Comparing the risk of hypothyroidism in HCV patients treated with different DAA drugs combinations (sofosbuvir + interferon + ribavirin and sofosbuvir + daclatasvir + ribavirin)
All authors contributed equally to this study.
Abstract
The recent development of direct-acting antiviral (DAA) drugs has revolutionized the area of hepatitis C virus (HCV) therapeutics but the efficacy and clinical outcome of interferon (IFN)-free therapy have not been extensively studied yet. We observed a dramatic increase in hypothyroidism among patients treated with sofosbuvir, IFN, and ribavirin. This is the first prospective study of the thyroid dysfunction in DAA drugs treated patients. This study compared the risk of hypothyroidism in two different groups of HCV patients treated with different DAA drugs regimens that were sofosbuvir + pegylated-IFN-α + ribavirin and sofosbuvir + daclatasvir + ribavirin. Our findings highlight the periodic screening of serum thyroid-stimulating hormone and T4 levels in HCV infected patients during the treatment and posttreatment.
Highlights
-
A mathematical model with threshold behavior in recovery and asymptomatic patients.
-
The efficacy and clinical outcome of interferon-free therapy have not been extensively studied yet.
-
Existing literature suggest that thyroid dysfunction is the most common endocrine disorder that occurs in HCV infected patients and interferon therapy has been associated with an increase in thyroid disorders.
-
Our findings highlight the need of periodic screening of serum TSH and T4levels in HCV infected patients treated with direct-acting antiviral drugs.
-
Furthermore, clinicians must recommend sofosbuvir, ribavirin, and daclatasvir instead of sofosbuvir, peg-IFN-a, and ribavirin for genotype 1 to avoid this increasing risk of hypothyroidism.
1 INTRODUCTION
Hepatitis C virus (HCV) is the major public health challenge in Pakistan and the prevalence is constantly increasing because of lack of awareness, unsafe medical practices, and community exposures such as barbershops, beauty salons, unqualified medical professionals, and traditional healers. Although the new oral medications against HCV are used in Pakistan a major challenge remains in optimization and risks associated with different treatment regimens.1 HCV has also been reported to cause extrahepatic manifestations (autoimmune disorders or malignant tumors). Autoimmune diseases are triggered by the loss of tolerance especially, autoimmune thyroid disorders, with T lymphocytes (cellular or humoral response) or autoantibodies reacting with self-antigens.2 Existing literature suggest that thyroid dysfunction is the most common endocrine disorder that occurs in HCV infected patients and interferon (IFN) therapy has been associated with an increase in thyroid disorders.3-6 This is the first-ever evidence to report thyroid dysfunction in two different groups of chronic hepatitis C (CHC) patients treated with two different direct-acting antiviral (DAA) drug combinations that are sofosbuvir + ribavirin + pegylated-IFN-α and sofosbuvir + ribavirin + daclatasvir.
2 METHODOLOGY
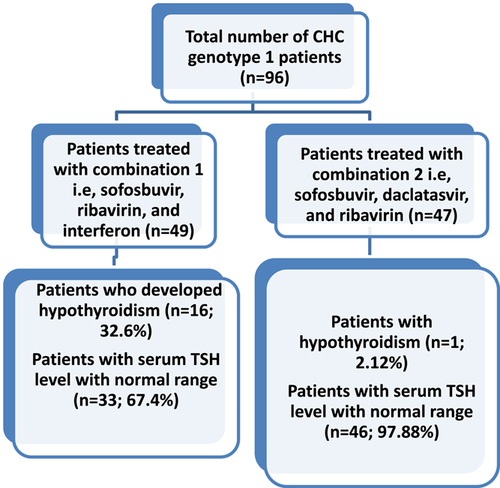
This study is multicentre clinical study conducted at the Genome Center For Molecular Based Diagnostics and Research, Pakistan. Total 96 patients with CHC were recruited from January 2016 to November 2017. All untreated patients and IFN treated patients were excluded. Only, oral DAA drug-treated patients were included in the study. Of total 96 patients, 49 patients were treated with sofosbuvir (400 mg once daily), ribavirin (600 mg daily), pegylated-IFN-α (180 μg weekly) whereas, 47 patients received sofosbuvir (400 mg once daily), ribavirin (600 mg daily), and daclatasvir (60 mg once daily). The diagnosis of HCV was based on advanced technology, that is, the Cepheid Xpert HCV Viral Load assay. In addition to this, hematological and routine biochemical tests were measured before and after treatment. Serum thyroid-stimulating hormone (TSH) and T4 levels was measured on state-of-the-art VIDAS BIOMERIEUX System using kits from bioMerieux France.
For statistical analysis, the Student t test was performed using IBM SPSS Version 25 (SPSS Inc, Chicago, IL) and P value less than .05 was considered significant.
3 RESULTS AND DISCUSSION
The mean age of patients was 43 years (range 17-64) and the overall prevalence of HCV was found higher in males (n = 57; 59.37%) as compared with females (n = 39; 40.62%) (Table 1). Our study published in Journal of Medical Virology has already confirmed the risk of hypothyroidism in DAA drug patients; however, the association of thyroid dysfunction with specific combinations of DAA drugs were not discussed in that study.7
| Combination 1 (sofosbuvir + ribavirin + interferon) | Combination 2 (sofosbuvir + daclatasvir + ribavirin) | |||||
|---|---|---|---|---|---|---|
| Parameter | Pretreatment | Posttreatment | Pretreatment | Posttreatment | P value | Normal range |
| AST | 45 | 34 | 39 | 24 | >.05 | 10-35 U/L |
| ALP | 127 | 119 | 212 | 168 | >.015 | 42-390 U/L |
| ALT | 44 | 36 | 45 | 37 | >.05 | 10-45 U/L |
| AFP | 4.5 | 5.7 | 4.9 | 6.1 | >.05 | <10 ng/ml |
| TSH | 1.12 | 5.6 | 2.1 | 2.7 | .025 | 0.25-5 µIU/ml |
| Free-thyroxine T4 | 0.95 | 0.38 | 0.96 | 1.1 | NS | 0.7-1.8 ng/dL |
| RBCs | 5.11 | 4.56 | 5.12 | 4.78 | >.05 | 3.50-5.50 mil/cm |
| Platelets | 187 | 230 | 210 | 160 | >.05 | 150-450 × 109/L |
| WBCs | 8.5 | 7.8 | 7.1 | 7.5 | >.05 | 4.00-11.00 × 109/L |
| Hb | 14.1 | 12.4 | 12.6 | 12.1 | >.05 | 13.5-17.5 g/d |
- Note: These two bold tests form the diagnostic basis of hypothyroidism.
- Abbreviations: AFP, α-fetoprotein; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Hb, hemoglobin; NS, not significant; RBCs, red blood cells; WBCs, white blood cells; TSH, thyroid-stimulating hormone.
In many public sector hospitals of Pakistan, a combination of sofosbuvir, peg-IFN-α, and ribavirin is prescribed to patients infected with HCV genotype 1 because of nonavailability and expensive cost of daclatasvir.8 Herein, we observe a dramatic increase in thyroid dysfunction among patients treated with sofosbuvir, ribavirin, and IFN. We observed that 16 (32.6%) of patients treated with sofosbuvir, ribavirin, and IFN had a very high TSH level whereas, serum TSH level of 97.6% of patients treated with daclatasvir, sofosbuvir, and ribavirin was within the normal range (Figure 1). It is necessary to mention that patients denied any family history of thyroid dysfunctions.
Accumulating evidence suggests that patients receiving IFN therapy experience thyroid dysfunctions because IFN induces direct inhibitory effects on thyroid hormone synthesis, release, and metabolism. According to recently published case series, two of three patients developed silent thyroiditis after experiencing hypothyroidism.8-10
The small sample size is the practical limitation of this study, further studies should be conducted on large sample size.
4 CONCLUSION
This study highlights the risk of hypothyroidism in DAA drugs treated patients. Our findings highlight the need for periodic screening of serum TSH and T4 levels in HCV infected patients during the treatment and posttreatment. Furthermore, clinicians must recommend sofosbuvir, ribavirin, and daclatasvir instead of sofosbuvir, peg-IFN-α, and ribavirin for genotype 1 to avoid this increasing risk of hypothyroidism because such associated complications may alter the treatment outcome with DAA drugs and worsen the condition of the patient.
Developing countries like Pakistan face substantial barriers in the provision of high-quality drugs furthermore, clinical and healthcare professionals should prescribe the treatment regimens in accordance with national and international guidelines. Researchers need to take proactive steps to optimize different treatment regimens and figure out the most efficacious, safe, and well-tolerated sofosbuvir-based treatment scheme.
ACKNOWLEDGMENT
The authors especially thank Genome Center for Molecular Based Diagnostics and Research, Pakistan.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
BW conceived the idea and wrote the manuscript. KS, SG, and SG planned the experiments. MI analyzed data and wrote the manuscript.
ETHICS STATEMENT
The ethical committee of the institution approved this study. Informed consent was obtained from patients.




