Kaposi's sarcoma-associated herpesvirus and extracellular vesicles
Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) represents the etiological agent for several human malignancies, including Kaposi's Sarcoma (KS), primary effusion lymphoma (PEL), and multicentric Castleman's disease (MCD), which develop mainly in immunocompromised patients. KSHV has established many strategies to hijack and thwart the host's immune responses, including through the use of extracellular vesicles (EVs). EVs represent a significant mode of intercellular communication as they carry a variety of molecules that can be delivered from cell-to-cell. EVs are now recognized as one of the major players in immune system development and function during both innate and adaptive immune responses. In the current mini-review, we summarize recent findings on how KSHV utilizes EVs to create favorable environments for viral spread and persistence while evading immune responses. We also discuss the limitations and unanswered questions in this field and the potential areas for related immunotherapies.
1 INTRODUCTION
Intercellular communication is a vital function of multicellular organisms. In the last two decades, extracellular vesicles (EVs) have become central to research in multiple fields as they represent a new mechanism of signal transduction. Previously, direct cell-to-cell contact and the transfer of secreted molecules were considered the only characterized mechanisms of intercellular communication. For some time, the release of EVs was thought to be used only as a way of disposing of unwanted “cellular debris.” However, EVs are now recognized as a necessary function of healthy cells. Among EVs, some vesicles are generated by budding from the plasma membrane and are referred to as microvesicles, ectosomes, shedding vesicles, or microparticles. Other vesicles are generated within the cell and once released into the extracellular matrix are termed as exosomes, and this process is analogous to virus biogenesis.1 Apoptotic bodies are also considered as EVs, which are generated through membrane blebbing and protrusion.
EVs can be released by different cell types and are enclosed by a phospholipid membrane. EVs can contain a variety of molecules, including proteins, lipids, and nucleic acids (mainly microRNAs (miRNAs), but also long noncoding RNAs (lncRNAs) and circular RNAs (circRNAs)).2, 3 The biochemical composition of EVs is different from those of their releasing cells, suggesting that the composition of EVs is actively packaged by specific molecular mechanisms,1 although there are similarities between EVs and their releasing cells. For example, EVs released by endothelial cells contain endothelial cell-specific proteins but at different concentrations and of a different type.4-6
Interestingly, the chemical characteristics of many EVs resemble these of enveloped viruses in many ways.7 Both of them have lipid membranes and carry genetic materials that can cause functional changes in recipient cells. Unlike viruses, EVs do not replicate or cause infections, although EVs from virus-infected cells affect the host's immune response during infection.7 Additionally, EVs are now recognized as one of the major players in immune system development and function during both innate and adaptive immune responses.
Kaposi's sarcoma-associated herpesvirus (KSHV) represents the etiological agent for several human malignancies, including Kaposi's sarcoma (KS), primary effusion lymphoma (PEL), and multicentric Castleman's disease (MCD).8 These KSHV-associated cancers develop mainly in immunocompromised patients, especially those infected with human immunodeficiency viruses (HIV).9 Further, the morbidity rate of KSHV-associated diseases is much higher in patients with compromised immune systems compared to those with competent immune systems. KSHV has two life stages: latency and lytic replication, so it can establish lifelong infection by using the host's machinery to replicate its episome and pass on its genetic material to daughter cells.10 If the reactivation occurs, the virus will start lytic replication, resulting in the production of new virions to infect new cells. To establish persistent infection, KSHV hijacks many aspects of the host's immune system, including using EVs as a powerful weapon.11 In this review, we will discuss how KSHV utilizes EVs to create a favorable environment for virus spread and cell transformation while evading immune surveillance. We will also highlight how KSHV manipulates the EVs signaling network to promote tumorigenesis and the potential areas for related immunotherapies.
2 EVs BIOGENESIS, RELEASE, AND UPTAKE
2.1 Biogenesis
Exosomes are formed through inward budding of the late endosomal membrane, which produces small intraluminal vesicles (ILVs).12 These late endosomal membranes are called multivesicular bodies (MVBs). The pathways that lead to the production of ILVs can be classified as endosomal sorting complex required for transport (ESCRT)-dependent and independent. ESCRT is composed of four protein complexes, ESCRT-0, -I, -II, -III, along with many other accessory proteins. The ESCRT process begins with ubiquitinated protein recognition to their specific domains of the endosomal membrane through the ubiquitin-binding subunits of ESCRT-0. Then ESCRT-I and -II will recruit ESCRT-III, which promotes the budding process, and forms the whole complex. Finally, the buds are cleaved to form ILVs, and the ESCRT-III proteins are stripped off the MVB membrane using Vps4.13 While there is some debate on whether exosome release is an ESCRT-dependent mechanism, there is a great prevalence of many ESCRT-related proteins in exosomes, with Tsg101 (an ESCRT-I component) and Alix (an accessory protein) being the most widely used markers for exosomes.12
Recent studies have found evidence in favor of an ESCRT-independent mechanism for exosome sorting. This mechanism seems to depend on raft-based microdomains that laterally segregate the endosomal membrane cargo. These microdomains are enriched with sphingomyelinases, which can give rise to ceramides through hydrolytic removal of the phosphocholine component.14 The cone-shaped structure of ceramides may cause spontaneous negative curvature of the endosomal membrane, thus promoting domain-induced budding. This ceramide-dependent mechanism emphasizes the importance of exosomal lipids in exosome biogenesis.15 Tetraspanins and other proteins also play important roles in exosome biogenesis and protein loading. Tetraspanin-enriched microdomains (TEMs) are specialized membrane platforms that organize receptors and signaling proteins in the plasma membrane.16 TEMs, along with tetraspanin CD81, have been shown to play a key role in sorting target receptors and intracellular compounds involved in exosome biogenesis and release.17
2.2 Release and uptake
Once formed, the MVBs can either follow the secretory or lysosomal pathway for exocytosis.18 In the secretory pathway, MVBs fuse with the plasma membrane then intraluminal vesicles (ILVs) are released as exosomes. The peripheral membrane of the MVBs is incorporated into the plasma membrane. In the lysosomal pathway, MVBs fuse with lysosomes and release ILVs into the lysosomal lumen for degradation. During this process, MVB trafficking is controlled by the Rab family of GTPases. RAB27A works for docking and fusing the MVBs to the cell membrane, whereas RAB27B functions in the transfer of vesicles from the Golgi to MVBs and in the transport of MVBs to the actin-rich cortex under the plasma membrane.19 Further, the mechanism by which MVBs fuse to the plasma membrane seems to be dependent on soluble NSF attachment protein receptors (SNAREs) proteins. However, this process was not fully characterized.20 Once released, EVs can bind to neighboring cells or the extracellular matrix or float freely in the bloodstream or other bodily fluids.
3 EVs AND KSHV IMMUNE EVASION
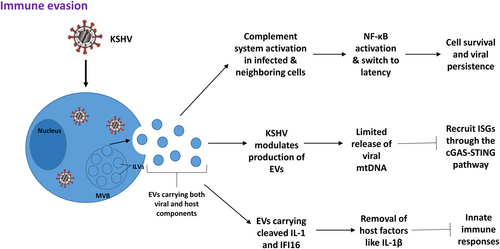
The complement system is a significant player in innate immunity that works to combat pathogens infection. The protein cascade induces inflammatory responses and opsonizes target pathogens. Complements have diverse additional functions, including modulating adaptive immunity, removing apoptotic cells, and regulating the coagulation system. The complement system also affects cell survival and tumor progression. KSHV is thought to suppress the complement system during infection using its complement inhibitor, KSHV complement control protein (KCP).21 A recent study found that EVs from KSHV-infected cells activated the complement system both in infected and neighboring cells during acute infection in human endothelial cells.22 However, activation of the complement system does not prevent KSHV infection, rather it promotes cell survival and allows for persistent viral infection through activation of the NF-κB pathway and KSHV switching to latency.
Although EVs from virus-infected cells do not cause infection and replication, they do affect the host's immune response during viral infection. Type I interferons (IFNs) and IFN-stimulated genes (ISGs) are key players used by vertebrates to control virus infection. Typically, de novo virus infection and reactivation from latency stimulates the host's antiviral immune response. However, KSHV has adapted multiple mechanisms to thwart Type I IFN response. Zhu et al.23 showed that de novo KSHV infection elicited a weak antiviral response. Interestingly, in a recent study, Jeon et al.24 demonstrated that EVs from KSHV-infected cells induced ISGs in human endothelial cells through the cGAS-STING pathway. Previous studies have shown that mitochondrial DNA (mtDNA) can trigger antiviral responses through cGAS and/or toll-like receptor 9 (TLR9). Jeon et al.24 found that KSHV-infected cells released tenfold more EVs compared to uninfected cells, which they proposed to be a mechanism to release excess mtDNA and maintain cell equilibrium. Thus, they suggest that the release of mtDNA through EVs may be the causative factor for the recruitment of ISGs and propose that KSHV can modify EV production to control early antiviral defenses.
Singh et al.25 also proposed another mechanism by which KSHV latency uses EVs to downregulate the host's innate immune response. They showed that cleaved interleukin-1 (IL-1) and interferon γ-inducible protein 16 (IFI16) were released from KSHV-infected cells within EVs. This exosomal release may be used by KSHV to remove host factors, like IL-1β, which typically trigger innate immune responses. Together, the strategies of KSHV manipulating EVs to mediate immune evasion have been summarized in Figure 1.
4 EVs AND KSHV TUMORIGENESIS
KSHV-associated malignancies, like KS and PEL, persist by establishing a favorable tumor microenvironment and reprogramming both infected cells and noninfected neighboring cells. One way that KSHV manipulates its microenvironment to favor tumorigenesis is through the use of miRNAs, which are small noncoding RNAs that influence downstream target gene expression. A previous study has revealed that viral infections cause changes in host miRNA profiles due to the expression of viral genes. KSHV is also known to produce viral miRNAs that alter host gene expression and promote viral persistence, immune evasion, and tumorigenesis.26-31 This oncogenic virus has been found to encode 12 pre-miRNAs, which are processed into 25 mature miRNAs. Chugh et al.32 characterized circulating cellular and viral miRNAs in exosomes from KS patient plasma, pleural effusions, and mouse models of KS. They found that KSHV-infected cells release EVs containing all of the viral miRNAs, and these viral miRNAs are present at high levels in EVs from KSHV + lymphoma in culture and in patients.32 Due to the presence of KSHV-encoded miRNAs in systemically circulating exosomes, they suggest that viral miRNAs can have effects far from the infected cell. They propose that these viral miRNAs could serve as highly specific biomarkers for KSHV-associated malignancies. Further, they found that hTERT-HUVECs treated with patient pleural fluid-derived exosomes led to enhanced endothelial cell migration and thus may play a role in disease progression and paracrine signaling utilized by KSHV for tumorigenesis. Interestingly, Hoshina et al. evaluated the miRNA profiles of exosomes released from KSHV- or EBV-infected cells and compared them with intracellular miRNAs.33 Next-generation sequencing revealed that specific viral miRNAs were sorted by EXOmotifs, which are nucleotide motifs that promote the loading of miRNAs into exosomes, and then these viral miRNAs accumulated in the exosomes from virus-infected cells.
To expand on this idea, McNamara et al.11 studied the effects of purified EVs from PEL on endothelial cell (EC) behavior. They found that EVs purified from PEL triggered long-term reprogramming in EC through the extracellular signal-related kinases (ERK1/2) pathway. This occurred without triggering innate sensors like interferon regulatory factor 3 (IRF3) and NF-κB, allowing KSHV to modify its immediate environment without causing innate immunity responses. KSHV-related EVs also enhanced the proliferation and migration of EC by upregulating the production of interleukin-6 (IL-6), an important pro-inflammatory cytokine.11 In addition, chronic exposure (measured at Day 10 and Day 12) to PEL-derived EV, which mimics the KS tumor microenvironment, leads to EC reprogramming through alterations in novel cellular pathways. Their findings were consistent with previous research showing KSHV miRNAs contribute to viral-induced reprogramming through silencing the cellular transcription factor MAF (musculoaponeurotic fibrosarcoma oncogene), promoting the endothelial-to-mesenchymal transition (EndMT), and inducing peroxisome biogenesis.26, 34, 35 These findings highlight the novel mechanisms KSHV can utilize to induce cellular reprogramming and promote oncogenesis.
EVs from KSHV-infected cells have also been shown to influence the behavior of B cells. Meckes et al.36 found that EVs from KSHV-infected cells carry host proteins that are involved in glycolytic metabolisms, such as lactate dehydrogenase. Thus, these enzymes can be delivered through EVs and alter the metabolism of recipient cells by promoting glycolysis.37, 38 In addition, KSHV EVs also carry proteins that are involved in epithelial adherens junction (AJ) remodeling, suggesting that they could influence cell anchorage and movement.36
Taken together, these findings (summarized in Figure 2) highlight multiple mechanisms by which KSHV can manipulate its microenvironment to promote angiogenesis and tumorigenesis using EVs. Not only can KSHV infect EC and B cells, but the virus can also influence the behavior of distant cells through the use of EVs carrying viral components.

5 CONCLUSION
The study of EVs and the role they play in viral pathogenesis is a rapidly expanding field. As an oncogenic virus, KSHV has developed multiple mechanisms using EVs to interfere with the host's immune responses and create favorable microenvironments for its pathogenesis and tumorigenesis. However, there are many challenges and unanswered questions within this field that need further investigation. For example, there is currently not a standard method for isolating and purifying EVs, and the most commonly used procedures, such as ultracentrifugation and size exclusion chromatography, do not adequately exclude viral particles from EVs.11 Refining EV isolation methods will lead to a clearer picture of how EVs are involved in viral infection, transmission, and persistence. Further, it remains unclear how some viruses, including KSHV, can manipulate EVs biogenesis and release, especially selectively “packing” viral components into EVs.
Recent studies have begun to explore the use of exosome-based vaccines. For example, Anticoli et al.39 found that intramuscular injection of exosomes engineered with the E7 protein of human papillomavirus (HPV) elicited a strong antigen-specific cytotoxic T lymphocyte (CTL) immune response. There is also evidence that exosome biogenesis and release can be controlled using selective inhibitors.40 With further understanding of the functions of EVs, inhibitors that interfere with EV production and release will be significantly facilitated as antiviral drug candidates in the future.1
ACKNOWLEDGMENTS
This study was supported by the Arkansas Bioscience Institute, the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000. Funding sources had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author on reasonable request.