COVID-19 and cancer: A clear change not only in daily clinical practice but also in clinical research management
The outbreak of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has literally engulfed the health systems of many countries around the world. Particular attention was paid to the possible relationship between cancer and coronavirus disease-19 (COVID-19) and to the fact that cancer patients are more susceptible to SARS-CoV-2 infection than the general population1-4; however, this increase ranged from 0.4%1 to 2%3 in cancer patients, again compared with the general population. The cause was hypothesized to be immunosuppression linked to cancer treatments, although the chemo-radiotherapy accounted for around 42% of patients with cancer.1 However, although the data indicate that cancer patients may have a higher risk to develop SARS-CoV-2, there are several biases in the reported data (test criteria, tests used, age, gender, and comorbidities among the population with cancer and the general population). The conclusion is that it is currently not possible to ascertain whether cancer patients are more susceptible to SARS-CoV-2 infection.5, 6 Different authors reported a drop in the number of new diagnosis of solid tumors during COVID-19 lockdown with a range from 15%7 to 17%.8 What is not reported in the literature is how the SARS-CoV-2 pandemic has affected also clinical research management. The aim of this paper is to investigate how the SARS-CoV-2 pandemic has influenced the enrollment and management of new patients at the Clinical Research Unit of our Medical Oncology Unit (with reference to oncological trials) before and after the adoption of COVID-19 lockdown in our Country.
A retrospective analysis of all new patients with solid tumors enrolled in oncological trials at our Medical Oncology Unit between January 13, 2020 and April 30, 2020 was performed. The case study has been divided into two cohorts: admitted from January 1, 2020 to March 8, 2020 (Group 1, before the adoption of COVID-19 lockdown in our Country) and admitted from March 9, 2020 to April 30, 2020 (Group 2) (during the adoption COVID-19 lockdown in our Country). We have decided to start at January 13, 2020 to have two groups balanced each other on concerning the number of working days.
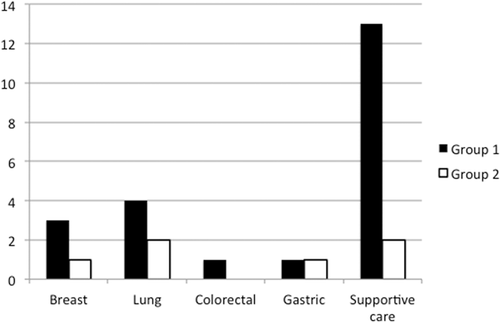
At the April 30, 2020, 29 oncological trials were active (11 regarding breast cancer, 5 colorectal cancer, 5 lung cancer, 2 gastric cancer, 2 head and neck cancer, 1 gynecological cancer, and 4 supportive care). No trials were activated during COVID-19 lockdown. Twenty-five patients belonged to Group 1 (4 (16.0%) lung, 3 (12.0%) breast, 3 (12.0%) head and neck, 1 (4.0%) colorectal, 1 (4.0%) gastric and 13 (52.0%) supportive care) and 6 patients belonged to Group 2 (2 (33.3%) lung, 1 (16.7%) breast, 1 (16.7%) gastric, and 2 (33.3%) supportive care), with an overall reduction of 76% in the new enrollment (Figure 1). No patients in Group 2 were COVID-19 positive.
Considering all active oncological treatments (not only patients in clinical trials), a reduction was evident during the adoption of COVID-19 lockdown (911 oncological treatments vs. 779 during the adoption of COVID-19 lockdown, for both infusive treatments [577 vs. 524] that orally treatments [334 vs. 255], with a reduction of 14.5% [in line with the reduction of new pathological diagnosis during COVID-19 lockdown7]).
From these data, it is evident that there was a clear reduction (−76%) in the enrollment of new patients in oncological trials during COVID-19 lockdown, more evident than the drop of new pathological diagnosis (−15%). Analyzing the data in more detail it is evident that the reduction is due to the gap in the enrollment in supportive care (13 vs. 2 patients); the others are small numbers (small variations on small numbers are more evident in percentage terms). To this, we must add that patients who are candidates for the enrollment in supportive care trials are not new diagnosis, but patients already in care. The COVID-19 lockdown has undoubtedly reduced the access of patients, especially those not in active cancer treatment (that are the candidate to be enrolled into supportive care trials). From the organizational point of view we also highlight a change in the monitoring activity of the clinical trials, that was suspended during the period of the COVID-19 lockdown and managed remotely.
In conclusion, the SARS-CoV-2 pandemic, as well as bringing about changes in daily clinical activity, has also heavily conditioned the clinical research (both in the enrollment that in the monitoring activity). Similarly to other authors,9 limiting enrollment in clinical trials to patients who are most likely to benefit from it and discussing palliative care and end-of-life for COVID-19 positive patients with advanced cancer to limit unnecessary hospital admissions and mechanical ventilation could be a good answer during a pandemic. In particular, referring to palliative care and end-of-life cancer patients it is well known that clinical and management monitoring (including dose titration in palliative sedation) should be determined by clinical situation: for patients that are imminently dying routine monitoring of vital signs should not be performed and the only critical parameters for ongoing observation are those pertaining to comfort, differently, for patients that are not imminently dying, monitoring may be undertaken to preserve physiological stability (if life-threatening obtundation with respiratory depression occurs, a lower treatment dose may be required). This kind of attitude is widely shared in the oncology field, well codify in International Guidelines10 and applied in daily clinical practice.11 So, similar to other authors,12 we believe that for palliative care and end-of-life cancer patients, whenever possible, the referral system should be to accommodate patients at freestanding hospice or home care centers, especially in this COVID-19 era, to withdraw life sustaining treatments and not life sustaining care.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.