Total antioxidant capacity as a marker of severity of COVID-19 infection: Possible prognostic and therapeutic clinical application
Abstract
The pathogenesis of SARS-CoV-2 infection, causative pathogen of the known COVID-19 pandemic is not well clarified. In this regard oxidative stress is one of the topics that need to be investigated. Therefore, the present research was performed to explore the relationship between the oxidant/antioxidant system and COVID-19 exacerbation. Sera were collected from 120 patients with COVID-19 infection and 60 healthy volunteers as the control group. The patient group consisted of 60 cases with mild disease and 60 severely ill patients. Serum levels of total antioxidant capacity (TAC) and nitric oxide (NO) as well as serum activities of the two main antioxidant defense enzymes, superoxide dismutase (SOD) and catalase (CAT), were measured. TAC levels were considerably lower in patients compared with healthy individuals (p < 0.05) and also between patients with mild and severe diseases (p < 0.05). A rather decreasing trend was also found in NO concentration as well as SOD and CAT activity, though, the observed differences were not statistically significant (p > 0.05). These findings suggest that COVID-19 patients may be susceptible to depleted total antioxidant capacity. Moreover, showing such variations in blood samples of infected individuals could be considered as a predictive marker of COVID-19 severity.
1 INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of a global serious pandemic, was first identified in late 2019 in Wuhan (China).1 The clinical manifestations of COVID-19 cover a broad range from asymptomatic to critically ill patients.2 Recent studies have demonstrated that a wide variety of factors may be associated with the severity and outcomes of COVID-19 infection including genetic background, immune system defense against coronavirus, several comorbidities such as diabetes, hypertension and asthma, old age, BMI, lifestyle, and even gender discrepancies in health and risk behavior.3-6 However, the rapid spread of the coronavirus throughout the world and its high mortality rates, have made it an increasingly urgent problem that needs particular attention by researchers of any profession.
Despite growing evidence on SARS-CoV-2 pathogenesis, the puzzle is yet to be completed and used for developing therapeutic strategies. The complicated mechanisms behind COVID-19 infection include repression of host antiviral immunity, oxidative stress, and inflammation due to excessive cytokine secretion or cytokine storm which causes acute lung disease, tissue microscopic fibrosis, coagulopathy, and pneumonia.7 Oxidative stress is basically characterized by a disruption in the balance between oxidant production and antioxidant protective responses that may be induced during natural metabolic processes or pathologic conditions.8 Respiratory viral infections in general, cause the imbalance of the oxidant antioxidant system by overproduction of reactive oxygen or nitrogen species (ROS or RNS) and particularly superoxide ions.9 Moreover, they may disturb the antioxidant defense potential against SARS-CoV-2 infection by a direct effect on the antioxidant molecules and enzymes or indirect influences such as suppressing the nuclear factor (erythroid-derived 2)-like 2 (NRF2) related defense 10 or activation of nuclear factor κB (NF-κB)-toll-like receptor (TLR) signaling pathways.11
Similarly, SARS-CoV-2 binding to the angiotensin-converting enzyme-2 (ACE2) receptor helps the virus enter cells, however, leads to the subsequent reduction of the bioavailability of ACE2.12 On the other side, the newly formed product, angiotensin II (Ang II), can interact with angiotensin type 1 (AT1R) and regulate the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) thereby ROS formation (superoxide radical anion (O2•−) and hydrogen peroxide (H2O2).12 Another possible mechanism of oxidative stress during COVID infection is mediated by iron release into the bloodstream.12 Indeed, SARS-CoV-2 may attack red blood cells (RBCs), lyze them, disturb oxygenation, and cause refractory hypoxemia as well as produce free Fe (III) ions that in turn can promote Fenton and Haber–Weiss reactions to induce oxidative stress.12, 13
Any imbalance between generation and scavenging of ROS, can impair viral-specific innate immune responses, and activate compensatory responses through the redox-sensitive transcription factor NF-κB that is by far less specific against SARS-COV-II.14 Furthermore, oxidation of proteins, lipids, and DNA by excessive ROS and necrosis of virus-infected cells may produce disease-associated molecular patterns sensed by adaptive immune cells.15 Overall, diversion of antiviral innate immunity under oxidative conditions triggers pathological inflammation and lung injury by overproduction of proinflammatory cytokines and chemokines such as TNF-α, IL-6, IL-8, MCP1, and MIP1-α that unleash a cytokine storm and also exacerbate ROS production by activating NOX.16-19 Moreover, oxidative stress may cause hyporesponsiveness of T lymphocytes via oxidation of important regulatory proteins in T cells.20 In this scenario, the antioxidant defense system may be of great value to reduce COVID-19 severity.
Human antioxidants contain two main parts; nonenzymatic molecules such as glutathione, vitamins A, C, and E), and enzymatic systems like superoxide dismutase (SOD) and catalase (CAT).21 The existing body of research on SOD suggests that it is the most powerful antioxidant enzyme and plays a key role in proper respiratory function.22 SOD detoxifies two molecules of superoxide anion radicals (O2•−) by converting them to hydrogen peroxide (H2O2) and oxygen molecules (O2).23 Then, CAT uses oxygen molecules to degrade H2O2.24 Aside from scavenging ROS, both enzymes are also essential factors for natural surfactants.24 Several lines of evidence have suggested that increased blood levels of ROS or RNS might result in less antioxidant capacity so that the severity of the disease is directly related to the extent of depletion of antioxidants.21 On the other side, there are various biomarkers that evaluate the redox status of tissues by detecting oxidant markers such as nitric oxide (NO) and free radicals.21 It is now well established from a variety of studies, that altering NO levels may be associated with endothelial dysfunction, thrombotic complications, immune responses, and antiviral activity.25
The present study investigated the total antioxidant capacity, NO concentration as well as SOD and CAT enzyme activities to further analyze the oxidative stress etiology in COVID-19 patients with different clinical manifestations and elucidate the significance of antioxidant status in SARS-CoV-2 pathogenesis.
2 MATERIALS AND METHODS
2.1 Study design and participants
This study was a cross-sectional comparative study carried out in one of the centers dedicated to COVID-19 patients in Mashhad, Northeastern Iran. The study population includes 120 patients who were diagnosed with COVID-19 and 60 healthy controls. The Diagnosis and Treatment Protocol for COVID-19 Patients (Trial Version 8) was used to organize patients into mild and severe groups. This protocol has summarized China's recent clinical experience on COVID-19 disease, as well as treatment guidelines issued by the World Health Organization (WHO) and others. According to this protocol, mild patients were characterized as 60 outpatients by low fever, slight fatigue, olfactory, and gustatory disorders, and no evidence of pneumonia in imaging tests. In the same way, based on the Trial Version 8 protocol, 60 severely ill patients were intensive care unit (ICU)-hospitalized patients developing signs of respiratory distress syndrome including dyspnea, hypoxemia (oxygen saturation ≤93% at rest), Shortness of breath (RR ≥ 30 times/min), and evidence of lung injury. Further characteristic features of critically-ill patients included moderate fever, progressive lung lesions, metabolic acidosis, coagulation dysfunction, and multiple organ failure.
The exclusion criteria for all groups comprise having any comorbidity such as chronic heart, liver, and kidney dysfunction, autoimmunity, immunodeficiency, cancer, and receiving antiviral therapy. Moreover, none of the participants received antioxidant therapy such as vitamins, green tea polyphenols, carnitines, zinc, folic acid, selenium, and glutathione. Clinical characteristics including symptoms and laboratory findings of all patients were collected from electronic medical records. The poor outcome was defined as meeting at least one of the following criteria: ICU admission, being in need of mechanical ventilation, and death for any reason. The study was reviewed and approved by the research ethics committee of Mashhad University of Medical Sciences (Ref. No.: IR.MUMS.MEDICAL.REC.1399.424). All of the participants took part in this study after giving their written consent.
2.2 Laboratory tests
SARS-CoV-2 viral nucleic acid was detected in nose swabs of all positive cases by real-time polymerase chain reaction test. Upon patients admission to the hospital, blood samples were collected from SARS-CoV-2 infected subjects as well as healthy controls to measure whole blood cell count, hemoglobin, hematocrit, c-reactive protein (CRP), erythrocyte sedimentation rate (ESR), alanine transaminase (ALT), aspartate transaminase (AST), lactate dehydrogenase (LDH), blood sugar (BS), blood urea nitrogen (BUN), creatinine, serum electrolytes (potassium, sodium, and calcium), arterial blood gas (ABG), prothrombin time (PT), activated partial thromboplastin time, NO, total antioxidant capacity (TAC) as well as SOD and CAT enzyme activities.
2.3 Assays to measure oxidative stress markers
Commercial kits from Zellbio GmbH were used to analyze serum levels of TAC and NO as well as serum activities of SOD and CAT enzymes. All the tests were performed according to the manufactures' instructions. TAC was analyzed by adding a chromogen reagent to all sera samples. After reading absorbance at two different times and calculating the ∆OD (OD2 − OD1), TAC concentration was estimated based on the standard curve provided by the assay kit.
Additionally, serum activities of two antioxidant enzymes were measured: SOD and CAT. Based on the protocol, SOD changes the superoxide anions into hydrogen peroxide and oxygen. The reaction proceeds to produce a chromogenic substance measured colorimetrically at 420 nm. The enzyme activity in international U/ml was estimated according to the standard curves in the kit manual. Similarly, to calculate CAT activity two reactions should have been conducted. First, CAT converts hydrogen peroxide to water and oxygen. All samples were incubated in a certain amount of hydrogen peroxide. The reaction continues for one minute and then CAT will be quenched with a quencher. At last, the kit-provided chromogen substance and remaining hydrogen peroxide interact with each other.
In the same way, serum concentrations of NO (in μmol/ml) were measured by detecting nitrate and nitrite levels that can produce a pink azo product from a chromogenic agent. To calculate the nitrate amount, endogenous nitrite should be subtracted from the total nitrite levels according to the kit protocol.
2.4 Data analysis and statistics
All analyses were carried out using Statistical Package for the Social Sciences (SPSS) version 16.0 and Graph Pad Prism 6. Mean for variables were compared across different groups using analysis of variance, Kruskal–Wallis and independent sample t test. To find the possible relationships between variables the Pearson and Spearman's correlations were used as appropriate. Logistic regression analysis was performed to determine risk factors of a worse prognosis. Receiver operating characteristic (ROC) curve analysis was carried out to evaluate the prognostic value of serum TAC on COVID-19 patients. A p < 0.05 was assigned as statistically significant.
2.5 Ethical considerations
The whole procedure was established based on the ethical standards and codes of the institutional and national research committee and with the Helsinki Declaration on human research and its later amendments. The study was reviewed and approved by the research ethics committee of Mashhad University of Medical Sciences (Ref No.: IR.MUMS.MEDICAL.REC.1399.424).
3 RESULTS
3.1 Baseline characteristics of the study population
Totally 180 participants were enrolled in this study, 60 healthy controls and 120 patients with COVID-19 disease including 60 critically ill patients and 60 outpatients with mild symptoms of infection. As shown in Table 1, the study population consisted of 99 males (55%) and 81 females (45%) aged from 20 to 60 years. Four out of 60 severe cases died during the study process.
| Variables | Mild disease (mean ± SD) | Severe disease (mean ± SD) | Healthy controls (mean ± SD) | p value |
|---|---|---|---|---|
| Age (years) | 36 ± 8.4 | 37.8 ± 8.2 | 35 ± 8.7 | 0.18 |
| Male | 30 (50%) | 38 (63%) | 31 (52%) | - |
| Female | 30 (50%) | 22 (37%) | 29 (48%) | - |
| Fever | 26.7% | 81.7 | 0 | <0.001 |
| Cough | 13.3% | 60% | 0 | 0.01 |
| Fatigue | 45% | 55% | 0 | 0.15 |
| Myalgia | 13.3% | 23.3% | 0 | 0.10 |
| Diarrhea | 8.3% | 25% | 0 | 0.06 |
| Shortness of breath | 8% | 43.3% | 0 | <0.001 |
| WBC (/µl) | 8230 ± 840 | 11600 ± 2630 | 5920 ± 1030 | <0.001 |
| RBC (/µl) | 4.9 * 106 ± 0.3 | 4.6 * 106 ± 0.4 | 4.9 * 106 ± 0.3 | 0.71 |
| Neutrophils (%) | 68.7 ± 7 | 82 ± 6.8 | 68 ± 6.2 | <0.001 |
| Lymphocytes (%) | 30.3 ± 6.7 | 15 ± 6.9 | 30.6 ± 6.3 | <0.001 |
| NLR (%) | 2.43 ± 0.81 | 8.3 ± 7.2 | 2.36 ± 0.76 | <0.001 |
| ESR | 26 ± 6.7 | 69 ± 30 | 5.75 ± 3.1 | <0.001 |
| CRP (mg/l) | 7.7 ± 1.3 | 89 ± 40 | 2.5 ± 0.97 | <0.001 |
| Hb (g/dl) | 13.01 ± 1 | 12.7 ± 1.3 | 13.1 ± 1.4 | 0.24 |
| HCT (%) | 39.4 ± 2.8 | 38.6 ± 4.3 | 39.6 ± 4.4 | 0.31 |
| PLT (/µl) | 279 * 103 ± 37 | 210 * 103 ± 42 | 258 * 103 ± 40 | 0.05 |
| PH | 7.34 ± 0.01 | 7.37 ± 0.02 | 7.34 ± 0.01 | 0.06 |
| PO2 (mmHg) | 88.1 ± 4.5 | 59.4 ± 10 | 90.2 ± 5.5 | <0.001 |
| PCO2 (mmHg) | 40.2 ± 3 | 52.4 ± 14 | 39.5 ± 2.8 | 0.05 |
| SaO2 (%) | 93.4 ± 1.3 | 66.7 ± 14.5 | 97 ± 1.4 | 0.01 |
| Na (meq/l) | 138 ± 5 | 138 ± 4.5 | 140 ± 3.4 | 0.64 |
| K (meq/l) | 4.3 ± 0.43 | 3.9 ± 0.3 | 4 ± 0.37 | 0.53 |
| Ca (mg/dl) | 9 ± 0.5 | 8.6 ± 0.5 | 9.3 ± 0.4 | 0.01 |
| BS (mg/dl) | 85 ± 11 | 100 ± 30 | 84 ± 5.8 | 0.96 |
| BUN (mg/dl) | 31 ± 7 | 35 ± 14 | 26 ± 6.8 | 0.71 |
| Cr (mg/dl) | 0.9 ± 0.2 | 0.9 ± 0.26 | 0.85 ± 0.34 | 0.34 |
| AST (u/l) | 25 ± 8 | 35 ± 11 | 22 ± 8.3 | 0.21 |
| ALT (u/l) | 26 ± 8 | 32 ± 10 | 25 ± 8.5 | 0.62 |
| LDH (u/l) | 297 ± 53 | 500 ± 300 | 244 ± 42 | 0.05 |
| PT (second) | 13 ± 0.28 | 14 ± 1.4 | 12.8 ± 0.36 | 0.65 |
| PTT (second) | 31 ± 4 | 35 ± 9 | 30 ± 4.3 | 0.61 |
- Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; CRP, c-reactive protein; ESR, erythrocyte sedimentation rate; HCT, hematocrit; LDH, lactate dehydrogenase; NLR, neutrophil-lymphocyte ratio; PLT, platelet; PT, prothrombin time; PTT, partial thromboplastin time.
3.2 Assessing oxidative stress indicators across the study groups
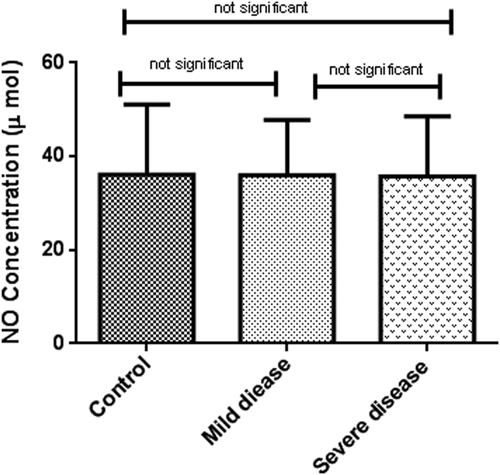
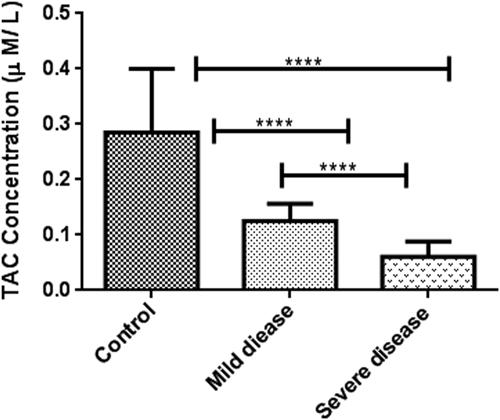
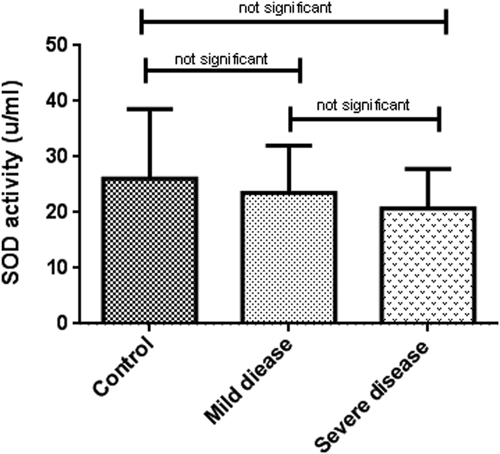
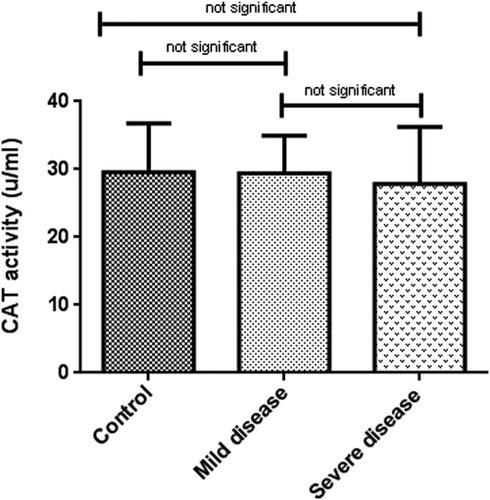
Oxidative stress markers were categorized into oxidant (NO) and antioxidant indicators (TAC, SOD, and CAT). As indicated in Table 2, TAC levels in patients with severe disease (0.06 ± 0.02, μM/L) was considerably lower than patients with mild symptoms (0.14 ± 0.03, μM/L) and healthy subjects (0.28 ± 0.11 μM/L) (p < 0.001). In the same way, a decreasing pattern in NO concentration and antioxidant enzyme activities were observed among the patient groups although it was not statistically significant (Figures 1-4).
| Marker | Healthy control | Mild disease | Severe disease | p value | |
|---|---|---|---|---|---|
| Anti-oxidants variables | TAC (μM/L) | 0.28 ± 0.11 | 0.14 ± 0.03 | 0.06 ± 0.02 | <0.001 |
| SOD (u/ml) | 26.11 ± 12.47 | 23.52 ± 8.49 | 20.75 ± 7.08 | 0.5 | |
| CAT (u/ml) | 29.60 ± 7.16 | 29.43 ± 5.53 | 27.84 ± 8.44 | 0.3 | |
| Oxidant marker | NO (μmol/ml) | 36.08 ± 15.02 | 35.95 ± 11.86 | 35.75 ± 12.77 | 0.9 |
- Note: The p value compares severe disease, healthy controls, and mild disease groups. Statistical significances (ANOVA and Kruskal–Wallis test) were only seen in the case of TAC among the three studied groups.
- Abbreviations: CAT, catalase; NO, nitric oxide; SOD, superoxide dismutase; TAC, total antioxidant capacity.
3.3 Association between serum TAC and clinical characteristics
The incidence of reduced TAC was 66% (80/120) in all the COVID-19 patients. Patients with lower TAC concentrations had higher levels of WBC, PMN, NLR, CRP, ESR, and PT while a lower amount of lymphocyte, PO2, SaO2, and calcium. Spearman/Pearson correlation analysis showed that the level of serum TAC was positively correlated with lymphocyte count, PO2, SaO2, and Ca. By contrast, negative associations were found between serum TAC and WBC, PMN, NLR, CRP, ESR, and PT levels (Table 3). In the same way, TAC levels were inversely related to the development of fever, cough, and shortness of breath in patients, although, these correlations were not statistically significant. Moreover, no significant correlation was detected for other laboratory parameters such as sodium, potassium, ALT, AST, LDH, Hb, HCT, PLT, BUN, Cr, and PTT (Table 1).
| Variable | Spearman/Pearson value | p value |
|---|---|---|
| WBC | −0.62 | <0.001 |
| PMN | −0.48 | <0.001 |
| Lymph | 0.49 | 0.001 |
| NLR | −0.36 | 0.003 |
| PO2 | 0.52 | 0.005 |
| SaO2 | 0.50 | 0.010 |
| Ca | 0.42 | 0.020 |
| CRP | −0.52 | <0.001 |
| ESR | −0.59 | <0.001 |
| PT | −0.49 | .020 |
| Fever | −0.22 | 0.09 |
| Cough | −0.07 | 0.54 |
| Shortness of breath | −0.17 | 0.18 |
3.4 Prognostic value of serum TAC for COVID-19 patients
Prognostic performance of TAC was analyzed as a marker of the severity and outcome of COVID-19 disease. Area under the ROC curve was 0.93 (95% CI: 0.89–0.97), and p < 0.001 (Figure 5). When the cutoff value of TAC was determined as 0.13 μM/L, the sensitivity was 84% and the specificity was 91%.
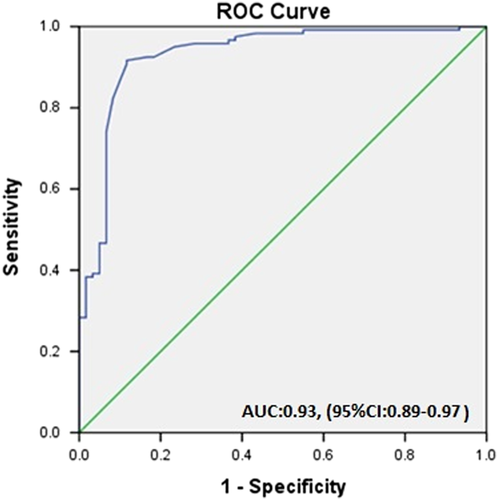
Data obtained from this study revealed that patients with poor outcomes had significantly lower levels of median serum TAC (0.06 (0.01–0.11) vs. 0.14 (0.09–0.24) for other patients (p = 0.0001).
4 DISCUSSION
The COVID-19 pandemic has altered the global normal pace of life.26 Serious health threats and remarkable morbidity and mortality rates of SARS-COV-II have inspired many researchers of various backgrounds to take quick actions to investigate possible guards against this world health problem.27 Up to now, far too little attention has been paid to the assessment of redox status in COVID-19 patients; therefore the present study investigated this phenomenon during COVID-19 infection and its connection with disease severity and ultimate mortality.
Comprehensive profiling of oxidative stress markers and redox status among COVID-19 patients is still unclear. To further illustrate the point, serum levels of TAC were measured. This study has shown that COVID-19 infected patients with reduced TAC are more prone to disease exacerbation. Afterward, serum NO concentration in addition to SOD and CAT antioxidant enzyme activities were assessed to explore the exact roles of these molecules in maintaining redox balance during SARS-COV-II infection. No significant differences were found between serum levels of NO and serum activities of SOD and CAT enzymes in COVID-19 infected patients compared with healthy individuals. It seems logical that the enzyme activities have not changed significantly yet, although a slight difference was observed because COVID-19 diseases are rapidly developing and there is not enough time to influence the enzyme activities. Furthermore, the compensatory mechanisms may keep the enzyme levels close to normal conditions.
The difference in TAC levels over various groups of this study could be attributed to high ROS production, acute inflammatory condition and infiltration of inflammatory cells into the different organs, multiorgan involvement, and declined oxygen saturation.14, 28-30 Although outpatients had normal oxygen levels, their amount of TAC was lower than healthy subjects; therefore it might be a stronger and more reliable prognostic factor. Additionally, oxygen levels may be improved following the O2 therapy for patients; in these cases, TAC could serve as a useful index to evaluate the patient's condition. Since the O2 saturation may change fast and mislead the physicians, a combination of O2 sat and TAC may indicate the patient's condition more accurately. Moreover, the patient quickly enters the severe phase of the disease, so, TAC can be used for monitoring the status of the patients. Upon treatment and correction of TAC, the patient's condition may become more stable. Furthermore, prognostic clinical applications can be considered to prevent the severe phase of the disease. Other laboratory findings in severe COVID-19 patients comprised lymphopenia, hypocalcemia, hypoxemia, and increased levels of WBC, CRP, ESR, and PT. These abnormalities are in accordance with previously published articles31 and in significant relation to TAC levels.
One source of weakness of the study might be confounder variables affecting TAC, although, major confounder factors were controlled and it seems unlikely that minor remaining confounders could alter the whole results. Several main confounders such as age, gender, and underlying background disease were considered by forming age and gender-matched groups without any background disease. Respiratory viral infections generally cause high cytokine secretion, inflammation, apoptosis, and several pathophysiological processes, which may be associated with redox imbalance.11 Numerous studies have postulated a convergence between aging, accumulative oxidative damage, and attenuated antioxidant defense system.32 Similarly, several investigations into the correlation between gender and oxidative stress have demonstrated that sex differences may be of great value in stress response; indeed male cells are often more sensitive to oxidative stress-induced cell death.33 This point justifies why men with COVID-19 are more prone to worse outcomes and death.34 In the same way, background diseases such as cardiovascular comorbidities may contribute to endothelial dysfunction, vascular injury, and NO production that might affect research findings on redox status.35, 36 Although, the patients with unmet inclusion criteria were excluded, more broadly research with larger study groups is needed to determine which components are the main factors of redox imbalance during SARS-COV-II infection.
The observed results suggest that oxidative stress may deteriorate COVID-19 disease whether it is induced by SARS-COV-II or existed before viral infection. This study program has raised important questions about the mechanisms behind redox imbalance as a leading factor in SARS-COV-II infection and severity. It also lays the groundwork for future research into the possible roles of other components of the oxidant/antioxidant system in COVID-19 exacerbation. The insights gained from this paper may be of assistance to develop diagnostic, prognostic, or therapeutic strategies for the SARS-COV-II virus.
5 CONCLUSION
A significant decrease in TAC levels was observed in patients, which could explain in part the pathogenesis of the infection and may be used for diagnostic, prognostic, and therapeutic purposes.
ACKNOWLEDGMENTS
This study was supported by a grant from Mashhad University of medical sciences (Grant No.: 990636). We thank all staff at the special COVID-19 ward for their kind assistance.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Conceptualisation, investigation, writing—original draft preparation, software, formal analysis: Neda Yaghoubi. Writing—review and editing: Masoud Youssefi. Data curation: Farahzad Jabbari Azad. Methodology, data curation, investigation, and validation: Faramarz Farzad. Data curation: Zahra Yavari. Project administration, funding, supervision: Farnaz Zahedi Avval.
Open Research
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.




