Full-length genomic sequencing and characterization of Borna disease virus 1 isolates: Lessons in epidemiology
Yujie Guo, Peng He, and Lin Sun contributed equally to this study.
Abstract
Borna disease virus 1 (BoDV-1) is a nonsegmented, negative-strand RNA virus that infects mammals including humans. BoDV-1 strains occur globally, dominate the species Mammalian 1 bornavirus, and display highly conserved genomes and persistent infection (brain, blood). Subclinical infections prevail but the rare fatal outcomes even in people need awareness and risk assessment. Although BoDV-1 strains were successfully isolated, only limited full genomic sequences are available. In this study, the entire genomes of two natural BoDV-1 isolates (Hu-H2, Equ-Cres) and one vaccine strain (DessVac) were sequenced. They were compared with 20 genomes and 20 single-gene sequences (N and P) of worldwide human strains from psychiatric and neurologic patients and animal strains from horses with Borna disease available at GenBank. Phylogenetic analyses confirmed a low divergence not exceeding 5.55%, 5.34%, and 4.94% at the genome, P-gene, and N-gene level, respectively, characteristic of BoDV-1. Human viruses tended to cluster at the country level but appeared to be independent of hosts’ diseases and/or time of isolation. Notably, our data also indicated that human viruses provided individual genetic signatures but exhibited no distinct genotypes that separated them from animal strains. Sequence similarities thus occurred between different host species and distant geographic regions, supporting global BoDV-1 prevalence. Overall low genetic divergence among BoDV-1 viruses shown here also argued against zoonotic concepts, requiring further clarification beyond sequence similarities. Finally, unlike shared sequence conservation, phenotyping of natural and laboratory variants revealed that they manipulated host cells differently, underpinning the authenticity of the human BoDV-1 strains.
1 INTRODUCTION
Borna disease virus 1 (BoDV-1) strains are the leading viruses of the species Mammalian 1 orthobornavirus. The family Bornaviridae comprises nonsegmented negative-strand RNA viruses replicating in the nucleus of the infected cell, and until now accounts for 8 species and 16 viruses in various bird, reptile, and mammalian species.1 Notably, BoDV-1 strains including human viruses are genetically closely related (>95% homology), in contrast to all other genetically distant Bornavirus species. Even the variegated squirrel bornavirus 1 (VSBV-1),2 species Mammalian 2 orthobornavirus, differs genetically by more than 30%. The BoDV-1 genome is approximately 8.9 kb long and contains six open reading frames (ORFs) encoding the nucleoprotein (N, p40), phosphoprotein (P, p24), X-protein (X), matrix protein (M), glycoprotein (GP), and polymerase (L).
The occurrence of BoDV-1 infection in humans has been controversial for decades,3-5 but human fatalities caused by BoDV-1 encephalitis in transplant recipients6 and patients without an immune-suppressive background7-9 clearly confirmed human infection and the risk of an even fatal outcome. Recently, a few further retrospectively analyzed human fatalities (14 between 1999 and 2019) were reported, thereby introducing the hypothesis that human BoDV-1 infection is a fatal zoonosis, transmitted in endemic regions in Germany by its reservoir host, the bi-colored white-toothed shrew.10 The shrew theory had been set up earlier in 2014.11 However, this view dismissed previous epidemiologic studies which indicated that healthy carriers exist and include humans, farm animals, and pets, and that the geographic distribution of BoDV-1 infection has a global range, including Central Europe, the Middle East, Japan, and China.12-17 Narrowing the clinical picture to fatal encephalitis10 also ignored previous studies suggesting that BoDV-1 infections may be associated with psychiatric disorders, whereas the majority of infections presented subclinically based on an estimated prevalence between 10% and 30% in healthy individuals.18-22 BoDV-1 predominantly infects neurons of the cortex and hippocampus, causing cognitive deficiencies and behavioral alterations.23
Despite the mounting significance of BoDV-1 infection, only a few human isolates exist to date, three recovered from peripheral blood mononuclear cells (PBMCs) of psychiatric patients in Germany,24 one recovered from the brain of a schizophrenic patient via a gerbil passage,25 and a recent one recovered from the brain of a human patient who had died from encephalitis.10 Likewise, only a limited number of full-length genomic sequences of Mammalian 1 bornavirus strains/isolates are registered in the GenBank, including those of the first fully sequenced reference animal laboratory strains V and He/80.26, 27
This study first aimed to fill the gap of full genome sequences of BoDV-1 strains, secondly to unravel the relationship of their genomes to others by phylogenetic analysis, and thirdly, to contribute to the poorly understood and currently controversial epidemiology. The entire genomes of three different BoDV-1 isolates were sequenced, namely a human PBMC-derived strain (Hu-H2),24 an equine strain (Equ-Cres), the first recovered from PBMCs, and a highly adapted vaccine strain (DessVac). They were compared with the complete genomes of total 20 strains/isolates, namely seven human strains6-8, 24, 25 and four horse strains,28 one bovine strain,29 and eight nonnatural laboratory-adapted strains of equine origin.26, 27, 30, 31 Furthermore, nine additional nucleotide sequences of the BoDV-1 P-gene were comparatively included,32-36 and 11 nucleotide sequences of parts of the N-gene.24, 33, 36-39 The study thus provides a phylogenetic analysis of total 20 full genomes and 20 sequences of either N or P-genes to classify three fully sequenced unique BoDV-1 strains.
Finally, the impact of the three isolates on the cell cycle and apoptosis of oligodendroglia (OL) cell lines was studied by flow cytometry. Previous studies have shown the opposite effects of natural human versus laboratory strains despite the genetic similarity.40, 41
2 MATERIALS AND METHODS
2.1 Virus strains and cell culture
Human OL cells and virus stocks of the natural human strain BoDV-1 Hu-H2 and equine strain Equ-Cres, the nonnatural vaccine strain DessVac and strain V propagated in OL cells were kindly supplied by Hanns Ludwig, Professor of Virology (Free University of Berlin, Berlin, Germany) in 2012 and 2010, respectively. These BoDV-1 strains were distinct strains of completely different origins, the histories of which are described in full detail in Tables 1 and 2.
| Template | Hu-H2 (abbreviation) | Equ-Cres (abbreviation) |
|---|---|---|
| Order | Mononegavirales | Mononegavirales |
| Family | Bornaviridae | Bornaviridae |
| Genus | Bornavirus | Bornavirus |
| Species | Mammalian 1 bornavirus | Mammalian 1 bornavirus |
| Virus | Borna disease virus 1 (BoDV-1) | Borna disease virus 1 (BoDV-1) |
| Strain | BoDV-1 Hu-H2 | BoDV-1 Equ-Cres |
| Isolation host | Homo sapiens (H. sapiens) | Equus ferus (E. ferus) |
| Isolation source | PBMCs | PBMCs |
| Sampling location | Germany (DE), Berlin | Germany (DE), Munich |
| Sampling year | 1994 (94) | 1995 (95) |
| Genetic variant | Patient H2, male, 37y, OCD, chronic for 13.9y (DSM IV 300.30); Berlin 1994, WK H2, sample H2-3-PBMC (antigen-positive), passaged in human oligodendroglial (OL-221) cells (virus p11, p13-p25) (stable virus titer from p13 on) | Horse Cresalo, acute (for 16 d) severe Borna disease with apathy, head-shaking, yawning, intermitting fever, respiratory symptoms, appetite loss, change of behavior; positive serology antibodies, Berlin 1995, PBMC sample (antigen-positive), passaged in human oligodendroglial (OL-221) cells (virus p8–p34) (stable virus titer from p14 on) |
| Suffix | Passaged in OL cells (-tc) | Passaged in OL cells (-tc) |
| Pathology in rabbits | Behavioral disease, no weight loss (3 wk p.i.), Hu-H2/OL p15 used | Not tested |
| Published GenBank accession no. | Prior partial sequences: | None, before this study |
| U58595, L76235, L76230, L76238 | ||
| Full designated name | BoDV-1 Hu-H2/H. sapiens-tc/DE/1994/Berlin-Hu-H2-94 WKs3-PBMC | BoDV-1 Equ-Cres/E. ferus-tc/DE Munich/1995/Berlin-Equ-Cres-95s2-PBMC |
| Medium-length designation | BoDV-1 HuH2/Hsap-tc/DE/94/Ber-HuH2-94 WKs3-PBMC | BoDV-1 Equ-Cres/Efer-tc/DE Mun/95/Ber-EquCres-95s2-PBMC |
| Passage history of virus stock provided | OL-221/HuH2-94/-96FR, passage p28, 1996, provided 2012 to CQMU | OL-221/EquCres-95/-98, passage 51, 1998, provided 2012 to CQMU |
| Passage grown at CQMU and used as virus source | OL-221/HuH2-94/-96FR, passage p29 in OL-221 | OL-221/EquCres-95/-98, passage 52 in OL-221 |
- Abbreviations: CQMU, Chongqing Medical University; DEU, Germany; OCD, obsessive compulsive disorder; OL, oligodendroglia cell line; PBMCs, peripheral blood mononuclear cells; p.i., post-infection.
| Template | DessVac (abbreviation) | Strain V (abbreviation)* |
|---|---|---|
| Order | Mononegavirales | Mononegavirales |
| Family | Bornaviridae | Bornaviridae |
| Genus | Bornavirus | Bornavirus |
| Species | Mammalian 1 bornavirus | Mammalian 1 bornavirus |
| Virus | Borna disease virus 1 (BoDV-1) | Borna disease virus 1 (BoDV-1) |
| Strain | BoDV-1 strain Dessau | BoDV-1 strain V |
| Isolation host | Equus ferus (E. ferus) | Equus ferus (E. ferus) |
| Isolation source | Brain | Brain |
| Sampling location | Germany (DEU), Dessau | Germany (DEU), Giessen |
| Sampling year | 1960 (60) | 1927 (27) |
| Genetic variant | Horse with fatal Borna disease (encephalomyelitis) (-hist), horse brain, passaged in rabbits (brain, 50-100×, “lapinization”), lyophilized vaccine strain “Dessau”, 1950 up to 1990 applied horse live vaccine in former DDR, cancellation of license 1992; OL-221 cells (virus p10 on) | Horse V, fatal Borna disease (encephalomyelitis) (-hist), Giessen-horse V-brain, passaged in rabbits (brain, 20-50×), rats (brain 6×), OL-221 cells (virus p10-p25) |
| Suffix | Passaged in rabbits (live vaccine) and OL cells (-lab) | Passaged in laboratory animal hosts and OL cells (-lab) |
| Pathology in rabbits | 0.5 g lyophilized vaccine (single vaccine dose s.c. as applied to horses) equivalent to 10 lethal doses for rabbits | Fatal disease (3 wk p.i.), strain V/OL p12 used |
| GenBank accession no. | Prior partial sequence data (unpublished) | U04608 (full-length) |
| Full designated name | BoDV-1 strain Dessau/E. ferus VECTOR/O.cuniculus-lab/DEU/1960/Dessau-vaccine strain-rabbit-brain | BoDV-1 strain V/E. ferus VECTOR/O.cuniculus-lab/Wistar rat-lab/DEU/1927/Giessen-horse V-brain |
| Medium-length designation | BoDV-1 str.DessVac/VECTOR/O.cun-lab/DEU/60/DessVac-rabbit-brain | BoDV-1 str.V/VECTOR/O.cun-lab/Wistar-lab/DEU/27/Gie-horse V-brain |
| Passage history of virus stock provided | OL-221/strain DessVac, specified as Dessau63, passage 63, 1998, provided 2010 to CQMU | OL-221/strain V, specified as TL103 FR_p103, passage p103, 1998, provided 2010 to CQMU |
| Passage grown at CQMU and used as virus source | OL-221/strain DessVac, passage p64 in OL-221 | OL-221/strain V, passage 104 in OL-221 |
- * Adapted as described previously.41
- Abbreviations: CQMU, Chongqing Medical University; DEU, Germany; p.i., post-infection; OL, oligodendroglia cell line.
For each virus strain, strictly separated lamina air flow facilities of our cell laboratory at Chongqing Medical University were used. OL cells were maintained in Dulbecco's Modified Eagle's Medium (DMEM; Invitrogen Life Technologies) with high glucose (4.5 g/L, Gibco), containing 1% penicillin, 1% streptomycin (Sigma-Aldrich), and 5% heat-inactivated fetal bovine serum (Gibco). Cells were incubated at 37°C in a 5% CO2 incubator. Cells were harvested after reaching 70% to 80% confluence.
2.2 Amplification of BoDV-1 genomes
For sequencing complete genomes of strains BoDV-1 Hu-H2, Equ-Cres, and DessVac, viral RNA was extracted from OL cells using Trizol (Invitrogen Life Technologies) and quantified by a NanoDrop spectrophotometer. RNA (500 ng) was reverse-transcribed by SuperScript I reverse transcriptase (Takara, Japan) using random hexamers. Eight pairs of primers were designed to amplify nucleotides (nt) 52 to 8788 of the BoDV-1 genome by overlapping polymerase chain reaction (PCR) (Table 3). To sequence the 5′ and 3′ termini, viral RNA was circularized by T4 RNA ligase (Promega, USA). The ligated RNA was reverse-transcribed using primer P9R, and amplified with primers P9F/P9R. Thereafter, 3′-RACE was performed to determine the 3′-terminus using the 3′-Full RACE Core Set (Takara, Japan), according to the manufacturer's instructions. Briefly, after RNA was reverse-transcribed into complementary DNA (cDNA) by the 3′-RACE adapter primer in this kit, nested PCR was performed using primers P10F/P10R and P11F/P11R for the inner and outer PCRs, respectively. All primers are listed in Table 3 and synthesized by the Shanghai Songon Biological Engineering Biotechnology Company.
| Primer | Sequence (5′→3′) | Region |
|---|---|---|
| P1F | GCAATGCCACCCAAGAGAC | 52-1619 |
| P1R | ACTTGGAGGGCGGACAG | |
| P2F | GGCCGAGAATAGCATGA | 1518-2891 |
| P2R | GGGAGTATGAACGCCTAAAT | |
| P3F | TGCTGCTCGGCGATTACAAC | 2606-3883 |
| P3R | AATAGTCACAGGATGCCAGGTTC | |
| P4F | GGTTGGGTGGGGTTCTATACTTA | 3657-4870 |
| P4R | CCATATCTAGGTCAGCAATTCTTA | |
| P5F | TCTCTTCGGTGCAGAAGTCA | 4547-5697 |
| P5R | ATTTTGGCTTGCAGATTTATGTA | |
| P6F | CAGGACTGGGTGCACACT | 5427-7271 |
| P6R | CTAATCACCGAATCAGCATA | |
| P7F | AAGTCAAGGGCTGCAAATCTAGTC | 6925-8498 |
| P7R | AAGGCTAACCTGAACGACTGTAAG | |
| P8F | TACCCCACAGTCGATCCC | 8101-8788 |
| P8R | GAAGCCCAACCAACAGAG | |
| P9F | AGCTTACAGTCGTTCAGGTTAGCCTTAATGGAC | 8473-323 |
| P9R | GCCGGTTTAAGGCTGCCATCATA | |
| P10R | TACCGTCGTTCCACTAGTGATTT | |
| P11R | CGCGGATCCTCCACTAGTGATTTCACTATAGG |
- Abbreviations: BoDV-1, Borna disease virus 1; F, forward, R, reverse.
PCR reactions were performed using 2 μL of cDNA with high fidelity Pfu DNA polymerase in a 25 μL reaction mixture applied to a Corbett Research Rotor-Gene 6000 Thermocycler (Corbett Research, Australia). Cycling conditions consisted of an initial hold for 10 minutes at 94°C, followed by 35 amplification cycles at 94°C for 30 seconds, at 56 to 60°C for 30 seconds, and at 72°C for 2 to 4 minutes, with a final extension step at 72°C for 10 minutes. Each PCR was independently repeated at least three times. All PCR products were directly sequenced after purification or cloned into the pGM-T Easy vector system (Promega, USA) supplied by the Shanghai Songon Biological Engineering Biotechnology Company, using an ABI3730xl DNA Analyser (Macrogen Inc, Amsterdam, The Netherlands). For quality control of sequences, firstly, the PCR products were analyzed by 1.5% agarose gel electrophoresis. Only PCR products in the expected position, demonstrating one bright product band without any nonspecific amplification, were selected for sequencing (Figure 1). Secondly, PCR products of each virus strain were repeatedly analyzed, at least three times. Based on the Overlap-Layout-Consensus algorithm, DNAMAN 8.0 (Lynnon Biosoft, USA) was used for sequence assembly.
2.3 Sequence alignment and phylogenetic analysis
Before phylogenetic analysis, multiple sequence alignments were conducted using ClustalW based on progressive alignment. The highly variable regions in alignments did not contain gaps which were manually verified.
Considering what model of nucleotide evolution might be most appropriate for analyzing our data, several phylogenetic trees were created by the MEGA 6.0 program using both the neighbor-joining (NJ) and the maximum-likelihood (ML) methods with different algorithms, thereby employing 1000 replicates of bootstrap resampling analysis for each tree. The tree with the highest supporting bootstrap values was chosen. The trees were rooted with the sequence of BoDV-2 No/98 (AJ311524).
In accord with the full-length BoDV-1 sequences (8910 nucleotides each; N = 23), an overview on accession numbers at GenBank, original hosts of isolated viruses and hosts’ diseases, biological sources of sequences, and the corresponding references are provided in Table 4. Sequences of published strains/isolates were retrieved from the NCBI nucleotide database. Additional analysis of 28 sequences based on P-protein, and 31 sequences based on N-protein acquired from the GenBank database by using BLAST are shown in Supporting Information Figure S1 and Table S1; Figure S2 and Table S2, respectively.
| Number | Accession number, strain, name, host, source, country, and year | Host | Disease | Source | Sequence source | Isolation | Year | Sequence | Year sequence | Reference cited |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MT375542_isolate_Hu-H2_Human_PBMC_DE_1994_CN_2019 | Human | OCD | PBMC | OL cells human | Germany | 1994 | China | 2019 | This study |
| 2 | MT375543_isolate_DessVac_Horse_Brain_DE_1960_CN_2019 | Horse | BD fatal | Brain | Rabbit brain | Germany | 1960 | China | 2019 | This study |
| 3 | MT375544_isolate_Equ-Cres_Horse_PBMC_DE_1995_CN_2019 | Horse | BD nonfatal | PBMC | OL cells human | Germany | 1995 | China | 2019 | This study |
| 4 | U04608_Strain_V_Horse_Brain_DE_1927_US_1994 | Horse | BD fatal | Brain | OL cells human | Germany | 1927 | USA | 1994 | 26 |
| 5 | MK574679_isolate_BoDV1-ER2_human_brain_DE_2019 | Human | GB like encephalitis | Brain | Human brain | Germany | 2019 | Germany | 2019 | 8 |
| 6 | MH190827_strain_112-16_human_brain_DE_2018 | Human | Encephalitis | Brain | Human brain | Germany | 2018 | Germany | 2018 | 7 |
| 7 | LT991983_isolate_recipient1_0201213_human_brain_DE_2018 | Human | Encephalitis | Brain | Human brain | Germany | 2018 | Germany | 2018 | 6 |
| 8 | LT991982_isolate_recipient1_02465_human_kidney_DE_2018 | Human | Encephalitis | Kidney | Human kidney | Germany | 2018 | Germany | 2018 | 6 |
| 9 | LT991981_isolate_recipient2_02464_human_brain_DE_2018 | Human | Encephalomyelitis | Brain | Human brain | Germany | 2018 | Germany | 2018 | 6 |
| 10 | KY002072_strain_8072/15_horse_brain_AT_2015_AT_2017 | Horse | BD fatal | Brain | Horse brain | Austria | 2015 | Austria | 2017 | 28 |
| 11 | KY002071_strain_1407/15_horse_brain_AT_2015_AT_2017 | Horse | BD fatal | Brain | Horse brain | Austria | 2015 | Austria | 2017 | 28 |
| 12 | KY490041_strain_272/16_horse_brain_AT_2015_AT_2017 | Horse | BD fatal | Brain | Horse brain | Austria | 2015 | Austria | 2017 | 28 |
| 13 | KY490040_strain_271/16_horse_brain_AT_2015_AT_2017 | Horse | BD fatal | Brain | Horse brain | Austria | 2015 | Austria | 2017 | 28 |
| 14 | AB032031_Isolate_Hu-BV_Human_Brain_JP_1999_JP_1999 | Human | Brain | Human brain | Japan | 1999 | Japan | 1999 | Unpublished | |
| 15 | AB258389_Isolate_huP2br_Human_Brain_JP_2000_JP_2007 | Human | Schizophrenia | Brain | OL cells human | Japan | 2000 | Japan | 2007 | 25, 29 |
| 16 | AB246670_Isolate_Bo/04w_Cow_Brain_JP_2002_JP_2007 | Cow | Encephalomyelitis | Brain | C6 cells rat | Japan | 2002 | Japan | 2007 | 29 |
| 17 | AY114163_Strain_CRNP5_Horse_Brain_DE_1980_US_2002 | Horse | BD fatal | Brain | MDCK canine | Germany | 1980 | USA | 2002 | 31 |
| 18 | AY114162_Strain_CRP3B_Horse_Brain_DE1980_US_2002 | Horse | BD fatal | Brain | MDCK canine | Germany | 1980 | USA | 2002 | 31 |
| 19 | AY114161_Strain_CRP3A_Horse_Brain_DE_1980_US_2002 | Horse | BD fatal | Brain | MDCK canine | Germany | 1980 | USA | 2002 | 31 |
| 20 | AJ311523_Strain_H1766_Horse_Brain_DE_1994_DE_2001 | Horse | BD fatal | Brain | MDCK canine | Germany | 1994 | Germany | 2001 | 30 |
| 21 | AJ311522_Strain_He/80/FR_Horse_Brain_DE_1980_DE_2001 | Horse | BD fatal | Brain | OL cells human | Germany | 1980 | Germany | 2001 | 30 |
| 22 | AJ311521_Strain_V/FR_Horse_Brain_DE_1927_DE_2001 | Horse | BD fatal | Brain | OL cells human | Germany | 1927 | Germany | 2001 | 30 |
| 23 | L27077_Strain_C6BV_Horse_Brain_DE_1980_US_1994 | Horse | BD fatal | Brain | C6 cells rat | Germany | 1980 | USA | 1994 | 27 |
- Abbreviations: BD, Borna disease; GB, Guillain Barré; OCD, obsessive compulsive disorder; OL, oligodendroglia cell line; MDCK, Madin-Darby canine kidney; PBMC, peripheral blood mononuclear cell.
2.4 Accession numbers of new full-length genomes
The new full-length genome sequences provided in this study have been submitted to GenBank (National Center for Biotechnology Information, USA; NCBI).
The genomic sequence of Isolate_Hu-H2_DE_1994_CN_2019 is accessible through accession number MT375542. The genomic sequence of Isolate_DessVac_DE_1960_CN_2019 is accessible through accession number MT375543. The genomic sequence of Isolate_Equ-Cres_DE_1995_CN_2019 is accessible through accession number MT375544 at GenBank.
2.5 Cell cycle analysis
Cell cycle analysis was performed as described previously.40 In brief: strains Hu-H2, Equ-Cres, DessVac, and strain V propagated in OL cells as well as uninfected OL cells were cultured without serum for 24 hours to induce a G1 arrest. After addition of 5% serum to finish arrest, the cells were harvested over a 24-hour period. Then, OL cells (1 × 106 cells) were washed with cold phosphate-buffered saline (PBS), fixed in cold 70% ethanol at 4°C for at least 2 hours, and stained with 0.5 mL propidium iodide (50 µg propidium iodide/mL, 100 U of RNase A/mL, PBS) solution for 30 minutes in the dark. Measurement of DNA content and analysis of cell cycle phases was performed by flow cytometry (BD Biosciences, USA). Flow cytometric analysis for DNA content cannot distinguish between G2 and M phases. Therefore, the percentage of cells in the G1, S, and G2/M phases was determined at different time points after release from G1 arrest.
Cell cycle analysis was used to determine the proliferation index (PI) which is defined as the percentage of cells in the S-phase + G2/M-phase. The PI was applied to assess proliferation rates.
2.6 Cell apoptosis assay
Apoptosis of OL cells infected with strain Hu-H2, Equ-Cres, DessVac, and strain V, as well as of uninfected control cells was measured using an Annexin V-FITC Apoptosis Detection Kit (Beyotime, China), according to the manufacturer's instructions as described previously.40 Briefly, cells were washed twice with PBS and re-suspended with binding buffer at a concentration of 1 × 106/mL, followed by the addition of 5 μL Annexin V-FITC and 5 μL propidium iodide. After gentle mixing, the cells were incubated for 15 minutes at room temperature in the dark. The cells were analyzed by flow cytometry within 1 hour. Experiments were repeated in triplicate.
2.7 Statistical analysis
Data were expressed as the means ± standard deviations. SPSS 19.0 software was used to analyze the experimental data. The cells were compared using ANOVA. Two-tailed Student t tests were applied to identify significant differences at a 95% confidence level. P < .05 was deemed to be statistically significant.
3 RESULTS
3.1 Profile and taxonomical classification of BoDV-1 strains
This study not only aimed to provide new sequence and phylogenetic data but also to profile three unique BoDV-1 strains. Two of them, BoDV-1 Hu-H2 and BoDV-1 Equ-Cres were isolated from PBMCs by long-term cocultivation with further passaging in human OL cells. Isolate Hu-H2 came from a patient with obsessive compulsive disorder (OCD), located in Berlin, Germany,24 and isolate Equ-Cres from a horse with nonfatal Borna disease, located in Munich, Germany. These viruses belong to the very few which could have been retrieved from blood instead of brain cells. In contrast to these natural isolates, BoDV-1 DessVac, and strain V, originally isolated from brains of horses with fatal Borna disease (BD) underwent multiple in vivo passaging in rabbits to become either an adapted vaccine strain for horses, or a laboratory strain by further passaging in rats and finally in vitro in cell culture.42, 43 Strain V was the first laboratory strain to become completely sequenced26 and served taxonomically as a reference strain of the new family Bornaviridae.1 A detailed description of the history of isolate Hu-H2, isolate Equ-Cres, strain DessVac, and strain V41 is provided in Tables 1 and 2, respectively.
3.2 Full-length genomic sequences of BoDV-1 strains and phylogenetic analysis
Multiple sequence alignments of full genomes of Hu-H2, Equ-Cres, and DessVac compared with 20 other BoDV-1 strains confirmed strikingly low divergence levels not exceeding a maximum of 5.55%.
Phylogenetic analysis revealed that human isolate Hu-H2 and horse isolate Equ-Cres were more distant from DessVac, but could be assigned to the strain V cluster. Other clusters could be assigned to the laboratory strain He/80 and USA derivatives of He/80, Japanese strains, Austrian horse strains, and German encephalitis cases (Figure 2).

As for human-derived isolates, the Hu-H2 genome isolated in 1994 from blood cells of a German OCD patient,24 displayed the largest distance to BoDV-1 sequences retrieved from German encephalitis cases of transplant recipients reported in 20186 (5.20%). Much lower levels were found compared to hP2br, isolated in 2000 from the brain of a Japanese schizophrenic patient25, 29 (1.89%), as well as compared to the two other German encephalitis cases (112-6 and ER2)7, 8 reported in 2018/2019 (1.88% and 2.31%), respectively.
Isolate Equ-Cres, originating from PBMCs of a Bavarian horse with nonfatal BD in 1995, displaying a very low divergence to Hu-H2, could also be assigned to the strain V cluster. Compared with other natural horse-derived strains like recent Austrian strains originating from the brain (8072/15, 1407/15, 272/16, 271/16),28 moderate divergence levels (between 2.18% and 2.28%) were found. The vaccine strain DessVac from 1960 displayed the largest genetic distance (5.22%) to the laboratory strain He/80 (C6BV and related derivatives)27, 30, 31 and a moderate distance (1.63%) to laboratory strain V26, 30 originating from 1927.43 A detailed divergence matrix is provided in Supporting Information Table S3.
3.3 Phylogenetic analysis based on nucleotide sequences of major BoDV-1 proteins
In contrast to full-length genomes, a higher number of P-gene and N-gene sequences are available, many of which originating from human patients differing by source, time of isolation, and country. Their phylogenetic analyses elicited valuable data in addition to complete genomes, as evaluated below. The corresponding figures and tables can be found in the Supporting Information, as detailed below.
3.3.1 Full BDV P-gene sequences
Twenty-five complete P-gene sequences (603 bp) available at GenBank and the new sequences from human isolate Hu-H2, equine isolate Equ-Cres, and laboratory vaccine strain DessVac, were phylogenetically compared (see Supporting Information Figure S1 and Table S1). We found a maximum genetic distance of 5.34%, which is close to that of full genomes. As for human viruses, an Australian, Japanese, and German cluster could be identified. The largest divergence within human viruses at the P-gene level (5.17%) was detected between the Australian major depressive disorder (MDD) patient DP87 and the Austrian chronic fatigue syndrome (CFS) patient.32 The new Hu-H2 sequence displayed a lower divergence to German encephalitis isolate ER28 (1.34%), the Japanese schizophrenic patient (huP2br)25, 29 (1.51%), and the Australian MDD patient (DP87) (1.68%) than to the Austrian CFS patient32 (3.4%) and the encephalitis cases of transplant recipients6 (2.71%). Thus, for the latter, the distance to Hu-H2 based on the P-gene was considerably lower than for full genomes.
Within a country and for natural equine viruses, a divergence level of 3% was found between isolate Equ-Cres originating from PBMCs (1995, nonfatal BD, Southern Germany) and WT-1 originating from the brain (1991, fatal BD, East Germany).36 Notably, a similar level (3.4%) was found for different batches of the nonnatural vaccine strain DessVac by comparing the here provided sequence of the 1960 batch with that of 1990.33 Moreover, the P-genes of those batches even belonged to different clusters.
As for all evaluated laboratory strains, clusters of He/80 (C6BV), strain V, and strain DessVac were identified. The new DessVac sequence displayed divergence levels between 2.71% and 2.88% compared to natural Australian viruses,34 of 1.17% to isolates Hu-H2 and Equ-Cres, of 1.34% to nonnatural strain V,26 and 2.19% divergence to the nonnatural strain He/80 (C6BV).27 To the latter, the distance at P-gene level was less than that based on full genomes.
3.3.2 Partial BDV N-gene sequences
Twenty-eight partial N-gene sequences (439 base pairs; nucleotides 279-717) available at GenBank and the new sequences from human isolate Hu-H2, equine isolate Equ-Cres, and the laboratory vaccine strain DessVac were phylogenetically compared (see Supporting Information Figure S2 and Table S2). We found a maximum genetic distance of 4.94%, a bit lower than revealed at the full genome and P-gene level. Notably, as for human viruses, different clusters could not only be identified for different countries (USA, Japan, Germany) but also within a country, four of which were in Germany. The US strains isolated from hippocampus sclerosis autopsies38, 39 were identified within the He/80 cluster, whereas isolate Hu-H2 was found in the strain V cluster, as expected. The encephalitis cases from transplant recipients6 clustered differently from those unrelated to transplantation.7, 8 Interestingly, N-sequences from the first German psychiatric patients37 (X84689, X84690, X84691) were found in three different N-gene clusters, only one of which (patient 1, OCD, X84691) in the strain V cluster. Except for patient 1 whose 10 months later retrieved PBMC sample (H2-3) had led to isolate Hu-H2,24 the two other patients were unrelated to those of whom isolates could be retrieved24 but all came from the same city.
Compared to all human-derived sequences, the new Hu-H2 sequence displayed the largest distance to patient 3 suffering from severe recurrent major depressive disorder (MDD) (X84689)37 (3.74%). Similar divergence levels were identified to a US hippocampus derived strain (AJ246857)38, 39 and to the recent German transplant recipient encephalitis cases6 (3.5%). Much lower levels (1.15% to 1.38%) were found between Hu-H2 and the other recent encephalitis cases,7, 8 as well as to the Japanese brain isolate (huP2br) from a schizophrenic patient25, 29 (1.61%).
As for natural German horse viruses, the new sequence of PBMC strain Equ-Cres displayed a higher divergence to WT-136 (4.22%) than found at the P-gene level. Likewise, the same was found for the different batches of the nonnatural strain DessVac (4.7% vs 3.4%). The new DessVac also formed a cluster different from that of strain V,26, 30 but at a much lower distance (1.61%). As for the He/80 cluster,27, 30, 31 the distance of DessVac at N-gene level reached 4.22% and thus was higher than at the P-gene level (2.19%) but lower than at the complete genome level (5.22%).
Focusing on human strains/sequences, we finally compared isolate Hu-H2 on the level of the complete genome, partial N-gene, and P-gene sequences with 22 human strains/sequences and four laboratory strains. Sequence data encompassed 25 years (1994-2019) originating from five countries and displayed nucleotide identities between 95% and 100%, which appeared unrelated to time and location (Table 5).
| Nucleotide identity % with MT375542_human isolate Hu-H2 | |||
|---|---|---|---|
| Accession number and description | Complete genome | N -gene (439 bp) | P-gene |
| MK574679_isolate_BoDV1-ER2_human_brain_DE_2019 | 97,70 | 98,60 | 98,70 |
| MH190827_strain_112-16_human_brain_DE_2018 | 98,20 | 98,90 | 98,50 |
| LT991983_isolate_recipient1_0201213_human_brain_DE_2018 | 95,00 | 96,50 | 97,30 |
| LT991982_isolate_recipient1_02465_human_kidney_DE_2018 | 95,00 | 96,50 | 97,30 |
| LT991981_isolate_recipient2_02464_human_brain_DE_2018 | 95,00 | 96,50 | 97,30 |
| AB032031_Isolate_Hu-BV_Human_Brain_JP_1999_JP_1999 | 98,10 | 98,40 | 98,30 |
| AB258389_Isolate_huP2br_Human_Brain_JP_2000_JP_2007 | 98,10 | 98,40 | 98,50 |
| AJ246850_clone_4720_human_brain_US_1996_DE_1999 | 96,70 | ||
| AJ246853_clone_4795_human_brain_US_1996_DE_1999 | 97,00 | ||
| AJ246855_clone_4796#1_human_brain_US_1996_DE_1999 | 96,00 | ||
| AJ246857_clone_4796#3_human_brain_US_1996_DE_1999 | 96,50 | ||
| X84689_Amplificate_Patient_3_Human_PBMC_DE_1993_DE_1995 | 96,30 | ||
| X84690_Amplificate_Patient_2_Human_PBMC_DE_1993_DE_1995 | 98,40 | ||
| X84691_Amplificate_Patient_1_Human_PBMC_DE_1993_DE_1995 | 99,80 | ||
| U58594_Isolate_Hu-H1_Human_PBMC_DE_1994_DE_1996 | 99,50 | ||
| U58595_Isolate_Hu-H2_Human_PBMC_DE_1994_DE_1996 | 99,30 | ||
| U58596_Isolate_Hu-H3_Human_PBMC_DE_1994_DE_1996 | 100,00 | ||
| AY686714_Amplificate_DP87_Human_Plasma_AU_2004_AU_2004 | 98,30 | ||
| L76234_Isolate_Hu-H1_Human_PBMC_DE_1994_USA_1996 | 99,30 | ||
| L76235_Isolate_Hu-H2_Human_PBMC_DE_1994_USA_1996 | 99,70 | ||
| L76236_Isolate_HuH3_Human_PBMC_DE_1994_USA_1996 | 99,30 | ||
| AF094478_Amplificate_CFS_Human_PBMC_AT_1998_AT_1998 | 96,60 | ||
| L27077_Strain_C6BV_Horse_Brain_DE_1980_US_1994 | 94,90 | 96,70 | 97,30 |
| U04608_Strain_V_Horse_Brain_DE_1927_US_1994 | 99,90 | 99,70 | 99,80 |
| MT375543_Isolate_DessVac_Horse_Brain_DE_1960_CN_2019 | 98,40 | 98,20 | 98,80 |
| AY374519_Strain_Dessau_Horse_Brain_DE_AT_1990_AT_2003 | 95,80 | 96,40 | |
- The last four lines represent sequences of laboratory strains.
- Abbreviations: BoDV-1, Borna disease virus 1; CFS, chronic fatigue syndrome; PBMC, peripheral blood mononuclear cells.
3.4 Effects of BoDV-1 strains on OL cell proliferation
To address the different effects of BoDV-1 strains on OL cell proliferation, we measured cell cycles by flow cytometry. In the cell cycle, the primary phases of cell proliferation are the S and G2 phases. We determined the cell proliferation index (PI), defined as the percentage of cells in the S-phase + G2/M-phase. Thus, cell proliferation rates could be assessed by estimating the relative cell numbers in the S + G2/M-phase fraction.
The percentages of S + G2/M-phase cells in natural viruses, namely OL/Hu-H2 and OL/Equ-Cres, were not significantly different from each other (t = 0.840, P = .448), but were significantly lower than those found in the nonnatural laboratory viruses OL/strain V and OL/DessVac, as well as in uninfected control OL cells (P > .05). In addition, the proliferation rate of laboratory OL/strain V was comparable to that of laboratory OL/strain DessVac (t = 0.261, P = .807) (Figure 3A).
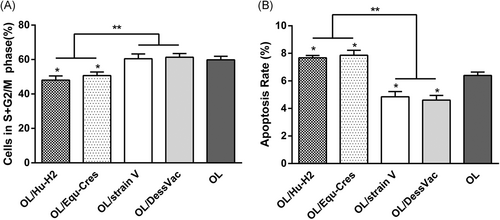
3.5 Effects of BoDV-1 strains on OL cell apoptosis
Next, we assessed the effects of BoDV-1 strains on OL cell apoptosis by using Annexin V-FITC staining. The apoptosis rates of OL/Hu-H2 and OL/Equ-Cres were significantly increased compared to uninfected OL cells (t = 4.220, P = .013; t = 3.317, P = .029, respectively). In contrast, significantly decreased apoptosis rates of OL/strain V and OL/DessVac were measured compared to that of uninfected OL cells (t = −3.399, P = .027; t = −4.229, P = .013, respectively). Furthermore, the apoptosis rates did not significantly differ between either natural viruses Hu-H2 and Equ-Cres (t = 0.456, P = .672) or laboratory strains V and DessVac (t = 0.485, P = .653) (Figure 3B). Moreover, we could show here that these opposite biological signatures were independent of the strains’ host background.
4 DISCUSSION
The family Bornaviridae has recently been re-classified by the International Committee on Taxonomy of Viruses (ICTV) based on known genomic and protein sequences, and biological characteristics.1 According to the new taxonomy, the genus Bornavirus now includes 8 species and 16 viruses infecting vertebrate hosts (reptiles, birds, and mammals). However, there is only one globally present virus, BoDV-1, prototype of the dominant Mammalian 1 bornavirus species, infecting humans and a broad variety of mammals including farm animals and pets. Unlike the other highly divergent bornavirus species, BoDV-1 viruses are genetically closely related (>95% homology). Recently, BoDV-1 was identified as the cause of deadly human encephalitis after organ transplantation, which markedly increased the significance of this infection in terms of human health and disease.6 Moreover, a current study retrospectively identified further rare human fatalities assigned to BoDV-1 infection, adding up to 14 in 20 years (1999 to 2019).10 However, until today, only a limited number of full-length genomic sequences of Mammalian 1 bornavirus strains are available at GenBank, the majority belonging to highly adapted laboratory strains, namely str. V and He/80 and derivatives.26, 27, 30, 31
This study first of all added entire genome sequences of two natural blood cell derived isolates of human and horse origin (Hu-H2 and Equ-Cres) and a laboratory-adapted vaccine strain (DessVac). Secondly, the study analyzed clustering in phylogenetic trees, focusing on full genomes of human strains from different countries, time points and clinical background. Thirdly, phylogenetic analyses compared human, equine and laboratory strains, including also N-gene and P-gene sequences, and whether they impacted infection epidemiology. Totally, 20 complete and 20 partial sequences were retrieved from GenBank to phylogenetically compare them to the three new sequences. Fourthly, the study addressed BoDV-1 phenotypes by analyzing how natural and nonnatural (laboratory) virus strains impacted host cell proliferation and apoptosis.
4.1 New full-length genomes
Full-length genomic sequencing of isolates Hu-H2 and Equ-Cres, and strain DessVac confirmed that they are authentic BoDV-1 viruses, displaying similarly high levels of sequence conservation (≥95%) characteristic of BoDV-1 viruses.6-8, 25-31
4.2 Phylogenetic trees’ analyses
Phylogenetic trees altogether revealed a maximum divergence of 5.55%, 5.34%, and 4.94% at the genome, P-gene, and N-gene levels, respectively. Notably, the genetic distance could be at the same level between different batches of the same strain (DessVac, 1960 and 1990) as between different strains, shown for the P-gene.
As for human viruses, apart from the level of divergence, phylogenetic trees suggested clustering trends by country and/or country region rather than by clinical diagnosis or year of isolation. At the genome or single-gene level, clusters could be assigned to countries (Japan, USA, Austria, Australia, and Germany) and German regions. In contrast, different diseases of patients (MDD, BP, OCD, CFS, and encephalitis) or time of isolation (1993 to 2019) turned out to be unrelated to genetic differences of virus isolates/strains.
4.3 BoDV-1 epidemiology
What our phylogenetic analyses suggested controverts the currently promoted hypothesis. The bottom line of the latter boils down to three points: (a) human disease is always fatal, (b) BoDV-1 infection is mainly restricted to single endemic clusters in Germany, and (c) transmission occurs through a reservoir animal, the bi-colored white-toothed shrew.10 In contrast and apart from global healthy carriers and psychiatric patients independently arguing against zoonosis,3, 17-22 this study here suggested that sequence similarities alone are likely inappropriate to establish circumscribed endemic areas, given the high genetic conservation of BoDV-1 (Table 5).
Regional clustering were determined earlier for animal viruses and shrews.11, 33 However, independently evaluated phylogenetic trees assigning Japanese macaque #33 strain44 to other GenBank sequences (N- and P-gene), revealed that the degree of homology was not only independent of host species and time of sampling, but also unrelated to countries.45
4.4 Overlapping genetic signatures
Furthermore, phylogenetic analysis unraveled that instead of distinct host-specific genotypes, human and animal BoDV-1 strains were differing by highly conserved individual genetic signatures. Single varying mutations were shared with other strains from either human or animal origin, but each signature's profile was unique. The view of characteristic signatures of human isolates assumed earlier24, 35 was supported by this study.
With respect to animal natural and nonnatural strains, we found a coincidental distribution among different human strains at more or less separate branches on either the P- or N-gene level. German psychiatric human and equine strains were more close to strain V than Australian viruses. Notably, partial sequencing of German isolates24 were done in an expert laboratory in the United States, which never dealt with strain V.35 At the N-gene level, the human encephalitis cases (transplant recipients) were more close to the American brain autopsy sequences, and at the P-gene level, also more close to those and the Austrian CFS patient as well as to laboratory strain He/80. Japanese human and bovine isolates were more close to strain H1766, another horse-derived laboratory strain30 than to DessVac (batch in 1960). Japanese isolates25 were, however, sequenced in collaboration with the above expert laboratory in the United States, which had sequenced laboratory strain C6BV (He/80)27 but not strain H1766. Thus, at various sequence levels, this study could rule out previous46 and currently repeated misconception10 which claimed that human isolates24, 25 are contaminants.
4.5 Phenotypes of BoDV-1 strains
Earlier findings had revealed that even very low genetic variability below 0.05% could be associated with significant differences in disease phenotype. He/80 variants differing by only two mutations each in the glycoprotein and the L polymerase induced a more severe neurologic disease in Lewis rats than nonmutated strains.31
Recently, our team could show that natural human strain Hu-H1 and nonnatural strain V were differently impacting host cell reproduction40 and metabolic signatures.41 This study finally indicated that different phenotypes are universally applicable to any natural vs nonnatural strain. We found significantly decreased proliferation and enhanced apoptosis by two other natural strains of either human or equine origin (Hu-H2 and Equ-Cres), respectively, compared to the opposite effects by nonnatural (laboratory) strains V and DessVac. Opposite phenotypes could clearly distinguish between natural and laboratory strains which are otherwise genetically closely related, and thus confirm their authenticity. Given the still existing contamination debate,10 this is an important finding. Nevertheless, the question of what mechanisms may cause these differences, remained unsolved. At least, the opposite phenotypes appeared to be dependent upon significantly different levels of virus strain adaptation, namely different manipulation of cell metabolism, rather than to be influenced by different host species. According to a previous study of our group, human strain Hu-H1 was shown to promote cell apoptosis via upregulating expression levels of proapoptotic protein Bax and downregulating antiapoptotic Bcl-2 protein, whereas laboratory str. V did the opposite, as shown by Western blot analysis.40 Another remarkable difference concerned the sensitivity to antiviral drugs. Although the in vitroreplication of human strain Hu-H1 could be inhibited by amantadine sulfate in a dose-dependent manner (0.2 to 1.2 µg/mL), no effects were observed using laboratory str. V.47 Notably, the high sensitivity of human BoDV-1 strains to amantadine held also true in infected depressed patients who benefitted significantly from low dose well-tolerated amantadine treatment vs placebo.47
5 CONCLUSION
In conclusion, comprehensive phylogenetic analyses revealed that human viruses are distinct strains within the species Mammalian 1 bornavirus, displaying similarly high levels of sequence conservation as animal viruses. They differed from animal viruses by individual genetic signatures, but could share varying single mutations. Sequence similarities could thus occur between viruses from different host species, geographic regions, and time of isolation, thereby supporting the global prevalence of BoDV-1. Human viruses tended to vary at country level and appeared genetically to be independent of hosts’ diseases. Overall low genetic divergence among the BoDV-1 viruses shown here also questioned zoonotic transmission as the main source of human infection. At least, conflicting epidemiological concepts will need further clarification beyond sequence similarities. Finally, despite low genetic divergence, opposite biological phenotypes between natural virus isolates and nonnatural laboratory strains were confirmed.
ACKNOWLEDGMENTS
We are particularly grateful to Professor Hanns Ludwig, Free University of Berlin, Germany, for kindly providing human OL cells infected with BoDV-1 strains Hu-H2, Equ-Cres, and DessVac used in this study. This study was supported by the National Key Research and Development Program of China (Grant No. YFA0505700), the Natural Science Foundation of China (Grant No. 81601207), and the Chongqing Basic and Frontier Research Project (Cstc2016jcyjA0159).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Yujie Guo, Peng He, Liv Bode, and Peng Xie were responsible for the conception of the study, data analysis, and data interpretation. Lin Sun, Xiong Zhang, Xiaoyan Xu, Tian Tang, Wei Zhou, Qi Li, and Dezhi Zou performed experiments, contributed to data acquisition, phylogenetic trees, and sequence analysis. Yujie Guo and Liv Bode wrote the final manuscript. All authors read and approved the final version of the manuscript.




