71 replication and can serve as a target for the development of anti-EV-A71 therapeutics.
1 INTRODUCTION
Human enterovirus A71 (EV-A71) is the causative agent of hand-foot-and-mouth disease (HFMD), which is a common infectious disease normally affecting children under 5 years of age.1 The majority of HFMD outbreaks have occurred in the Asia-Pacific region, including Malaysia, Vietnam, China, and India.2 Upon primary infection in the gastrointestinal tract, EV-A71 can enter the bloodstream, systematically spread, and lead to complications such as respiratory symptoms, skin rashes, and even more severe neurologic disorders.3, 4 As a neurotropic enterovirus, EV-A71 has emerged as a major public health concern over the past years.5 Although three inactivated EV-A71 vaccines have been approved and used in China since 2016, more consistent and systemic consideration of the protection afforded by EV-A71 vaccinations is required before a conclusion can be reached.6 In addition, no specific antiviral drugs are available against EV-A71 infection, thus the clinical treatment is basically supportive.7
EV-A71 is a single-stranded, positive-sense, non-enveloped RNA virus that belongs to the Piconaviridae family.8 The viral genome is approximately 7.5 kb with a single open reading frame (ORF), which is flanked by the 5′ and 3′ untranslated region (UTR).9 The ORF encodes four structure proteins (VP1, VP2, VP3, and VP4) and seven nonstructure proteins.10 Upon entry, viral RNA is released into the cytoplasm for the synthesis of positive-sense genomic viral RNA, enhancing viral protein synthesis, whereas the production of host protein is shut down as the virus hijacks the host translational machinery.11 The 5′ UTR of EV-A71 genomic viral RNA contains a cloverleaf structure involved in viral RNA replication and an internal ribosomal entry site (IRES) that directs the initiation of viral protein translation.12 A number of cellular proteins have been reported to interact with EV-A71 IRES and mediate the initiation of viral protein translation, including heterogeneous nuclear ribonucleoprotein A1 (hnRNP A1), far-upstream element-binding protein 1 (FBP1), and FBP2.13-15 In addition, protein kinases have been implicated in EV-A71 virogenesis. For example, Misshapen NIKs-related kinase (MINK) has been identified as an IRES-mediated protein involved in EV-A71 viral replication.16 Protein kinases exert influence on the activity, localization, and function of downstream effectors via reversible phosphorylation of their target proteins and thus play important roles in almost all cellular processes, including proliferation, survival, motility, metabolism, angiogenesis, and evasion of antitumor immune responses.17, 18 Apart from the multiple functions of protein kinases in cellular processes, many viruses are highly dependent on cellular kinases for viral replication. For example, serine-arginine protein kinase 1 is essential for Ebola virus transcription through regulating the phosphorylation of VP30.19 Similarly, protein kinase C βII is a specific regulator of late endosome function required for influenza virus entry and infection.20 As such, we sought to identify cellular kinases required for EV-A71 viral growth.
Rhabdomyosarcoma (RD) cells are widely used to study EV-A71 and its interaction with host cells and to identify host-dependent factors.21, 22 However, they cannot fully recapitulate the cellular diversity or mimic complicated functions in the human body.23 In addition, these cells no longer retain epigenetic and genetic features after long-term culture, limiting direct comparison with in vivo study.24 Thus, concerns have been raised about the clinical relevance of findings obtained by the use of selected drugs discovered from this platform.25 In recent years, adult stem cell-derived three-dimensional (3D) human intestinal organoids that comprise multiple intestinal epithelium cell types (enterocyte, goblet cell, enteroendocrine cell, and Paneth cell) have been established.26 These human intestinal organoids can stimulate the morphological and functional complexity of human intestinal epithelium, and thus have become the most popular model for studying enteric infections in the past few years.27-29 Moreover, human intestinal organoids can be grown in 2D monolayer cultures that recapitulate most of the features of 3D-cultured organoids, making them more favorable to study host-pathogen interactions.30
As aforementioned, no antivirals have been identified against EV-A71 infection. Notably, great progress has been made in the development of protein kinase inhibitors in past years, with 52 approved kinase inhibitors and over 200 kinase inhibitors in different stages of clinical trials, targeting various cancers or other inflammatory diseases treatment.31 Drug repurposing of these kinase inhibitors may shorten drug development time and reduce risks to safety and pharmacokinetic properties, thereby increasing the likelihood of success. In this study, we combined 2D human intestinal organoids and RD cells screening to identify targets against EV-A71 infection using a kinase inhibitor library with a total of 765 kinase inhibitors. Several inhibitors were then identified against EV-A71 infection in the initial screening and further validated via viral reduction assays. Finally, we found that a Rock inhibitor, GSK269962A, efficiently suppressed the replication of EV-A71 in both RD cells and human intestinal organoids. Specifically, we identified Rock 1 to be a novel host dependency factor of EV-A71.
2 MATERIALS AND METHODS
2.1 Cell culture and virus propagation
RD cells (ATCC, CCL-136) were cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco) supplemented with 10% fetal bovine serum (FBS) (Gibco) and 100 units/ml penicillin and streptomycin at 37°C with 5% CO2. Clinical isolate of EV-A71 (GenBank accession number DQ341368.1) was propagated and titrated in RD cells with TCID50 assay as described elsewhere.32 All experiments with live viruses were conducted in biosafety level 2 laboratories upon institutional approval.
2.2 Establishment, maintenance, and differentiation of two-dimensional (2D) and three-dimensional (3D) human intestinal organoids
After ethics approval by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (UW13-364), human intestinal organoids were previously established using the human small intestinal tissues from patients who underwent surgical resection.27 The 3D human intestinal organoids were maintained in the expansion medium (Table S1) and passaged every week. To induce 3D human intestinal organoids differentiation, 4 days after passage, the expansion medium was replaced with a differentiation medium (Table S1), in which the organoids were incubated for 5 days. Photomicrographs of the organoids were acquired using Nikon Eclipse TS100 Inverted Routine Microscope.
The establishment of the differentiated 2D human intestinal organoids was performed as described previously.28 In brief, 7 days after passage, 3D human intestinal organoids were dissociated into single-cell with TrypLE, seeded onto the 96-well plates, which were coated with 1 mg/ml of collagen IV diluted 1:30 in cold H2O and incubated at 37°C for 90 min. After 24 h, the expansion medium was switched to differentiation medium to induce the differentiation for 3 days and then refreshing the culture with differentiation medium every other day until use.
2.3 Z'-factor calculation
(σ = standard deviation, µ = mean, p = positive control, n = negative control).
According to Z'-factor value, the screening platform can be discerned. A Z'-factor less than 0 means that signal from positive and negative control overlap, making the assay not very useful or screening purposes. In contrast, Z'-factor of 1 represents zero standard deviation and mean values of negative and positive control can be of any values; such value is an ideal hit. The Z'-factor between 1 and 0.5 suggests separation band is large and worth investigating further. The Z-factor between 0.5 and 0 indicates separation band is small.
2.4 Kinase inhibitor library screen
The kinase inhibitor library containing a total of 765 kinase inhibitors, mainly targeting human protein kinases, lipid kinases, and carbohydrate kinases, was obtained from MedChemExpress (HY-LD-000001298) for pharmacological screening with antiviral activity. Pirodavir (HY-13784) was also purchased from MedChemExpress.
The primary screening was based on cytopathic effect inhibition assay to identify inhibitors that could protect cells from viral infection. In brief, RD cells and 2D human intestinal organoids were inoculated with EV-A71 at a multiplicity of infection (MOI) of 0.01 and 1.25, respectively. After 2h incubation at 37°C, the inoculum was removed, followed by the addition of dimethyl sulfoxide (DMSO) or indicated kinase inhibitor-containing medium (10 μM). At 48 h postinfection, viability of DMSO and inhibitors treatment cells or 2D organoids was determined using the CellTiter-Glo® luminescent cell viability kit (Promega).
Secondary screening was performed with viral load reduction assay as described previously.33 In brief, differentiated 3D human intestinal organoids were sheared mechanically and incubated with EV-A71 at an MOI of 0.01 for 2 h at 37°C. After the inoculum was removed, the 3D human intestinal organoids were rinsed with phosphate-buffered saline (PBS), re-embedded in Matrigel and maintained in the differentiation medium with DMSO or indicated kinase inhibitor (10 μM). At 48 h postinfection, cell lysates and cell-free culture media were collected and applied for viral gene copy quantification by quantitative reverse-transcription polymerase chain reaction (RT-qPCR) assay.
2.5 Small interfering RNA (siRNA) knockdown
ON-TARGETplus siRNA of human Rock1, Rock2, and control were obtained from Dharmacon Research. Transfection of indicated siRNA on RD cells was performed using Lipofectamine RNAiMAX Transfection Reagent (Thermo Fisher Scientific), following the manufacturer's manual. In brief, RD cells were transfected with 50 nM specific siRNA for two consecutive days. At Day 3 after siRNA transfection, the cells were inoculated with EV-A71 at an MOI of 0.01 for 1 h at 37°C. At 24 h postinfection, cell lysates and supernatant were collected for the following experiment.
2.6 Quantification of cellular messenger RNA (mRNA) transcript and viral load by RT-qPCR assay
Detection of cellular mRNA expression and viral load was performed as described previously.34, 35 In brief, cell lysates and supernatant were applied to RNA extraction, followed by reverse transcription using oligo(dT) or the virus-specific primer. The resultant cDNAs were used for qPCR assay to measure mRNA expression level of cellular gene and viral load. The primer sequences used in qPCR assay were shown in Table S2.
2.7 Virus titration
The viral titer in the indicated samples was determined by a TCID50 assay. In brief, serially diluted samples from 10−1 to 10−8 in DMEM were inoculated to RD cells in 96-well plates, and the cells were incubated for 2 days at 37°C. TCID50 were calculated by counting the wells with cytopathic effect in infected RD cells using the Reed–Muench method.36
2.8 Immunofluorescence staining
Immunostaining analysis was performed according to the standard protocol as described elsewhere.37 In brief, RD cells seeded on glass coverslips and 3D human intestinal organoids were inoculated with EV-A71 at an MOI of 0.1. At 24 h postinfection, the infected or mock-infected cells were fixed with 4% paraformaldehyde and labeled with house-made mouse anti-VP1 antibody, followed with FITC-conjugated goat anti-mouse IgG (Life Technologies). For organoid imaging, nuclei and actin filaments were counterstained with 4′-6-diamino-2-phenylindole (DAPI) (Thermo Fisher Scientific) and Phalloidin-647 (Sigma Aldrich), respectively. Slides were mounted with ProLong Gold antifade reagent (Themo Fisher Scientific) and imaged with a Carl Zeiss LSM 800 confocal microscope.
2.9 Western blot analysis
The whole-cell extracts of specific siRNA transfected RD cells were separated in SDS-PAGE and transferred onto a nitrocellulose membrane. After blocking, the membranes were probed with antibodies against Rock1 (Abcam, ab134181), Rock2 (Abcam, ab125025), or β-Actin antibody (Sigma, A5441), followed by corresponding secondary staining. The blots were visualized by Luminata Classico Western HRP Substrate (Millipore, WBLUC0500) and imaged with an Alliance Q9 Mini Analyzer (UVITEC).
2.10 Statistics
Unpaired t test was performed for data analysis using GraphPad Prism 6. A p value < 0.05 was considered to be statistically significant. Data are presented as the mean and standard deviation (SD) of representative experiments.
3 RESULTS
3.1 Development of a screening assay for EV-A71 replication in RD cells and 2D human intestinal organoids
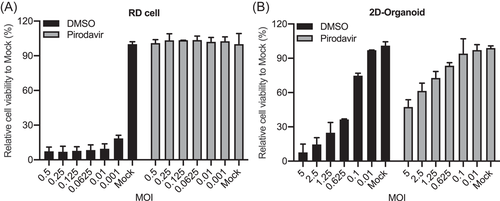
We selected a cytopathic effect (CPE) inhibition assay to screen potential host kinases involved in EV-A71 replication. First, we had to determine infection conditions, specifically MOI for inoculation, with which the impact of any virus-inhibitory agent could be maximally exemplified. To do this, Pirodavir, a broad-spectrum antipicornavirus agent targeting viral capsid protein VP1, was used as a control. In addition, the Z'-factor, a standard measurement of robustness of high throughput assays, was determined to verify the reproducibility and reliability of infection conditions. As shown in Figure 1, the correlation between MOI and cell viability were tested in DMSO-treated RD cells and 2D human intestinal organoids as negative control, in comparison with those treated by Pirodavir as positive control. The Z'-factor of RD cells and 2D human intestinal organoids were 0.748 at an MOI of 0.01 and 0.609 at a MOI of 1.25, respectively, indicating that the assay quality was high enough for the antiviral screening.
3.2 Kinase library screening and validation of kinase inhibitors against EV-A71 replication
A kinase inhibitor library containing a total of 765 kinase inhibitors was utilized in the primary screen in RD cells and 2D human intestinal organoids to identify positive hits that could protect cells from EV-A71-induced CPE. As shown in Figure 2A, 29 kinase inhibitors displayed significant cell protection in both EV-A71-infected RD cells and 2D human intestinal organoids with a cut-off of over 60% cell viability. The identified kinase inhibitors were shown in Table S3.
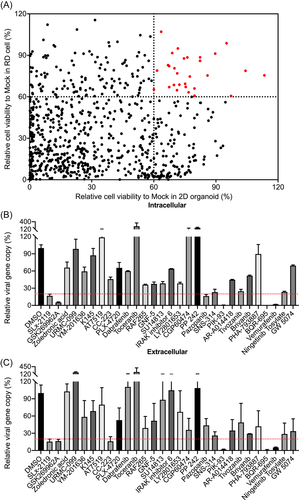
To verify that these identified kinase inhibitors indeed suppressed EV-A71 replication, we performed a second-round screen using the viral reduction assay in 3D human intestinal organoids. Among these identified kinase inhibitors, 5 hit inhibitors (SLx-2119, GSK269962A, PIK-93, BQR-695, and Vemurafenib) suppressed EV-A71 replication in both cell lysate (Figure 2B) and supernatant (Figure 2C), with a cut-off over 80% viral inhibition. The 5 specific inhibitors targeted 3 cellular genes, SLx-2119 and GSK269962A for Rock, PIK-93 and BQR-695 for PI4K, and Vemurafenib for Raf. Of the 3 genes, phosphatidylinositol 4-kinase IIIβ (PI4KB) was previously demonstrated to be critical for enterovirus genome RNA replication.21, 38 Likewise, Raf was required for the replication of EV-A71 through mediating the activation of the Raf/MEK/ERKs signaling cascade.39 Thus, one of Rock inhibitors, GSK269962A, but not SLx-2119, was selected for further study owing to its superior antiviral activity.
3.3 Rock inhibitor GSK269962A suppresses the EV-A71 replication in RD cells and 3D human intestinal organoids
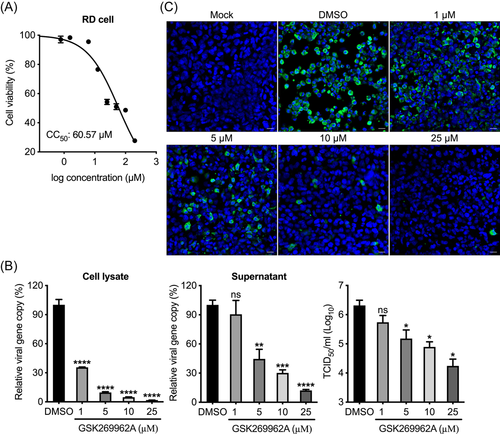
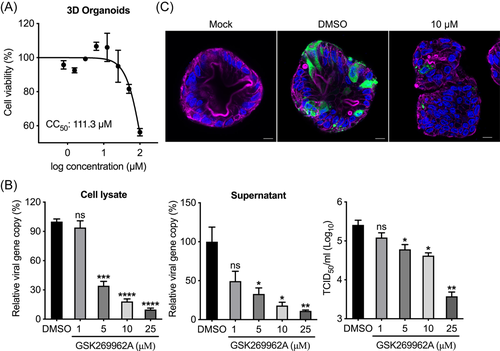
We evaluated the antiviral effect of the selected Rock inhibitor GSK269962A in RD cells. First, we measured the 50% cytotoxic concentration (CC50) of GSK269962A in RD cells to exclude the confounding effect of cytotoxicity on viral replication (Figure 3A). We then used various noncytotoxic concentrations of GSK269962A to test antiviral potency. As shown in Figure 3B, THE viral load and viral titer of EV-A71 were significantly reduced in a dose-dependent manner by GSK269962A in cell lysates and supernatant, respectively. Moreover, GSK269962A greatly reduced EV-A71 antigen expression in a dose-dependent manner (Figure 3C). Accordingly, GSK269962A suppressed EV-A71-induced CPE in RD cells in a dose-dependent manner (Figure S1A).
We further examined the antiviral effect of GSK269962A in 3D human intestinal organoids. Interestingly, the CC50 of GSK269962A in 3D human intestinal organoids turned out to be much higher than that in RD cells, suggesting that the tolerance of cytotoxicity is related to cellular diversity and complex structure (Figure 4A). As expected, efficient antiviral effect by GSK269962A was reproduced in the 3D human intestinal organoids. As shown in Figure 4B, THE viral load and viral titer of EV-A71 were also significantly reduced in a dose-dependent manner by GSK269962A in cell lysates and cell-free media, respectively. Meanwhile, GSK269962A dose-dependent inhibition of EV-A71 induced CPE was demonstrated in 3D human intestinal organoids (Figure S1B). In addition, EV-A71 antigen expression was drastically decreased in GSK269962A treated 3D human intestinal organoids, in comparison with those mock-treated by DMSO (Figure 4C). Overall, these experiments confirmed that the Rock inhibitor GSK269962A efficiently suppressed the replication of EV-A71 in both RD cells and 3D human intestinal organoids, suggesting it might be a good candidate for repurposing as therapeutic for EV-A71.
3.4 Rock1, but not Rock2, is required for EV-A71 replication
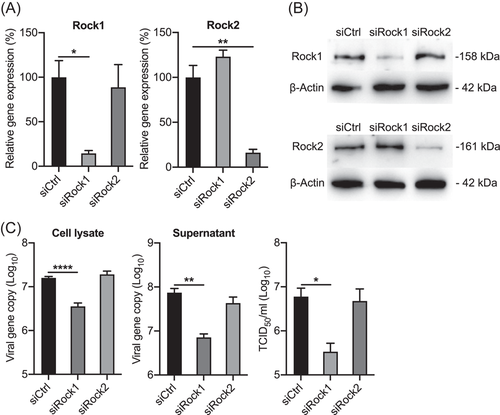
Two isoforms of Rock, Rock1 and Rock2, have been identified as serine/threonine kinases activated by RhoA GTPases.40 However, the functional gene involved in EV-A71 replication is not defined as the Rock inhibitor, GSK269962A, targets both Rock1 and Rock2. Therefore, to dissect the role of Rock1 and Rock2 in EV-A71 replication, we depleted Rock1 or Rock2 by siRNA knockdown in RD cells and examined the effect on EV-A71 replication. The effective depletion of Rock1 and Rock2 were shown by RT-qPCR assay at 48 h post siRNA transfection, with the mRNA expression levels of both genes were reduced to 20-30% relative to those in the control cells (Figure 5A). The reduced protein expression levels of Rock1 and Rock2 were also verified by Western blot (Figure 5B). Subsequently, we infected the transfected cells with EV-A71, and harvested the cell lysates and supernatant at 24 h postinfection. As shown in Figure 5C, a significant viral reduction was observed in the Rock1 depleted cells, rather than the cells with depleted Rock2, as shown by significantly reduced viral loads in cell lysate and culture medium. Viral titers in the culture medium further verified that depletion of Rock1 significantly decreased viral replication. To further verify whether Rock1 is involved in the replication of other enteroviruses, we compared infections by enteroviruses from species A to D, including human rhinovirus, coxsackievirus B2, poliovirus, and enterovirus D68 in Rock1-depleted RD cells. However, the results showed that apart from EV-A71, the replication of these viruses was not affected by Rock1 depletion (data not shown). Therefore, Rock1 may be an essential host protease for EV-A71 replication.
4 DISCUSSION
Since the first clinical case was identified in the United States in 1969, EV-A71 has been a common cause of HFMD disease in infants and young children.41 To date, there remains no approved target available for the treatment of EV-A71 infections. Host kinases are key regulators of multiple signaling pathways in response to a wide variety of stimuli, including viral infections. Leong et al. have found that Misshapen NIKs-related kinase (MINK), an IRES-mediated protein, is involved in EV-A71 replication, suggesting that a family of host kinases may play roles in facilitating EV-A71 infection and may serve as a target for the development of antiviral agents against EV-A71 infection.16 Notably, several protein kinase inhibitors, such as entrectinib, erdafitinib, pexidartinib, and fedratinib, have been approved for clinical use to treat cancers, suggesting the potential of repurposing these drugs as therapeutics against EV-A71 infection.31
Traditionally, immortalized cell lines are widely used as screening platform in the development of antiviral drugs.21, 22 However, drugs selected by this platform may not be appropriate for the in vivo study as cell lines cannot fully recapitulate the cellular diversity and complex functions of human physiology and disease pathology.23 Human enteroviruses are mainly transmitted via the fecal-oral route and the intestinal epithelium is the primary target of viral invasion. Besides, it is still unclear how EV-A71 occasionally involves the central nervous system (CNS) and induces diverse neurological complications. Notably, human adult stem cell-derived intestinal organoids that exhibit a similar cellular composition and many functional, region-specific aspects of the human gastrointestinal epithelium have been successfully established.26, 28 In this study, we aimed to identify potential crucial host kinases involved in EV-A71 replication by screening the kinase inhibitor library using the RD cells and 2D human intestinal organoids, a more physiologically relevant model.
First, we developed a sufficiently robust and reliable screening platform for identifying targets involved in EV-A71 replication using the RD cells and 2D human intestinal organoids (Figure 1). From the screen, we successfully identified several kinase inhibitors that displayed potent inhibition of EV-A71 infection at non-cytotoxic concentrations (Figure 2). While some of these kinase inhibitors may serve as lead molecules for future antiviral design, others can be useful tools in aiding our understanding of host-pathogen interactions for EV-A71 or picornaviruses in general. For example, PI4KB has been previously demonstrated to be critical for enterovirus genome RNA replication.21, 38
Furthermore, we demonstrated that the selected Rock inhibitor, GSK269962A, efficiently suppressed the replication of EV-A71 in RD cells and 3D human intestinal organoids in a dose-dependent manner by viral load detection and verified by viral titration (Figures 3 and 4). GSK269962A has been characterized as a potent Rock inhibitor by competitively binding to the Rock ATP pocket, with IC50 of 1.6 and 4 nM for recombinant human Rcok1 and Rock2, respectively.42 It is generally believed that Rocks are modulators of processes involving cytoskeletal rearrangement such as focal adhesion formation, membrane ruffling, and cell motility.43 Although the two Rock isoforms are very similar based on the major common activators and substrates and the high sequence homology in kinase domain, growing evidence suggests that individual knockdown of Rock1 and Rock2 have nonredundant functions in in vitro and in vivo study.44, 45 We thus moved on to resolve the specific effect of the two cytosolic isoforms on viral growth via genetic depletion. Interestingly, siRNA depletion of Rock1, but not Rock2, significantly reduced EV-A71 replication in both cell lysates and supernatant, indicating that Rock1 is a potential host factor involved in EV-A71 replication (Figure 5). Notably, previous studies have shown that Rock1, which mainly localizes to the nucleolus, is translocated to the nucleus during human cytomegalovirus infection.46 The results suggest that antiviral activity of Rock1 in EV-A71 infection may be associated with activation of the actomyosin network and inhibition of capsid egress out of the nucleus.
Besides, there are some limitations in our experiments. On the one hand, two Rock inhibitors have so far been approved for clinical use in Japan (fasudil and ripasudil) and the fasudil in China, suggesting that Rock inhibitors have potential therapeutic applicability in a wide variety of pathological conditions, but safety of GSK269962A is not clear. Future studies should evaluate the safety profile of GSK269962A by side-by-side comparison in in vitro and in vivo studies. On the other hand, the mechanism of action of this Rock inhibitor/kinase in restricting EV-A71 replication in RD and 3D organoids is still elusive, and future study is warranted.
In summary, human intestinal organoids may provide a useful new model of the human intestinal epithelium to study host-pathogen interactions. By combining human intestinal organoids and RD cells to screen the kinase inhibitor library, we found that several kinase inhibitors exhibited potent antiviral activity. Further experiments demonstrated that selected Rock inhibitor, GSK269962A, efficiently suppressed the replication of EV-A71 in both RD cells and 3D human intestinal organoids in a dose-dependent manner. Meanwhile, siRNA depletion of Rock1, but not Rock2, significantly reduced EV-A71 replication. This led us to believe that Rock1 is a host dependency factor potentially involved in regulating EV-A71 replication. The knowledge obtained from this study will provide a scientific basis for identification of host factor-based targets for development of prophylactics and therapeutics against enterovirus infection.
AUTHOR CONTRIBUTIONS
Jie Zhou and Pengfei Wang designed and supervised the study. Xiaoyu Zhao, Cun Li, Man Chun Chiu, and Rui Qiao performed the experiments. Xiaoyu Zhao, Shibo Jiang, Pengfei Wang, and Jie Zhou analyzed the data and wrote the manuscript. All the authors reviewed, commented, and approved the manuscript.
ACKNOWLEDGMENTS
We thank the Center of PanorOmic Sciences and Electron Microscope Unit, Li Ka Shing Faculty of Medicine, University of Hong Kong, for assistance in confocal imaging flow cytometry, and electron microscopy. This study was partly supported by funding from Health and Medical Research Fund (HMRF, 17161272 and 19180392), commissioned programme for control of infectious diseases CID-HKU1-of the Food and Health Bureau of the HKSAR government to J.Z.; General Research Fund (GRF, 17105420) and Collaborative Research Fund (CRF, C7042-21G) of the Research Grants Council and Health@InnoHK, Innovation and Technology Commission, of HKSAR government to J.Z. This study was also sponsored by Shanghai Rising-Star Program (22QA1408800) to P.W.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.