Protective role of overexpressed MUC5AC against fibrosis in MHV-68-induced combined pulmonary fibrosis and emphysema mouse model
Abstract
Mucins have long been regarded to play a role as a barrier to prevent mucosal infections; however, some studies report that overexpression of mucins induces obstruction and inflammation of airways. We investigated whether the secretion of overexpressed mucin, mucin5ac (MUC5AC), could improve protection against pathogens. To examine the possible roles of mucin hypersecretion in augmenting host defense against disease-promoting muco-obstructive lung disease, a mouse model that overexpressed MUC5AC was generated. We had previously proved that murine gammaherpesvirus-68 (MHV-68) infection could induce emphysema in mice, which later developed into combined pulmonary fibrosis and emphysema (CPFE). We further explored whether increased MUC5AC secretion could provide benefits against MHV-68 induced fibrosis. We initially developed a pcDNA3.1-MUC5AC mouse model. Next, the experimental mice were randomly divided into five groups: normal control, pcDNA3.1 control, pcDNA3.1-MUC5AC, CPFE, and pcDNA3.1- MUC5AC + CPFE. Morphometric analysis of each group was performed by hematoxylin and eosin staining and Masson trichrome staining. MUC5AC levels in lung tissues were analyzed by immunohistochemical staining, real-time polymerase chain reaction, and Western blot analysis. The airway inflammation was determined by differential cell counts of bronchoalveolar lavage fluid (BALF) and measurement of cytokines and chemokines in BALF by enzyme-linked immunosorbent assay. MUC5AC hypersecretion alone was not sufficient to drive goblet cell metaplasia to induce obvious mucus plugging and airway inflammation. However, MUC5AC overexpression served as a protective barrier against MHV-68 virus infection in vivo. Infectivity of MHV-68 was decreased in the pcDNA3.1-MUC5AC + CPFE group compared with that in CPFE group. Meanwhile, a reduction of MHV-68 virus attenuated the expressions of chemokine (C-C motif) ligand 2 (CCL2), chemokine (C-X-C motif) ligand 5 (CXCL5), interleukin-13 (IL-13), and transforming growth factor-β1 (TGF-β1), and weakened airway inflammation and fibrosis in the pcDNA3.1-MUC5AC + CPFE group. Overexpression of MUC5AC appears to exhibit a protective role against MHV-68 infection in mice with emphysema that subsequently developed into CPFE and to further decrease airway inflammation and fibrosis induced by MHV-68 by decreasing the expressions of CCL2, CXCL5, IL-13, and TGF-β1.
Highlights
Overexpression of MUC5AC appears to further decrease airway inflammation and fibrosis induced by MHV-68 by decreasing the expressions of CCL2, CXCL5, IL-13, and TGF-β1.
Abbreviations
-
- CPFE
-
- combined pulmonary fibrosis and emphysema
-
- IL-13
-
- Interleukin-13
-
- MHV-68
-
- murine gammaherpesvirus-68
-
- MUC5AC
-
- mucins5ac
-
- TGF-β1
-
- transforming growth factor-β1
1 INTRODUCTION
Respiratory tract surfaces are exposed to billions of particles and pathogens daily. The process of mucus production and secretion is central to the normal defense of the lungs. Mucus is comprised of several components, mainly secretory mucins. The secreted airway mucins (MUC5B, mucins5ac [MUC5AC], and MUC2) form a mucous gel that interacts with inhaled particles, including pathogens. The most abundantly secreted mucins in conducting airways are MUC5B and MUC5AC.1 Insights into the differential functions of MUC5B and MUC5AC in homeostasis of airway epithelial cells come from the study of transgenic mice that lack or express MUC5B and MUC5AC. MUC5B is required for airway defense by controlling infections in the airways and maintaining immune homeostasis in the lungs.2 MUC5B−/− mice develop severely impaired mucociliary clearance (MCC) and chronic bacterial infections with obstruction to airflow and inflammation of the airways.2 In contrast, the role of MUC5AC is relatively modest. MUC5AC−/− mice fail to recruit neutrophils following acute lung injury.3 Interestingly, increased expression of MUC5AC in airway epithelial cells appears to exhibit a protective role against influenza infection but does not cause mucus metaplasia or airway obstruction.4 This is a very recent opinion, as it was previously thought to play a pathologic role similar to that of MUC5B.5-8 Postmortem and airway biopsy studies suggest a significant pathophysiologic effect of mucus hypersecretion in airway obstruction and inflammation as observed in severe forms of asthma and chronic obstructive pulmonary disease.5, 7 However, new evidence suggests that MUC5AC hypersecretion alone cannot cause mucus obstruction and/or inflammation.4 Therefore, we believe that suitably upregulated MUC5AC would have a positive effect on particular inflammatory respiratory diseases, such as emphysema and pulmonary fibrosis.
The term “combined pulmonary fibrosis and emphysema” (CPFE) is reserved for the coexistence of radiological findings of emphysema and pulmonary fibrosis, both in varying extents and locations within the lung parenchyma as well as multiplicity pathologically confirmed case. Emphysema is usually encountered in the upper lobes, which precedes fibrosis in the lower lobes; the patients are smokers, predominantly males. It is known that murine gammaherpesvirus 68 (MHV-68) infection in aging mice may result in severe pneumonitis and fibrosis.9 We have previously proved that MHV-68 infection could induce emphysema in mice, which later developed into CPFE in the infected mice.10 In this study, a mouse model that overexpresses MUC5AC was constructed to explore whether increased MUC5AC secretion could provide benefits against fibrosis induced by MHV-68.
2 METHODS
2.1 Experimental animals
Forty-six to 48-week-old-male C57BL/6 mice which were purchased from the Center of Experimental Animals, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China). All experiments were approved by the Institutional Animal Care and Use Committee of Tongji Medical College, Huazhong University of Science and Technology. These mice were randomly divided into five groups: group A (normal control group, n = 8); group B (pcDNA3.1 control group, n = 8); group C (pcDNA3.1-MUC5AC group, n = 8); group D (CPFE group, n = 8); and group E (pcDNA3.1-MUC5AC + CPFE group, n = 8). They were fed a standard rodent diet and had free access to water.
2.2 Experimental design
Mice in CPFE group were subjected to passive cigarette smoke for 1 hour twice daily for 13 months as described previously10 and were then administered intranasally with 1 × 105 plaque-forming units of MHV-68. Mice in pcDNA3.1-MUC5AC + CPFE group were intranasally administered pcDNA3.1-MUC5AC plasmids, and transfection reagent ExGen500 was injected into the lungs of each mouse 48 hours before a MHV-68 virus challenge.9, 11 Twenty-eight days after the MHV-68 virus challenge, the mice were killed. A pcDNA3.1 plasmid that did not carry any mouse genes was used as control.
2.3 Bronchoalveolar lavage
Whole lung lavage was performed for each mouse. Sterile saline (0.7 ml) at room-temperature(20℃-25℃) was infused twice into the lungs to total lung capacity and bronchoalveolar lavage fluid (BALF) was later collected. Cells present in the lavage fluid were isolated by centrifugation at 300g/min for 10 minutes. The supernatants were stored at −80°C for subsequent assessment of cytokine levels by enzyme-linked immunosorbent assay (ELISA).
2.4 Differential cell counts in BALF
A single-cell suspension (200 µL) of washed and resuspended BALF was obtained by centrifugation using a Cytospin (Hettich, Germany) at 300g/min for 10 minutes and then stained with Wright-Giemsa solution. Three hundred cells were counted in at least twenty different fields based on standard morphological criteria and staining properties at a magnification ×1000. Total leukocyte count and proportions of macrophages, lymphocytes, and neutrophils were calculated.
2.5 Histological and immunohistochemical examinations
The left lungs of the treated mice were fixed with 4% paraformaldehyde (Sigma-Aldrich) and embedded in paraffin. Five-micrometer sections were stained with hematoxylin and eosin or Masson's trichrome stain to assess histology, and periodic acid-Schiff (PAS) reagent was used to detect goblet cells. Emphysematous changes were assessed by measuring both the mean linear intercept and destructive index, as described previously.10, 12 For each animal, eight fields at a magnification of ×100 were captured randomly from the three different zones of the left lung. Collagen area of the basal membrane of the airways was analyzed in 3 to 5 bronchioles on each slide. The result was expressed as collagen-stained areas per micrometer length of basement membrane of bronchioles, as described previously.10 The number of PAS-positive cells per 100 μM basement membrane length was counted in random fields.
Cellular localization of MUC5AC-positive cells was determined by immunohistochemistry by the streptavidin-peroxidase method using a kit obtained from Boster (Wuhan, China) according to the manufacturer's instructions. Phosphate buffered saline was used to replace polyclonal rabbit polyclonal anti-MUC5AC antibody (1:100; Boster) to serve as a negative control.
2.6 Detection of protein content of MUC5AC in lung tissues by Western blot analysis
Lung tissue was homogenized in Lysis buffer L13 (0.01 mol/L Tris-HCl pH 7.4, 100 mmol/L NaCl, 10 mmol/L EDTA, 0.1 mmol/L phenylmethylsulfonyl fluoride, 5 μg/mL aprotinin). Samples were centrifuged (600g) for 5 minutes; the proteins in the supernatants (75 mg per lane) were separated by electrophoresis on a 12% sodium dodecyl sulfate polyacrylamide gel and were transferred to nitrocellulose membranes. After blocking for 2 hours at room temperature, the membranes were incubated overnight with primary antibodies (rabbit polyclonal anti-MUC5AC antibody 1:200 and rabbit polyclonal anti-β-actin 1:400). Finally, the membranes were incubated with horseradish peroxidase-conjugated immunoglobulin G (1:4000) for 2 hours at room temperature and the protein bands were visualized using an ECL kit (Boster). The relative amount of immunoreactive MUC5AC present in each sample was quantified by densitometry (AMBIS Radioanalytic and Visual Imaging System, Ambis Inc). The densitometric data from each blot was normalized to a control value that was set equal to one for each experiment. All the tests were repeated three times.
2.7 Real-time polymerase chain reaction
Total RNA was extracted from 40 lung tissue samples from mice using the TRIzol reagent (Invitrogen) according to the manufacturer's protocol. RNA was reverse-transcribed to complementary DNA (cDNA) using an real-time polymerase chain reaction (RT-PCR) kit (TaKaRa, Japan) according to the manufacturer's instructions. The cDNA was analyzed immediately or stored at −20°C. Quantitative PCR amplification was performed by the ABI 7900HT Fast Real-Time PCR System (ABI Company), and the SYBR Green I fluorescent dye method was used to quantify cDNA. The primer pairs were as follows (5′ to 3′): MHV-68 gp150 (GenBank: NC_001826.2, Forward: CAACAATCCCACTACAATTATGCG, Reverse: TGCGGGCGTCTTTGTGT; amplicon size, 71 base pair [bp]), MUC5AC (GenBank: NM_010844, Forward: CCATGCAGAGTCCTCAGAACAA, Reverse: TTACTGGAAAGGCCCAAGCA; amplicon size, 106 bp), and β-actin (GenBank: NM_007393, Forward: CTGTCCCTGTATGCCTCTG, Reverse: ATGTCACGCACGATTTCC; amplicon size, 218 bp). All the primers were synthesized by Shanghai Bio-engineering Co, Ltd, China. The final volume of the RT-PCR reaction was 10 μL and included SYBR Premix Ex Taq 5.0 μL, primer (10 μmol/L) 0.2 μL each, 50 × ROX Reference Dye 0.2 μL, template 1.0 μL, adjust to 10 μL with distilled water. PCR cycling conditions consisted of an initial denaturing step for 10 seconds at 95°C, followed by 40 cycles of 5 seconds at 95°C and 30 seconds at 60°C. β-Actin from the same sample was used as an internal control, and the relative contents of the copy numbers of the target gene messenger RNA (mRNA) were calculated, from which we could determine the gene expression level and the trend of its change. Specificity of each reaction was controlled by melting curve analysis. A negative PCR control containing water instead of cDNA was performed. Real-time PCR was conducted in triplicate in three independent experiments.
2.8 Measurement of BALF cytokines and chemokines by enzyme-linked immunosorbent assay
Levels of cytokines and chemokines were determined using commercial ELISA kits in accordance with the manufacturer's instructions. ELISA kits for the detection of macrophage chemoattractant protein-1/chemokine (C-C motif) ligand 2 (CCL2), epithelial neutrophil activating peptide 78/chemokine (C-X-C motif) ligand 5 (CXCL5), interleukin-13 (IL-13), and transforming growth factor-β1 (TGF-β1) in supernatants of BALF were obtained from Boster with a detectable concentration range of 15.6 to 1000 pg/mL.
2.9 Statistical analysis
Values are expressed as mean ± SEM. GraphPad Prism v5 (San Diego, CA) was used for statistical analyses. Differences among groups were assessed using one-way analysis of variance followed by Tukey's post hoc test or the Kruskal-Wallis test with post hoc Dunn's test. A value of P < .05 was considered statistically significant. All evaluations were performed by investigators blinded to the experimental groups.
3 RESULTS
3.1 Morphometric analysis and immunohistochemical staining
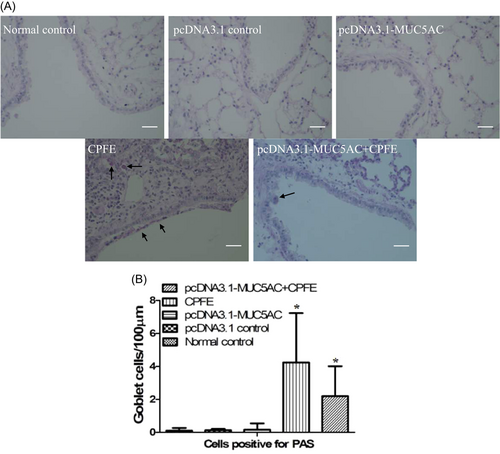
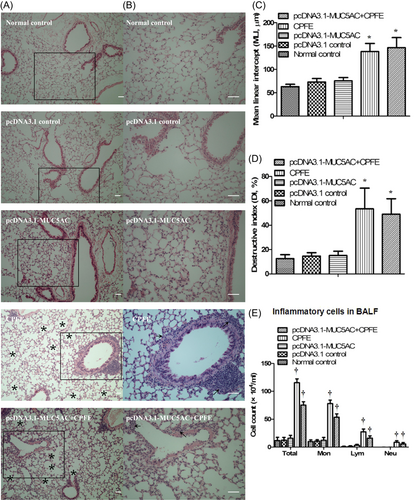
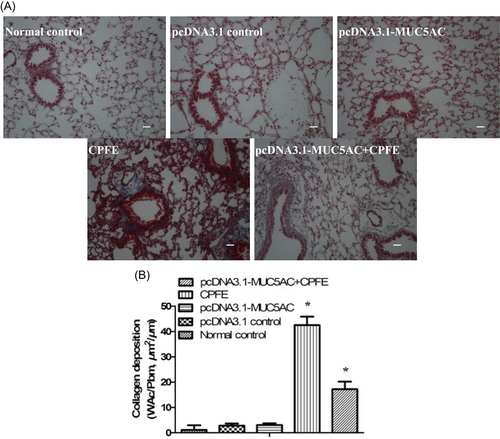
MUC5AC was expressed at high levels in the pcDNA3.1-MUC5AC group compared with normal control group and pcDNA3.1 control group (Figure 1). However, mucin hypersecretion alone was not sufficient to drive goblet cell metaplasia (Figure 2) to cause obvious mucus plugging and to induce airway inflammation (Figure 3). Hematoxylin and eosin staining demonstrated that the alveolar walls were noticeably broken down and merged together, and cavities of the alveoli were enlarged in the CPFE group and pcDNA3.1-MUC5AC + CPFE group. Moreover, thick-walled cystic lesions were observed in the CPFE group (Figure 3A-D). Masson trichrome staining showed collagen deposition as indicated by blue staining, with severe pneumonitis in the CPFE group; however, collagen fibers around bronchi and vessels were decreased significantly combined with slight pneumonitis in the pcDNA3.1-MUC5AC + CPFE group (Figure 4).
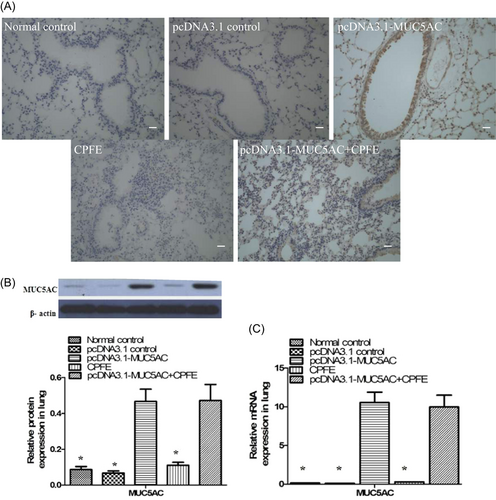
3.2 Determination of MUC5AC messenger RNA and protein in lung tissues by real-time PCR and Western blot analysis
The levels of MUC5AC mRNA and protein were higher in the lung tissues of the MUC5AC plasmid group compared with those in normal control group, pcDNA3.1 control group, and CPFE group (Figure 1B,C).
3.3 Infectivity of MHV-68 altered by MUC5AC
The level of MUC5AC mRNA in the pcDNA3.1-MUC5AC + CPFE group was higher than that in the CPFE group, but the level of MHV-68 gp150 in the pcDNA3.1-MUC5AC + CPFE group was lower than that in CPFE group (Table 1).
| Groups | n | MHV-68 gp150 |
|---|---|---|
| Normal control | 8 | 0.0244 ± 0.0019 |
| pcDNA3.1 control | 8 | 0.0472 ± 0.0041 |
| pcDNA3.1-MUC5AC | 8 | 0.0919 ± 0.0032 |
| CPFE | 8 | 24.1019 ± 5.1232* |
| pcDNA3.1-MUC5AC + CPFE | 8 | 13.2007 ± 3.8556* |
- Abbreviations: CPFE, combined pulmonary fibrosis and emphysema; MHV, murine gammaherpesvirus; mRNA, messenger RNA; MUC5AC, mucins5ac.
- * P < .05, compared with the other four groups differently.
3.4 Airway inflammation
The airway inflammation in the CPFE and pcDNA3.1-MUC5AC + CPFE groups was much more severe than that in pcDNA3.1-MUC5AC and the other two control groups. The majority of cells present in the BALF of CPFE and pcDNA3.1-MUC5AC + CPFE groups were macrophages with a relatively smaller number of neutrophils and lymphocytes. Total inflammatory cells, macrophages, neutrophils, and lymphocytes in the BALF increased significantly in pcDNA3.1-MUC5AC + CPFE and CPFE groups compared with the other three groups following MHV-68 infection. However, the total numbers of BALF inflammatory cells, macrophages, neutrophils, and lymphocytes were decreased in pcDNA3.1-MUC5AC + CPFE group than in CPFE group (Figure 3E). These data indicated that MUC5AC overexpression might serve as a protective barrier against MHV-68 virus infection in vivo, leading to a decrease in airway inflammation.
3.5 Levels of cytokines and chemokines in BALF
The levels of CCL2, CXCL5, IL-13, and TGF-β1 in BALF were increased in pcDNA3.1-MUC5AC + CPFE and CPFE groups than in the other three groups. Furthermore, their levels were lower in the pcDNA3.1-MUC5AC + CPFE group compared with CPFE group. However, the levels of CCL2, CXCL5, IL-13, and TGF-β1 were not different among the other three groups (Table 2). These results, coupled with the absence of inflammatory cells in histological sections of the lungs of mice in pcDNA3.1-MUC5AC group, indicate that MUC5AC hypersecretion alone does not initiate inflammation. However, MUC5AC hypersecretion alone can serve as a protective barrier against MHV-68 virus infection to further decrease airway inflammation and fibrosis induced by MHV-68 by decreasing the expression of CCL2, CXCL5, IL-13, and TGF-β1.
| Groups | n | CCL2 | CXCL5 | IL-13 | TGF-β1 |
|---|---|---|---|---|---|
| Normal control | 8 | 174.90 ± 5.12 | 17.74 ± 4.67 | 16.04 ± 4.02 | 65.65 ± 9.23 |
| pcDNA3.1 control | 8 | 170.27 ± 6.38 | 18.31 ± 5.01 | 17.13 ± 3.78 | 68.29 ± 8.37 |
| pcDNA3.1-MUC5AC | 8 | 179.22 ± 4.56 | 18.95 ± 5.82 | 19.55 ± 5.21 | 76.04 ± 9.75 |
| CPFE | 8 | 260.71 ± 20.22* | 56.63 ± 8.04** | 109.45 ± 19.02** | 317.75 ± 27.98** |
| pcDNA3.1-MUC5AC + CPFE | 8 | 249.75 ± 18.67* | 38.28 ± 7.94** | 78.46 ± 17.19** | 254.03 ± 24.23** |
- Abbreviations: BALF, bronchoalveolar lavage fluid; CCL2, chemokine (C-C motif) ligand 2; CPFE, combined pulmonary fibrosis and emphysema; CXCL5, chemokine (C-X-C motif) ligand 5; IL-13, interleukin-13; TGF-β1, transforming growth factor-β1.
- * P < .05, compared with normal control group, pcDNA3.1 control group, and pcDNA3.1-MUC5AC group differently.
- ** P < .05, compared with the other four groups differently.
4 DISCUSSION
Progressive lung damage and subsequent pulmonary fibrosis could be caused by derangement in the delicate pulmonary epithelial cells with microbes and particulate matter acting as a trigger. The luminal surfaces of the conducting airways interact with exogenous microorganisms and particles to prevent them from entering the lower layer of epithelial cells.1 Mucins have long been regarded to play a role as a barrier to prevent mucosal infection, but some studies report that overexpression of mucins induces obstruction and inflammation of airways.5, 7, 8 Hence, we initially created a pcDNA3.1-MUC5AC mouse model, and subsequently demonstrated a relationship between increased expression of a single mucin (MUC5AC) and mucus plugging, goblet cell metaplasia, and airway inflammation and fibrosis.
Although the MUC5AC protein was increased in pcDNA3.1-MUC5AC group, PAS staining of the lung sections did not show a metaplastic or enlargement of goblet cells. These data suggested that the airway cells produced more MUC5AC in pcDNA3.1-MUC5AC group, but did not induce goblet cell metaplasia. Moreover, hypersecretion of mucin did not cause mucus plugging. A previous study by Liu et al13 proved that increased secretion of mucin combined with a slowdown of MCC could induce mucin plugging.14 Our data revealed that pcDNA3.1-MUC5AC increased the level of MUC5AC but did not induce mucus plugging, suggesting that pcDNA3.1-MUC5AC did not decrease MCC. Furthermore, our results also indicated that there was no evidence of increasing airway inflammation, despite an approximately five-fold increase in MUC5AC protein expression.
Emphysema and pulmonary fibrosis have dissimilar physiological effects; however, there is an increasing clinical, radiologic, and pathologic recognition of the coexistence of emphysema and pulmonary fibrosis in the same patient, resulting in a clinical syndrome known as “combined pulmonary fibrosis and emphysema” (CPFE). Previous studies have reported that CPFE mostly develops in male patients due to long-term smoking.15-17 These data suggest that CPFE is related to age, smoking duration, and sex. It is known that long-term cigarette smoke exposure in male mice could lead to the development of emphysema in these mice, ultimately developing into pulmonary fibrosis.18 A study by Torres-González showed an increased susceptibility to lung injury due to herpesvirus infection, resulting in pulmonary fibrosis.9 Our previous data19 demonstrated that secondary damage to alveolar type II cells by rAAV-SPA-TK could induce pulmonary fibrosis in rats with emphysema. Based on these data, we propose that long-time passive cigarette smoke in old male mice having emphysema could develop into CPFE mice after being infected by MHV-68. Morphometric data support this finding, in which collagen deposition as displayed by the blue staining (Figure 4) indicates severe pneumonitis in the CPFE group.
The level of MHV-68 gp150 in the pcDNA3.1-MUC5AC + CPFE group was lower than that in CPFE group. These data suggest that MUC5AC overexpression serves as a protective barrier against MHV-68 virus infection in vivo. Previous studies have also speculated that increased MUC5AC with its sialic acid residues protected mice against specific influenza infections by acting as a “decoy” to bind virus in a mobile phase.4 This action prevented access of the virus to the airway surfaces, a necessary process to mediate cell infection. Inflammatory cells (including macrophages, lymphocytes, and neutrophils) as well as inflammatory factors (CCL2, CXCL5, IL-13, and TGF-β1) were reduced in pcDNA3.1-MUC5AC + CPFE mice than in CPFE mice, which indicated a direct anti-infectivity effect or a similar “anti-inflammatory” role of MUC5AC.
Various inflammatory factors play important roles in the pathogenesis of lung emphysema or fibrosis.20 Also, it has been proved that many cytokines and chemokines are responsible for the recruitment of specific types of cells in emphysema and pulmonary fibrosis. In this study, significantly greater numbers of inflammatory cells, especially macrophages, lymphocytes, and neutrophils were observed in BALF of CPFE and pcDNA3.1-MUC5AC + CPFE groups. CCL2 is a potent monocyte chemotactic protein. In addition to its critical role in mononuclear cell recruitment, CCL2 plays important roles in modulating fibroblast phenotype and activity.21 CXCL5 is a proinflammatory chemokine that stimulates neutrophil recruitment following secretion by a variety of cell types, including lymphocytes, macrophages, fibroblasts, and endothelial cells. Additionally, CXCL5 has been shown to be significantly elevated in patients with idiopathic pulmonary fibrosis (IPF).22 Thus, in our study, we investigated the roles of CCL2 and CXCL5 in the lungs of the treated mice. The higher levels of CCL2 and CXCL5 in pcDNA3.1-MUC5AC + CPFE and CPFE groups, and their lower levels in pcDNA3.1-MUC5AC + CPFE group compared with those in CPFE group revealed their role in the process of lung fibrosis. During chronic lung remodeling, numerous immune cell types can produce IL-13. IL-13 is a Th2-type cytokine that promotes lung fibrosis in a number of experimental settings.23-28 Both the IL-13 receptor subunits (IL-13Rα1 and IL-13Rα2) are highly expressed by IPF fibroblasts,29, 30 either in a TGF-β-dependent or -independent manner.31-34 Although the data concerning the induction of IL-13 is compelling, further studies will be required to determine if IL-13 cooperates with TGF-β to induce emphysema in the infected mice to further develop into CPFE by using IL-13 antibody or siRNA. TGF-β1 is a key mediator of pulmonary fibrosis. Consequently, overexpression of TGF-β1 induces epithelial-mesenchymal transition activates fibroblasts and fibroblast-like cells to synthesize excessive collagen, eventually leading to pulmonary fibrosis.35 The previous study showed an interplay between TGF-β1, IL-13, and CCL2 in IPF, wherein these three mediators feedback act on each other via feedback loops, promoting fibrotic pathogenesis.36 Further research is needed to determine whether this kind of interplay exists in the CPFE model in our study. In summary, our data show that CCL2, CXCL5, IL-13, and TGF-β1 contribute to the pathogenesis of pulmonary fibrosis through a poorly defined but targetable mechanism.
5 CONCLUSION
In conclusion, approximately five times more secretion was observed in the lungs of pcDNA3.1-MUC5AC mice than in the normal control mice, and the excess mucus could be efficiently cleared from the lungs, thereby demonstrating that mucin overexpression could occur without obstruction or inflammation of the airways. Consequently, at least in part, resistance to MHV-68 infection in mice overexpressing Mus5ac was proved. Therefore, future therapeutics aimed at reducing mucin production with the purpose of ameliorating airway obstruction may require careful monitoring to avoid compromising host defense. Moreover, in old male patients suffering from emphysema due to long-term smoking, MUC5AC may decrease the risk of developing CPFE.
ACKNOWLEDGMENTS
The authors thank the staff at the Key Laboratory of Respiratory Diseases, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China and also thank Health Commission of Hubei Province (WJ2017M241) for the funding support.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
LH and SSW conceived and designed the study. SSW and BZ performed the animal experiments. All authors completed the acquisition, analysis, and interpretation of data. LH and JHG wrote the manuscript. QQF, QZ, and JHG study supervised and coordinated and all authors read and approved the final manuscript.
ETHICS STATEMENT
The animal protocols were approved by the Institutional Animal Care and Use Committee of Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology.
Open Research
DATA AVAILABILITY STATEMENT
The data sets supporting the results of this article are included within the article.




