Re-emerging human monkeypox: A major public-health debacle
Abstract
A multicountry outbreak of the monkeypox virus has gained global attention. As of May 25, 250 confirmed human monkeypox cases have been reported globally. Monkeypox is caused by the Monkeypox virus, which belongs to the Orthopoxvirus genus and Poxviridae family. Monkeypox is often a self-limiting infection, with symptoms lasting 2–4 weeks with the case fatality ratio around 3%–6%. Monkeypox is transmitted to humans by direct contact with an infected person or animal or contact with virus-contaminated material. Human monkeypox infections may lead to various medical complications such as fever, rash, and lymphadenopathies. Pneumonitis, encephalitis, sight-threatening keratitis, and subsequent bacterial infections are all possible complications of monkeypox. An antiviral agent developed to treat smallpox has also been approved for use in the treatment of monkeypox in the United States. Vaccines used in the smallpox eradication program also provided immunity to monkeypox. Newer vaccines have been developed, one of which has been approved for monkeypox prevention. In this study, we provide information about the recent outbreaks of human monkeypox, epidemiology, transmission pattern, possible diagnosis techniques, therapeutics, and available preventive strategies.
1 INTRODUCTION
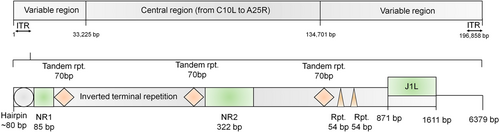
Monkeypox (MPX) is a viral zoonotic disease that is mostly prevalent in tropical rainforests in Central and West Africa, but can also be found in other parts of the world.1 Monkeypox is caused by the Monkeypox virus (MPXV), which belongs to Orthopoxvirus (OPXV) genus. Poxviruses are members of the Poxviridae family, a vast and diverse group with double-stranded DNA that replicate in the cytosol of infected cells. When seen under an electron microscope, poxviruses exhibit brick-shaped or oval structures of 200–400 nm. The size ranges of MPXV are 200 by 250 nm.2 The outer membrane protects membrane connections, as well as a tightly packed core containing enzymes, transcription factors, and a double-stranded DNA genome. The MPXV genome is made up of double-stranded linear DNA ∽197 kb, mainly composed of hairpin loops, some open reading frames (ORFs), and tandem repeats, while the inverted terminal repeats are made up of tandem repeats, hairpin loops, and some ORFs (Figure 1). Despite the fact that MPXV is a DNA virus, it spends its complete life cycle in the cytoplasm of infected cells.3 The MPXV is divided into two genetic clades: the Central African, which is the Congo Basin clade, and the West African clade. The Central African clade was assumed to be highly infectious and was known to cause more severe sickness in the past. However, Cameroon has been shown to exhibit both viral clades.4 The exploitation and control of host defenses are one of the reasons for poxvirus's broad host range and subsequent evolution. The MPXV has been found to be contagious in a variety of animal species. Rope squirrels, Gambian pouched rats, tree squirrels, nonhuman primates, dormice, and other animals fall within this category.5
Monkeypox is generally a short-term illness with symptoms lasting 2–4 weeks. The incubation time of monkeypox, or the time between infection and development of symptoms, is generally 6–13 days, but it can vary from 5 to 21 days.6 The rash appears to be focused more on the face and extremities than the trunk. The infection of MPXV can be divided into two distinct phases: the invasion period (0-5 days), which is marked by, intense headache, lymphadenopathy, fever, back pain, intense asthenia, and muscle aches, the skin eruption generally starts 1–3 days after the fever appears. The cheeks, palms of the hands, and soles of the feet are all affected by monkeypox. Oral mucosal membrane, conjunctivae, genital organs, and even the cornea, are also impacted. Secondary infections, sepsis, bronchopneumonia, encephalitis, and inflammation of the cornea with resulting visual loss are all possible outcomes of monkeypox. Monkeypox has a case fatality rate that has fluctuated from 0% to 11% in the general population, with a greater rate among small children. Their incident mortality ratio has been approximately 3%–6% in previous years.7
2 CASE DEFINITION OF HUMAN MONKEYPOX
According to the Centers for Disease Control and Prevention (CDC), the person under investigation (PUI) for MPX are individuals who are suspicious and have not been tested yet in the Laboratory Response Network (LRN). Possible cases of MPX can be explained as a person who meets one of the epidemiologic criteria and experiences fever or new rash and at least other signs and symptoms 21 days after the last exposure. The probable case of MPX is defined as a person who meets the criteria of a possible MPX case along with a new rash with or without fever. In addition, the probable case includes the presence of detectable levels of anti-orthopoxvirus IgM antibody for 4–56 days after the onset of rash. However, the confirmed orthopoxvirus case is defined as the person who meets the possible case definition and demonstrates the PCR positive for orthopoxvirus DNA or demonstrates the presence of orthopoxvirus by immunohistochemistry or electron microscopy. The confirmed monkeypox cases are defined as the person who meets the possible case definition along with the presence of monkeypox virus DNA by PCR or next-generation sequencing of clinical samples or isolation of MPXV in culture from a clinical specimen.8
3 OUTBREAKS OF MONKEYPOX
MPXV was initially discovered in monkeys held at a research center in Copenhagen, Denmark, in 1959 as an epidemic of a pox-like illness.9 Human monkeypox was discovered in a 9-year-old kid in the Democratic Republic of Congo in 1970, in a location where smallpox had already been controlled in 1968. Since then, the majority of the cases were recorded from the Congo Basin's rural, rainforest regions, mainly in the Democratic Republic of Congo, and human outbreaks were reported throughout Central and West Africa.10 MPX has been documented in 11 African nations since 1970: Cameroon, Benin, Central African Republic, Gabon, Democratic Republic of Congo, Nigeria, Cote d'Ivoire, Liberia, Sierra Leone, Republic of Congo, and South Sudan. MPX is a worldwide public health concern since it affects not only countries in Central and West Africa, but also the entire world.11 Over 70 incidences of MPX were documented in the United States as a result of this epidemic. Travelers from Nigeria to Israel in September 2018, the United Kingdom (UK) in December 2019, May 2021, and May 2022, Singapore in May 2019, and the United States in July and November 2021 have mostly been confirmed to have monkeypox. Additional outbreaks of MPX were confirmed throughout many non-endemic countries in May 2022.12
4 EPIDEMIOLOGY OF MONKEYPOX
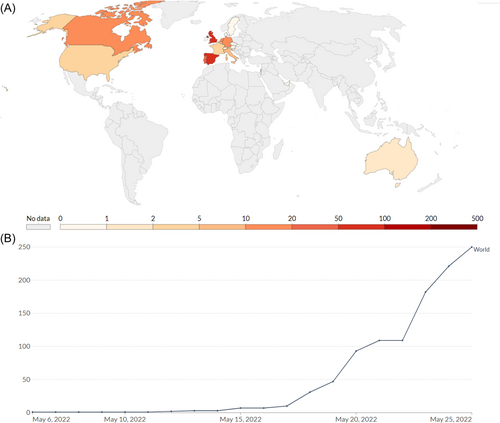
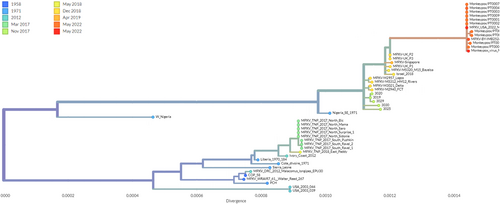
Since early May 2022, a monkeypox outbreak has been spreading across many countries. A number of nine incidents have been reported in the UK since the infection was first reported on May 7, 2022. According to the European Centre for Disease Prevention and Control, the UKHSA (UK Health Security Agency) reported a familial cluster of two cases of monkeypox in the UK on May 14, 2022.4 A record of 219 known cases has been documented from nations where the infection is not considered endemic as of May 25, 2022. Australia (2), Argentina (1 suspected case), Canada (15), the UK (71), Israel (1), Switzerland (2), the United States (9), the United Arab Emirates (1 with a travel history to West Africa), and Morocco are among the countries reporting cases outside the EU/EEA (3 suspected cases; Figure 2A,B).13, 14 These recently re-emerged strains of MPXV were analyzed based on the numbers of mutations and found to be genotypically distinct from their predecessor's strains of MPXV (Figure 3).15
5 TRANSMISSION OF MONKEYPOX VIRUS
MPXV transmission can take place in two ways: from animals to humans or from humans to humans. Inter-human transmission has been attributed to respiratory droplets and exposure to bodily fluids, as well as infected patients' surroundings and sick person's skin sores.16 Immediate contact with the blood, body fluids, or epidermal or mucosal lesions of infected animals can result in pandemic (animal-to-human) transmission. Many animals in Africa have been reported to have been infected with the MPXV, including rope squirrels, Gambian pouched rats, tree squirrels, dormice, and others. Monkeypox's natural reservoir has yet to be established, while rats are the most likely suspect.17 Close contact with respiratory secretions, skin sores of an infected person, or previous objects that are contaminated can result in human-to-human transmission. Transmission via droplet respiratory particles usually requires prolonged face-to-face contact, which puts health workers, household members, and other close contacts of active cases at greater risk.18 In addition, zoonotic transmission could occur by direct contact with the blood, body fluids, and inoculation from mucocutaneous lesions of an infected animal.19
6 DIAGNOSIS FOR MONKEYPOX
MPXV infections have various clinical signs and symptoms that are closely related to smallpox, chickenpox, measles, bacterial skin infections, scabies, medication allergies, and syphilis. Therefore, an early differential diagnosis is important to recognizing and restricting the virus spread to the community. In laboratory, MPXV can be diagnosed by using techniques like viral culture/isolation, polymerase chain reaction (PCR, real-time PCR), electron microscopy, immunohistochemistry, and serological analysis for specific antibodies (IgG and IgM based).20 Although, serology and antigen-based methods are not recommended for diagnosis due to the cross-reactivity with other Orthopoxviruses. Among the diagnostic tests, PCR is the preferred laboratory test given its accuracy and sensitivity. According to the WHO, the confirmation of MPXV infection depends on nucleic acid amplification testing (NAAT), using real-time or conventional polymerase chain reaction (PCR), for the detection of distinct sequences of viral DNA. For diagnosis of MPX, PCR can be performed alone, or in combination with sequencing.21 The primers/probes sequence for the panPox-real-time PCR has been developed which are listed in Table 1.22 In addition, some detection assays have been developed which are specific toward MPXV Congo Basin strain and MPXV West African strains along with a generic MPXV that works on broad range.23 Apart from this, recent studies have demonstrated that antiviral antibody and T-cell response increases at the time of infection, indicating that the development of new highly sensitive immunological approaches might enhance MPX detection during an epidemic.24 Changes in the Monkeypox virus MPXV-BY-IMB25241 genome sampled during May 2022 have been listed in Table 2.15
| MPXV West African specific (G2R_WA) assay23 | |
| Forward primers | 5′-CACACCGTCTCTTCCACAGA-3′ |
| Reverse primers | 5′-GATACAGGTTAATTTCCACATCG-3′ |
| Probes | 5′-FAM-AACCCGTCGTAACCAGCAATACATTT-BHQ1-3′ |
| MPXV Congo Basin specific (C3L) assay23 | |
| Forward primers | 5′-TGTCTACCTGGATACAGAAAGCAA-3′ |
| Reverse primers | 5′-GGCATCTCCGTTTAATACATTGAT-3′ |
| Probes | 5′-FAM-CCCATATATGCTAAATGTACCGGTACCGGA-BHQ1-3′ |
| MPXV generic (G2R_G) assay23 | |
| Forward primers | 5′-GGAAAATGTAAAGACAACGAATACAG-3′ |
| Reverse primers | 5′-GCTATCACATAATCTGGAAGCGTA-3′ |
| Probes | 5′-FAM-AAGCCGTAATCTATGTTGTCTATCGTGTCC-BHQ1-3' |
| panPox-real-time PCR primers/probes22 | |
| Forward primers | F1:5′-CCDCAYCARYTVGCIACIBTIGAYT-3′ |
| Reverse primers | R1:5′-GMDATIAYIGTYTTICCTGAICCCAT-3′ |
| R2:5′-GCCACGAATGTCTTACCACTTCCCAT-3′ | |
| Probes | A: 5′-FAM-WYRTGAAAYAWYADDRCDST-MGB-3′ |
| E: 5′-FAM-TYATGAAAYADYAWNRCWYT-MGB-3′ | |
| C: 5′-FAM-ATRTGRAAHARYARHACRCTYYTRT-MGB-3′ | |
| hGC: 5′-FAM-ATGTGRAASAGVARSAYRCT-MGB-3′ | |
| MPXV-BY-IMB25241 | |
|---|---|
| Collection date | 2022-05-19 |
| Host | Human |
| Outbreak Associated | Yes |
| Country | Germany |
| Region | Europe |
| Accession | ON568298 |
| Sequence changes observed (from root): | |
| Nt | Changes (391): G3108T, G3120A, G3429A, G3459A, G3531A, C3827T, A4700G, −4702C, -4703T, -4704T, -4730A, -4731T, -4732G, C7780T, C8902A, G14009T, G15437A, G18321A, C19376T, G21732A, C23573T, G25670A, G30376A, G31062A, -32218G, -32219A, -32220C, C33052T, C33152T, -33202A, -33417T, C33972T, T34014C, G34304A, G34468A, C34694T, T35868C, T36741C, G37211A, G38076A, G38369A, C38671T, C39128T, C39148T, G46904A, A48147G, C48536A, G52894A, C53216A, G54126A, G54644A, A55759G, C55814T, C58476T, T58491G, G64306A, G66589A, C70407A, C72371T, C73075T, G73248A, G74214A, G77392A, C80995T, G81284A, C82382T, G82460A, C83335T, C84596T, G87239A, G87306A, G91737A, T92525C, G95043A, G99221A, G99692A, C100571T, G104994A, T105135C, C106124T, A107241G, T107375G, T107403C, G109391A, A109589G, C119305T, C121329T, G124139A, G124683A, C124746T, C124997G, G125258A, T125523G, G126264A, G128085A, C128707T, C130855T, G131557A, C132728A, -132820T, T133550C, A133693G, C133744T, C133977T, G134895A, G135717A, A136457G, G136509A, -136566T, -136569G, G136615A, C136652T, G136698A, G136881A, G137526A, G137638A, -140116A, -140117T, -140118A, -140119A, -140120C, -140121A, -140122A, -140123T, -140124T, A140783G, G141311A, C141375T, A142348G, C142461A, T146698C, C147871T, T147937A, G148135A, A148137G, G148423A, T148553C, A149889G, C150480T, A151472C, A153221C, G154416A, G155806A, T156092C, A156173C, -156425C, -156426A, C156437T, A156459G, G156523A, -156557A, -156558G, G156965A, C156989T, T157284G, T157291C, G157448A, C157501T, -157683A, -157684T, -157685G, -157686A, -157687T, -157688A, -157689A, -157690A, -157691A, -157692A, -157693T, -157694T, -157695T, -157696T, -157697A, -157698A, -157699G, -157700C, -157701G, -158038A, -158039T, -158040A, -158177A, C158186A, G158409A, G158414A, G158522A, G158679T, T158992C, G159426A, A159458C, T159975G, T160131C, C160163T, G160878A, C161585T, A161771G, G161810A, G162254A, G162302A, C162342T, C162839T, -163202A, -163203A, -163206A, -163207A, C163478T, C164843T, C164859T, C165315T, -166102A, -166103A, -166104T, -166105A, -166106A, -166107T, -166108T, -166109A, -166110A, -166111T, -166112A, -166113A, -166114T, -166115T, G166410T, -166666T, -166667T, T167137C, G167667T, C167869T, C168131T, C168288A, -168453T, -168454A, C168599G, G169585A, G169614A, -169820A, -169821A, -169822G, G170259A, G170273A, A170294G, G170446A, -170925G, A171079G, G171686A, -172090T, G172199A, C172535T, G172991A, G173161A, -173357A, -173358T, -173359A, -173360T, -173361A, -173362T, -173363A, -173364T, -173365A, -173366T, -173367A, -173368T, -173369A, -173370T, -173371A, -173372T, -173373A, -173374T, -173375A, -173376T, -173377A, -173378T, -173379A, -173380T, -173381A, -173382T, -173383A, -173384T, -173385A, -173386T, -173387A, -173388T, -173389A, -173390T, -173391A, -173392T, -173393A, -173394T, -173395A, -173396T, -173397A, -173398T, -173399A, -173400T, -173401A, -173402T, -173403A, -173404T, -173405G, C173528T, -173585T, -173586A, A173631G, A173728G, -173827T, G173896A, C173897T, T173969C, C174257T, -174606G, -174607A, -174608T, -174609G, -174610A, -174611A, -174612G, -174613A, -174614T, -174615G, -174616A, -174617A, A175152C, A175483G, C176247T, G177160A, G177254T, G178220A, C178362T, T178372C, G178482A, G178510A, G178627A, A178974G, A179159T, T179163G, T179438C, -180484A, -180485A, -180486A, -180487A, -180488A, -180489A, -180490T, -180491T, A181091G, -181117T, -181118A, -181119T, -181120A, -181121T, -181122T, -181123C, -181124C, -181125T, -181261A, G181365A, G181995A, G182009A, A182426C, G182458A, G182506A, T182615C, C183023T, C183534T, G183710A, C183711T, G184152A, G186593A, C187169T, C187443T, T189288C, G189433A, -189754T, G189826T, G190113T, A190787C, -192501T, -192502C, -192503A, -192530A, -192531A, -192532G, T192534C, G193407A, C193703T, C193775T, C193805T, C194114T, C194126A, C194634T, C195963T |
| Reversions to root (28): T146873T, A146874A, T146875T, T146876T, T146877T, T146878T, A146879A, T146880T, A146881A, T146882T, T146883T, T146884T, T146885T, A146886A, T146887T, A146888A, T146889T, T146890T, T146891T, T150569T, G150571G, A150572A, T150573T, A150574A, T150575T, G150576G, A150577A, T150578T | |
| Gaps (3 regions, 68 bp): 173285.173293 (9 bp), 173325.173356 (32 bp), 179164.179190 (27 bp) | |
| MPXV-UK_P2-003 | Changes (1):D264N |
| MPXV-UK_P2-012 | Changes (1):A423D |
| MPXV-UK_P2-025 | Changes (1):S36F |
| MPXV-UK_P2-031 | Changes (1):R48C |
| MPXV-UK_P2-033 | Changes (4):V37M, X314R, X315H, X319* |
| MPXV-UK_P2-034 | Changes (3):X1M, X72F, X76* |
| MPXV-UK_P2-037 | Changes (1):P78S |
| MPXV-UK_P2-038 | Changes (1):G438R |
| MPXV-UK_P2-039 | Changes (1):T118A |
| MPXV-UK_P2-040 | Changes (2):E125K, A323V |
| MPXV-UK_P2-041 | Changes (1):E353K |
| MPXV-UK_P2-055 | Changes (2):L108F, W411L |
| MPXV-UK_P2-056 | Changes (1):D56N |
| MPXV-UK_P2-058 | Changes (2):V441I, F459S |
| MPXV-UK_P2-067 | Changes (1):R620Q |
| MPXV-UK_P2-072 | Changes (1):A178E |
| MPXV-UK_P2-075 | Changes (1):D196N |
| MPXV-UK_P2-076 | Changes (2):S30L, D88N |
| MPXV-UK_P2-077 | Changes (1):M142I |
| MPXV-UK_P2-081 | Changes (1):E162K |
| MPXV-UK_P2-087 | Changes (1):A12T |
| MPXV-UK_P2-088 | Changes (1):S734L |
| MPXV-UK_P2-092 | Changes (1):H740Y |
| MPXV-UK_P2-101 | Changes (2):R256K, S413N |
| MPXV-UK_P2-106 | Changes (1):A29V |
| MPXV-UK_P2-108 | Changes (2):N97H, V514I |
| MPXV-UK_P2-119 | Changes (1):D98N |
| MPXV-UK_P2-122 | Changes (1):A17T |
| MPXV-UK_P2-128 | Changes (5):E62K, R243Q, T264I, P348A, E435K |
| MPXV-UK_P2-133 | Changes (2):D100N, S307L |
| MPXV-UK_P2-137 | Changes (7):T14M, A343T, T355I, X370I, X371I, Y408H, X510* |
| MPXV-UK_P2-143 | Changes (5):X113N, -114N, -115N, X116Y, X146* |
| MPXV-UK_P2-145 | Changes (2):E67K, A88V |
| MPXV-UK_P2-147 | Changes (1):I46V |
| MPXV-UK_P2-151 | Changes (1):D31G |
| MPXV-UK_P2-153 | Changes (2):T145M, V167D |
| MPXV-UK_P2-154 | Changes (1):A30T |
| MPXV-UK_P2-155 | Changes (1):Y285C |
| MPXV-UK_P2-157 | Changes (2):N24D, H221Y |
| MPXV-UK_P2-161 | Changes (2):G14R, G66V |
| MPXV-UK_P2-162 | Changes (3):I22T, A167T, E177D |
| MPXV-UK_P2-165 | Changes (1):R506C |
| MPXV-UK_P2-167 | Changes (4):C80F, X165Y, X166F, X169* |
| MPXV-UK_P2-169 | Changes (2):R108I, L263F |
| MPXV-UK_P2-170 | Changes (4):X71V, X72I, S120C, X222* |
| MPXV-UK_P2-171 | Changes (3):X47K, X48E, X101* |
| MPXV-UK_P2-172 | Changes (2):D71N, R133Q |
| MPXV-UK_P2-173 | Changes (1):E230K |
| MPXV-UK_P2-175 | Changes (2):C99Y, A156T |
| MPXV-UK_P2-176 | Changes (2):M5I, H6Y |
| MPXV-UK_P2-177 | Changes (2):K178N, K289E |
| MPXV-UK_P2-178 | Changes (2):R169W, R473H |
| MPXV-UK_P2-179 | Changes (3):P39S, V42A, V79I |
| MPXV-UK_P2-180 | Changes (1):I53T |
| MPXV-UK_P2-181 | Changes (1):R187G |
| MPXV-UK_P2-182 | Changes (7):D209N, R363H, G379D, P722S, P781S, A928T, M1741I |
| MPXV-UK_P2-184 | Changes (1):D74N |
| MPXV-UK_P2-185 | Changes (1):R1M |
| MPXV-UK_P2-186 | Changes (1):E121D |
| MPXV-UK_P2-188 | Changes (1):D264N |
| MPXV-UK_P2-189 | Changes (1):S54F |
| MPXV-UK_P2-190 | Changes (1):S105L |
7 ANTIVIRALS FOR MONKEYPOX
Currently, no antiviral drug is licensed for the treatment of human monkeypox infection. Monkeypox clinical management should be properly adjusted to reduce symptoms, manage complications, and minimize long-term consequences. To maintain proper nutritional status, patients should be given water and food. Secondary bacterial infections should be treated according to the guidelines.25 Recently, European Medical Association licensed the Tecovirimat for the treatment of monkeypox in 2022 based on findings from animal and human data indicating the drug was safe and tolerable with only minor side effects. Tecovirimat is not generally available and is primarily designed for the treatment of smallpox. Cidofovir and Brincidofovir have proven activity against poxviruses in in vitro and animal studies. However, currently, no evidence is available to claim the effectiveness of both drugs for the treatment of human cases of monkeypox. Similarly, vaccinia immune globulin (VIG) was also tested for the treatment of monkeypox infection. However, its effectiveness against monkeypox in human subjects is still not well established.26
8 VACCINES FOR MONKEYPOX
Several observational studies have shown that smallpox vaccination is nearly 85 percent effective in preventing monkeypox. As a result, earlier vaccination against smallpox may result in a milder sickness during monkeypox infection. In 2019, a novel vaccine based on a modified attenuated Vaccinia virus (Ankara strain) was authorized for monkeypox prevention. This is a two-dose vaccine that is still in short supply. Due to the cross-protection provided by the immune response to Orthopoxviruses, smallpox and monkeypox vaccines are created in formulations based on the Vaccinia virus.25-28 In the United States, JYNNEOS™ (also known as Imvamune or Imvanex) has been approved to prevent monkeypox and smallpox. A clinical investigation of the immunogenicity of JYNNEOS™ and efficacy data from animal studies was used to determine the effectiveness of JYNNEOS™ against monkeypox. Another vaccine called ACAM2000, which includes a live Vaccinia virus, is approved for use in adults over the age of 18 who are at high risk for getting smallpox. This vaccine also provides protection against monkeypox.25-27
9 CONCLUSIONS AND FUTURE PERSPECTIVES
Monkeypox is a zoonotic disease caused by the monkeypox virus, an orthopoxvirus related to the smallpox virus. It was initially discovered in Central Africa in 1970 and has historically afflicted some of the world's poorest and most marginalized people. Fever, rash, and lymphadenopathy are all symptoms of the clinical condition. Pneumonitis, encephalitis, sight-threatening keratitis, and subsequent bacterial infections are all possible complications of monkeypox.29 The human-to-human transmission of monkeypox is well documented, including nosocomial and household spread. There are currently no approved treatments for human monkeypox; however, in the United States, two orally bioavailable medicines, Brincidofovir and Tecovirimat, have been approved. Although vaccines are available, studies have shown that smallpox vaccination is nearly 85% effective in preventing monkeypox. Monkeypox control strategies should emphasize intersectoral coordination, but not necessarily under the One Health umbrella. Future research and studies should focus on integrative techniques that combine human, animal, and environmental efforts to better understand the diverse parts of this disease system and offer appropriate solutions to preserve public health.
AUTHOR CONTRIBUTIONS
Shailendra K. Saxena conceived the idea and planned the study. Saniya Ansari, Vimal K. Maurya, Swatantra Kumar, and Shailendra K. Saxena collected the data, devised the initial draft, reviewed the final draft, and contributed equally to this study as the first author. Shailendra K. Saxena, Saniya Ansari, Vimal K. Maurya, Swatantra Kumar, Amita Jain, Janusz T. Paweska, Anil K. Tripathi, and Ahmed S. Abdel-Moneim finalized the draft for submission. All authors read and approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors are grateful to the Vice Chancellor, King George's Medical University (KGMU) Lucknow, for the encouragement for this study. ASA also acknowledges the support of Taif University Researchers Supporting Project no. TURSP-2020/11.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




