Low rates of active hepatitis B and C infections among adults and children living with HIV and taking antiretroviral therapy: A multicenter screening study in Lesotho
Abstract
Lesotho presents the second-highest adult human immunodeficiency virus (HIV) prevalence globally. Among people living with HIV, data on hepatitis B virus (HBV) or hepatitis C virus (HCV) coinfection are limited. We report HBV and HCV coinfection data from a multicentre cross-sectional study among adult and pediatric patients taking antiretroviral therapy in 10 health facilities in Lesotho. Among 1318 adults screened (68% female; median age, 44 years), 262 (20%) had immunologically controlled HBV infection, 99 (7.6%) tested anti-HBs positive and anti-HBc negative, indicating vaccination, and 57 (4.3%) had chronic HBV infection. Among the patients with chronic HBV infection, 15 tested hepatitis B envelope antigen (HBeAg) positive and eight had detectable HBV viremia (median, 2 477 400 copies/mL; interquartile range, 205-34 400 000) with a mean aspartate aminotransferase-to-platelet ratio index of 0.48 (SD, 0.40). Prevalence of HCV coinfection was 1.7% (22 of 1318), and only one patient had detectable HCV viremia. Among 162 pediatric patients screened, three (1.9%) had chronic HBV infection, whereby two also tested HBeAg-positive, and one had detectable HBV viral load (210 copies/mL). Six of 162 (3.7%) had anti-HCV antibodies, all with undetectable HCV viral loads.
Overall prevalence of chronic HBV/HIV and HCV/HIV coinfection among adults and children was relatively low, comparable to earlier reports from the same region. But prevalence of immunologically controlled HBV infection among adults was high. Of those patients with chronic HBV infection, a minority had detectable HBV-DNA.
Highlights
In this multicentre cross-sectional study from Lesotho, prevalence of chronic HBV/HIV and HCV/HIV co-infection among adults and children taking ART was relatively low. Those with co-infection showed low HBV and HCV activity. However, the prevalence of immunologically controlled HBV infection among adults living with HIV was high.
Abbreviations
-
- ALT
-
- alanine aminotransferase
-
- anti-HBc antibodies
-
- anti-HB core antibodies
-
- APRI
-
- aspartate aminotransferase-to-platelet ratio index
-
- ART
-
- antiretroviral therapy
-
- CI
-
- confidence interval
-
- HBeAg
-
- hepatitis B envelope antigen
-
- HBsAg
-
- hepatitis B surface antigen
-
- HBV
-
- hepatitis B virus
-
- HCV
-
- hepatitis C virus
-
- HIV
-
- human immunodeficiency virus
-
- IQR
-
- interquartile range
-
- RR
-
- relative risk
-
- TDF
-
- tenofovir disoproxil fumarate
-
- WHO
-
- World Health Organization
1 INTRODUCTION
Globally, hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are major causes of acute and chronic liver disease, resulting in about 1.4 million deaths annually with a disproportionally high burden for low- and middle-income countries.1 Persons living with the human immunodeficiency virus (HIV) are a key population most affected by HBV and HCV infection.2, 3 Coinfection with HIV has been identified as an additional factor for fibrosis progression and liver carcinogenesis.4 The estimated global prevalence of HIV/HBV coinfection is 7.6%, leading to 2.7 million individuals simultaneously carrying HIV and HBV. The highest numbers are found in sub-Saharan Africa (69% of all cases; 1.9 million).2 For HIV and HCV, global coprevalence is 2.4%3 and a recent systematic review shows a coprevalence of 4.6% in southeast Africa.5
Published prevalence data for HBV and HCV in Lesotho, a small country in Southern Africa with the second-highest HIV prevalence globally,6 are limited. Two older and rather small retrospective cohort studies in Lesotho found hepatitis B surface antigen (HBsAg) positivity among HIV-positive individuals in 5.5% and 10.5% of the patients, and prevalence of anti-HCV antibodies of 0.5%.7, 8 Both studies, however, did not assess HBV or HCV activity, and thus, the clinical relevance remains unclear.
In this study, we report results from a multicentre screening of HBV and HCV coinfection among children and adults living with HIV and taking antiretroviral therapy (ART) at 10 health facilities in Lesotho, including the assessment of HBV/HCV activity based on viral load measurement and the aspartate aminotransferase-to-platelet ratio index (APRI).
2 MATERIALS AND METHODS
The study “Comorbidities and Virologic Outcome Among Patients on Antiretroviral Therapy in Rural Lesotho (CART-1)” was a registered observational cohort study assessing comorbidities and virologic outcomes among patients on ART in 10 health facilities in two districts in Lesotho, Southern Africa. In the CART-1 study, all patients on first-line ART for 6 months or over, who attended routine follow-up visits between 5 May and 17 June 2014, received multidisease screening including for HBV and HCV after informed consent. The study setting, as well as the inclusion and exclusion criteria, have been described in detail elsewhere.9, 10 At the time of the study, no direct-acting antivirals for the treatment of HCV were available in Lesotho. Here, we present the results of the HBV/HCV screening, including viral load results and APRI score in those screened positive.
HBsAg, Hepatitis B envelope antigen (HBeAg), anti-HBs antibodies, and anti-HCV antibodies were measured at the study sites using the point-of-care test manufactured by SD BIOLINE. Blood was collected, transported within 8 hours to the hospital laboratories of the two districts (St Charles Mission Hospital in Seboche, Paray Hospital in Thaba-Tseka), centrifuged, and plasma-frozen at −40°C. After a maximal storage period of 3 weeks, these plasma aliquots were shipped on dry ice to a reference laboratory in Switzerland for determining HCV and HBV viral loads and anti-HBV core (anti-HBc) antibodies. HBV-DNA and HCV-RNA were quantified on a Roche AmpliPrep-Taqman system (AmpliPrep/COBAS TaqMan HBV Test, v2.0; AmpliPrep/COBAS TaqMan HCV-Test v2.0).
Data are presented separately for children and adults (≥16 years). Gender differences in adults were assessed using the Pearson χ2 test, differences in nonnormally distributed APRI means using the Mann-Whitney test, and differences in HBV suppression using the Fisher exact test. Unless otherwise specified, results are presented as relative risk (RR) with 95% confidence intervals (CI). All analyses were performed using Stata (version 14; Stata Corporation, Austin, TX). For all tests, we used complete case analysis and two-sided P values with α .05 level of significance.
The study has been approved by the National Health Research and Ethics Committee of Lesotho (ID 01-2014) and the “Ethikkomission Nordwest- und Zentralschweiz” in Switzerland (ID 2014-029). Before enrollment, all patients or caregivers of children had provided individual verbal and written informed consent.
3 RESULTS
3.1 Participant characteristics
Figure 1 displays the patient flow and screening results of adult and pediatric patients. Overall, 1563 adult patients were enrolled and from 1318 (84%) of them, documentation of HBsAg and anti-HCV antibodies results are available. The median age was 44 years (interquartile range [IQR] 35-54) and 899 (68%) were female. Median time since starting ART was 3.8 years (IQR: 2.0-5.5), 833 (63%) were taking an ART regimen containing tenofovir disoproxil fumarate (TDF), lamivudine plus either efavirenz or nevirapine, and the remaining took a regimen containing zidovudine (AZT) or abacavir instead of TDF. At the time of the study, 1207 (92%) had a suppressed HIV viral load (<100 copies/mL).
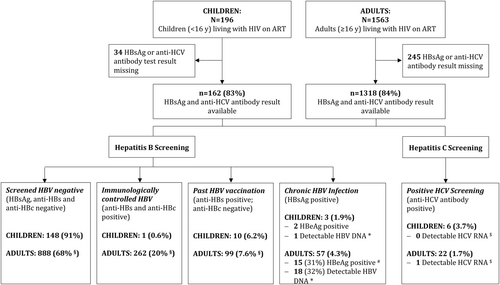
Of the 196 enrolled HIV-positive children (<16 years), complete screening test data are available for 162 (83%); the median age was 10 years (IQR 8-13) and 81 (50%) were female. Median time since starting ART was 3.1 years (IQR 2.4-5.4), 142 (88%) were taking an ART regimen containing AZT, lamivudine plus either efavirenz or nevirapine; six were on a TDF-based regimen. At the time of the study, 133 (82%) had a suppressed HIV viral load (<100 copies/mL).
3.2 Hepatitis screening results in adult patients
Among the 1318 adults screened, 262 (20%) had an immunologically controlled HBV infection (anti-HBs and anti-HBc antibodies positive), 99 (7.6%) tested anti-HBs positive and anti-HBc negative, indicating past vaccination, and 57 (4.3%; 95% CI, 3.35-5.57) had a chronic HBV infection (HBsAg-positive), as shown in Figure 1.
Men were more likely to test HBsAg-positive (RR, 2.07; 95% CI, 1.25-3.44; P = .004). HBeAg testing was successful for 49 of the 57 HBsAg carriers, and 15 (31%) were positive for both parameters. Polymerase chain reaction quantification was successful in 56 of 57 HBsAg carriers, and 18 (32%) of these had detectable HBV-DNA at 20 copies/mL or more (median, 2 477 400; IQR 205-34 400 000). Among the patients tested HBsAg-positive, those taking a TDF-containing regimen were more likely to have undetectable HBV-DNA (31 of 35 [89%] vs 7 of 20 [35%], RR 2.53; 95% CI, 1.38-4.65; P < .001). In the four patients with detectable HBV-DNA despite taking a TDF-containing ART regimen, HIV was suppressed (<100 copies/mL), indicating good adherence. The detectable HBV-DNA levels were low (max 308 copies/mL). Two of the patients had normal and two slightly elevated alanine aminotransferase (ALT) levels (59 and 83 IU/L). Table 1 displays the mean APRI score stratified by HBsAg, HBeAg, and HBV viremia. One patient with chronic HBV infection presented APRI > 2, the cut-off recommended by the World Health Organization (WHO) indicating cirrhosis and thus one of the antiviral treatment initiation criteria.11 Five chronic HBV patients presented APRI > 1.5, an alternative cut-off for cirrhosis suggested by a leading meta-analysis.12
| N | Mean APRI | SD | 95% CI | P value | z Value | |
|---|---|---|---|---|---|---|
| HBsAg-negative | 1252 | 0.42 | 0.63 | 0.38-0.45 | ||
| vs HBsAg-positive | 57 | 0.48 | 0.40 | 0.38-0.58 | .28 | −1.07 |
| HBV-undetectable | 38 | 0.45 | 0.41 | 0.32-0.58 | ||
| vs HBV-detectable | 18 | 0.57 | 0.39 | 0.39-0.75 | .29 | −1.06 |
| HBeAg-negative | 1286 | 0.42 | 0.63 | 0.39-0.45 | ||
| vs HBeAg-positive | 15 | 0.59 | 0.45 | 0.36-0.82 | .15 | −1.45 |
- Abbreviations: APRI, aspartate aminotransferase-to-platelet ratio index; ART, antiretroviral therapy; CI, confidence interval; HBeAg, hepatitis B envelope antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus.
The anti-HCV antibody prevalence among adult patients was 1.7% (95% CI, 1.10-2.52), and only one patient had detectable HCV-RNA (2 567 760 copies/mL), indicating chronic HCV infection (Figure 1). This 52-year-old female participant had an ALT level of 28, and an APRI of 0.37. Three patients were HIV/HBV/HCV coinfected.
3.3 Hepatitis screening results in pediatric patients
Among the children, overall, three of 162 (1.9%; 95% CI, 0.59-5.65) screened HBsAg-positive and two, also HBeAg-positive (Figure 1). One had low detectable HBV-DNA (210 copies/mL). This patient, an 11-year-old boy, had an unsuppressed HIV viremia (2,847 copies/mL), ALT 42 IU/L, APRI 0.6, and was on an AZT-containing ART. Ten out of the 162 (6.2%) children showed past vaccination (anti-HBs positive and anti-HBc negative) and one immunologically controlled HBV infection. Six of the 162 (3.7%; 95% CI, 1.66-8.06) children had anti-HCV antibodies, all with undetectable HCV-RNA.
4 DISCUSSION
This cross-sectional study conducted in 10 health facilities in Lesotho assessed the prevalence of HBV and HCV coinfection among adult and pediatric HIV patients taking ART. We found high rates of immunologically controlled HBV infection among adults (20%), and chronic HBV infection was rather rare (4.3%). Of those patients with chronic HBV infection, a minority had detectable HBV-DNA or an APRI indicating liver cirrhosis. None of the patients taking TDF as part of their ART regimen had high HBV-DNA levels. Among pediatric patients, HBsAg prevalence was low with 1.9%. Active HCV infection appears to be rare in both study populations, similar to a previous study from Lesotho.7
Our data are in line with the HBsAg prevalence among adults living with HIV reported in a smaller earlier study in Lesotho7 and in contrast to a second study conducted in Lesotho, which reported a considerably higher HBsAg prevalence of 10.5%.8 The latter data came, however, from a single health facility in a predominantly urban setting. A recent meta-analysis noted a co-seroprevalence of 4.4% for the Southern African region.2
Earlier studies found that patients with HBV coinfection are at a higher risk to develop liver fibrosis and cirrhosis.4, 13 Although the diagnostic accuracy of APRI is inferior to an ultrasound-guided assessment of liver elasticity (fibroscan) or liver biopsy, the WHO recommends APRI for the clinical management of HBV.11, 12, 14 In our adult cohort, the APRI score did not significantly differ between HBsAg-positive and -negative individuals. Several explanations are possible: First, the size of our cohort was insufficient to show a significant difference. Second, HIV-coinfected individuals were mainly taking TDF-containing ART. Previous studies demonstrated that TDF significantly decreases the risk of cirrhosis and its complications, and can even induce a regression of fibrosis and cirrhosis.15 Third, HIV alone might be independently associated with the APRI score.16
The WHO guidelines on HBV treatment recommend TDF or entecavir for long-term suppressive antiviral therapy.11 At the same time, TDF is part of the recommended adult first-line ART in Lesotho.17 In line with these recommendations, among those patients taking TDF-containing ART, only four had detectable HBV-DNA and all at low levels.
Unexpectedly, only 7.6% of the adults and 6.2% of the children tested anti-HBs antibody positive and anti-HBc antibody negative, indicating past vaccination. This is in sharp contrast to the more optimistic official figures of national immunization coverage rates, estimating an HBV vaccination coverage of 67% to 93%.18
Our study has several limitations. First, it was carried out in two predominantly rural districts of Lesotho, and may, therefore, not be generalizable for the entire country. Second, it exclusively involved patients, who were on ART for 6 months or longer and attended routine follow-up visits for HIV. HBV and HCV prevalence rates could be different among patients, who do not regularly attend medical care or who have been lost to follow-up. Third, ultrasound as a standard method was not available for assessing fibrosis stages among patients with chronic HBV or HCV infection.
Overall, this study covers a broad cohort of representative individuals living with HIV and taking ART in Lesotho, covering a wider data range than previous studies for the assessment of HBV/HCV activity.
In conclusion, this study finds the overall prevalence of HBV/HIV and HCV/HIV coinfection to be rare, comparable to earlier reports from the same region. HBV viremia was low, especially among patients taking tenofovir-containing ART.
ACKNOWLEDGMENTS
The authors would like to express their gratitude to all staff of the health facilities in the districts of Thaba-Tseka and Butha-Buthe, who were involved in the study and data collection process and to all patients who participated in the study. The study was funded by the Swiss Foundation for Excellence and Talent in Biomedical Research through a grant obtained by NDL. AA receives his salary through a grant from the PhD program of the Swiss National Science Foundation (Grant number 323530_177576). NDL receives part of his salary from the Eccellenca Professorship program of the Swiss National Science Foundation (PCEFP3_181355). This study was embedded in the SolidarMed country program and thus benefited from logistics and human resources provided by SolidarMed Lesotho. The funding sources had no role in the design of the study and were not involved in data collection, data analysis, interpretation of the results, or writing of the manuscript.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
NDL was the principal investigator of the CART-1 study. AA and NDL analyzed the data and drafted the manuscript. MC contributed to the study design and was responsible for blood sample processing and laboratory analyses in Lesotho. TIL contributed to the study design and was responsible for data collection. TK contributed to the study design and was responsible for all virologic analyses performed in Switzerland. MB, BLN, and MAH provided technical support and intellectual input. All authors read, revised, and approved the final manuscript.




