CD4/CD8 ratio, comorbidities, and aging in treated HIV infected individuals on viral suppression
Abstract
The progression of the human immunodeficiency virus (HIV) infection to acquired immunodeficiency syndrome (AIDS) can be efficiently interrupted by antiretroviral therapy (ART). However, even successfully treated HIV-infected individuals are prone to develop non-AIDS-related diseases that affect the metabolism and several organs and systems. Biomarkers that predict the occurrence of comorbidities may help develop preventive measures. Current research shows that CD4+ T cell counts and viral load do not predict the development of non-AIDS-related diseases. The CD4/CD8 ratio has been indicated as a suitable marker of persistent immune dysfunction and the occurrence of non-AIDS-related events in treated HIV-positive patients. In this study, we explored the relationship between CD4/CD8 ratios, comorbidities, and aging in ART-treated HIV patients on viral suppression. We collected and evaluated data from 352 HIV-positive adults who were virologically suppressed (<40 copies/mL) on ART and with CD4 counts above 350 cells/mm3. The median age for participants was 46 years, 218 individuals had at least one comorbidity, and 239 had inverted CD4/CD8 ratios (<1). Current CD4/CD8 ratios were predicted by baseline CD4/CD8 ratios and nadir CD4 counts. Despite the high rates of inverted CD4/CD8 ratios and prevalence of comorbidities, no association between them was observed. The prevalence of comorbidities was significantly higher in older individuals, though aging alone did not explain the rate of all individual comorbidities. Low CD4/CD8 ratios were linked to neurocognitive disorders, suggesting that persistent T cell dysfunction contributes to neurocognitive decline.
Highlights
-
Low CD4/CD8 ratios were associated with neurocognitive disorders in HIV infection.
-
CD4/CD8 ratios were not associated with age in HIV infection.
-
Aging and extremely low CD4/CD8 ratios were associated with the presence of comorbidities in HIV infection.
-
Older HIV-infected individuals are nearly three times more prone to develop comorbidities.
-
The chance of having multimorbidity is nearly twice as high for older HIV-infected individuals.
1 INTRODUCTION
The natural course of infection by the human immunodeficiency virus (HIV) culminates in the acquired immunodeficiency syndrome (AIDS) and subsequent death by opportunistic infections, neoplasias, and other life-threatening AIDS-related diseases.1-3 By infecting immune cells and using their apparatus to replicate and thrive, HIV deeply compromises the immune system. Once the infection is diagnosed, progression to severe immunosuppression can be routinely interrupted by antiretroviral therapy (ART). Increased globalization and access to ART, allied to continuing advances in the efficacy and tolerability of anti-retroviral drugs, has turned HIV infection into a manageable, although so far incurable, chronic disease.4
The chronicity of HIV infection has not come without challenges. ART has approximated the life expectancy of HIV-positive individuals to that of uninfected individuals, increasing the number of older people living with HIV worldwide.5 Currently, the increased incidence of non-AIDS-related diseases is a reason for concern in the management of older HIV-positive patients. Recently, it has been acknowledged that HIV-infected individuals are more prone to develop inflammatory diseases that directly affect many organs, metabolic cellular pathways and the circulatory and nervous systems.6 Moreover, HIV infection appears to promote the development of such comorbidities earlier in life for those living with HIV.7
Despite preventing progression to AIDS, extending the life span, and improving the life quality of HIV infected individuals, ART does not prevent non-AIDS-related conditions.8 In HIV infected individuals, chronic inflammation and immune activation persist, and one explanation could be residual viral replication in cryptic or immune-privileged sites.9 The theory that HIV infection accelerates aging is based on a series of biological events affecting both HIV infected and noninfected elderly individuals.10-12 Persistent immune activation, chronic low-grade inflammation, T cell dysfunction, and the fact that many non-AIDS related diseases are usually age-related contribute to this theory.7, 12, 13
Biomarkers that predict these comorbidities are important to the development and practice of preventive measures. Although viral load and CD4+ T cell counts predict disease progression and ART success, the CD4/CD8 ratio has been recently indicated as a reliable marker of T cell dysfunction. In many cases, the CD4/CD8 ratio remains reduced upon virological control and CD4 normalization, indicating that circulating CD8+ T cell counts continue elevated.18, 20, 21
Naïve CD8+ T cells play an important role in the immune response against viral infections, and their clonal expansion favors the increase in the numbers of memory CD8+ T cells.14 CD8+ T cell counts rise as they respond both to the virus and the CD4+ T cell depletion in the gut15, 16 undergoing clonal expansion followed by apoptosis or senescence.17 Many CD8+ T cells persist in a senescent state characterized by phenotypical changes, including loss of CD28 expression and release of proinflammatory mediators. The accumulation of CD8+ T cells with such a phenotype resembles the process of normal aging.18 This expansion of the CD8+ T cell subset is suggestive of poor immune recovery,19 as well as lower CD4/CD8 ratios.17, 20 Also, it has been linked to an increased risk of multimorbidity and serious non-AIDS related diseases.7, 21, 22
Here, we explored the relationship between CD4/CD8 ratios, non-AIDS-related comorbidities, and aging in a population of treated HIV patients on viral suppression.
2 METHODS
We conducted a retrospective case-control study using information collected from patients’ charts from the Archive department at Hospital Universitário de Santa Maria (HUSM). Ethics Committee approval was granted by the Universidade de Santa Maria (UFSM) (protocol #65777517.0.0000.5346).
2.1 Study population
The sampling frame was a 6-month list of patients with viral load and CD4+ and CD8+ T cell counts performed at the HUSM Clinical Analyses Laboratory.
2.2 Inclusion criteria
The inclusion criteria were age over 18 years, ART use for 2 years or more, absence of active coinfection and AIDS-related symptoms, vertical transmission, undetectable viral loads (<40 copies/mL) for at least a year, and CD4+ T counts above 350 cells/mm3. Patients with AIDS-defining diseases or those with complications resulting from opportunistic infections or ART toxicity were excluded from the study.
2.3 Data collection
Data such as age, sex, diagnosis, ART commencement, current regimen, viral suppression, and time with CD4/CD8 inversion were collected and recorded in the database. Baseline CD4/CD8 ratios and nadir CD4 counts were documented when available on the charts. The last available CD4+ and CD8+ T cell counts and the CD4/CD8 ratios were recorded. The baseline and current CD4/CD8 ratios and the nadir CD4 counts were analyzed as continuous and categorical variables. Nadir counts and baseline CD4/CD8 ratios were examined as smaller than the first quartile, smaller than the median, and below the third quartile. Baseline CD4/CD8 ratios below 1 (inverted) were also considered. Current CD4/CD8 ratios were evaluated as below the first quartile, lower than the median, and smaller than 1 (inverted).
2.4 Comorbidities
Comorbidities included non-AIDS-defining diseases occurring after HIV diagnosis. Multimorbidity was defined as more than one comorbidity.
2.5 Statistical analysis
Data were expressed in numbers, percentage, mean ± standard error (SE), median (±interquartile range [IQR]), and odds ratios (confidence intervals of 95%). The means between the different groups were compared using the Mann-Whitney test. Fischer χ2 was applied to investigate associations between categorical variables (minimum of five observations per cell).
Exploratory regression models were performed to estimate the collective effect of categorical and continuous dependent variables on an independent variable. The final models included variables identified as theoretically relevant to this and previous studies and had significant results on the univariate analysis. Sex and age were included as dependent variables in all models. A minimum of five observations per cell was required for the entrance of categorical variables in the models. All regression models met the assumptions of interaction, collinearity, number of outliers, normality of residuals, and statistical significance (Durbin-Watson for linear regression and Wald test for logistic regression).
P values smaller than 0.05 were considered statistically significant. The analyses were made using IBM®SSP v26.
3 RESULTS
3.1 Sex and age of participants
Three hundred and fifty-two patients participated in this study. The age of participants ranged from 21 to 86 with a median of 46 years (IQR: 15). One hundred and seventy-eight women (50.6%) and 174 (49.4%) men participated in the study. One hundred and sixty-nine participants (48%) were over the age of 46. The general descriptive data for the population of participants are featured in Table 1.
| Statistics | |
|---|---|
| Female | 178 (50.6%) |
| Male | 174 (49.4%) |
| Age | 46.00 (15) |
| ART exposure | 9 (7) |
| Time on viral suppression | 7 (6) |
| Time from beginning of ART to viral suppression | 1 (2) |
| CD4+ T cell counts | 678.5 (334) |
| CD8+ T cell counts | 900.5 (475) |
| Nadir CD4 counts | 265 (209) |
| Baseline CD4/CD8 ratio | 0.280 (0.31) |
| Inverted baseline CD4/CD8 ratio (<1) | 224 (97%) |
| Current CD4/CD8 ratio | 0.814 (0.56) |
| Current inverted CD4/CD8 (<1) | 239 (67.9%) |
| Overall time with inverted ratios | 6 (3-8) |
| Time with inverted ratios after beginning ART | 4 (2-7) |
| Comorbidities (overall) | 218 (61.9%) |
| Dyslipidemia | 124 (35.2%) |
| Cardiovascular diseases | 55 (15.6%) |
| Mental disorders | 49 (13.9%) |
| Diabetes | 45 (12.8%) |
| Neurocognitive diseases | 37 (10.5%) |
| Metabolic syndrome | 31 (8.8%) |
| Thyroid disease | 20 (5.7%%) |
| Bone disease | 15 (4.3%) |
| Renal disease | 14 (4.0%) |
| Pulmonary disease | 9 (2.6%) |
| Liver disease | 7 (2%) |
| Cancer | 5 (1.4%) |
| Multimorbidity | 105 (29.8%) |
| NNRTI-based ART regimen | 184 (52.3%) |
| PI-based ART regimen | 163 (46.3%) |
| Other ART regimens | 5 (1.4%) |
- Note: The parameters were expressed in absolute numbers and percentages or medians and interquartile ranges. N = 352, except for nadir CD4 counts (n = 238), baseline CD4/CD8 ratio (n = 231), and time with inverted ratios (n = 224, for overall and after commencement of ART). Age and time were measured in years and T cell counts and nadir in cells/mm3
- Abbreviation: ART, antiretroviral therapy; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
The median age for men was 48 (IQR: 11), significantly higher than the median age for women which was 44 (IQR: 15) (P < .001). Men were significantly more likely to age over 46 years (odds ratio [OR] = 1.77, P = .01). Therefore, age and sex were added to all logistic regression models.
3.2 Viral suppression and ART exposure
The median for ART exposure was 9 years (IQR: 7) and the median for the time on viral suppression was 7 years (IQR: 6). The median for the time elapsed from the beginning of ART to viral suppression was 1 year (IQR: 2). Two hundred and forty-three participants (69%) reached viral suppression within a year of ART.
3.3 ART regimens
The most representative ART regimen was the non-nucleoside reverse transcriptase inhibitor-based 184 (52.3%), followed by protease inhibitor-based 163 (46.3%; Table 1).
3.4 Nadir and current CD4 and CD8 counts
The median CD4 nadir count was 265 (IQR: 96) cells/mm3. The lowest nadir CD4 count was 2, 25% of the cases were below 152 (Q1), and 25% of the cases were above 361 cells/mm3 (Q3).
The median was 678.5 (IQR: 334) for current CD4 and 900.5 cells/mm3 (IQR: 475) for current CD8+ T cell counts. These results appear in Table 1.
3.5 CD4/CD8 ratios
The median for baseline CD4/CD8 ratios was 0.28 (IQR: 0.31) (Table 1). The lowest observation for baseline CD4/CD8 ratios was 0.01, 25% of the cases were below 0.16 (Q1), 25% of the cases were above 0.47 (Q3), and the largest observation was 1.74. The current CD4/CD8 ratio median was 0.8 (IQR: 0.56) (Table 1). The lowest value for current CD4/CD8 ratios was 0.19, Q1 was 0.57, Q3 was 0.113, and the largest observation was 2.76.
Baseline CD4/CD8 ratios were not correlated to the age at baseline (R = −0.61, P = .327). The average baseline CD4/CD8 ratios for men (0.304 ± 0.023) and women (0.358 ± 0.025) were not significantly different (P = .113). Current CD4/CD8 ratios were also not correlated to current age (R = −0.63, P = .203), but the mean ratio for men (0.750 ± 0.030) was significantly lower when compared to women (0.893 ± 0.030). Current CD4/CD8 ratios correlated positively with CD4 counts (R = 0.54, P < .001), baseline CD4/CD8 ratios (R = 0.68, P < .001) and nadir CD4 counts (R = 0.50, P < .001), and inversely with CD8 counts (R = −0.70, P < .001; Figure 1).
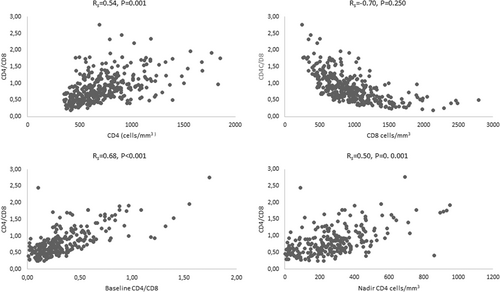
Multiple linear regression models were built to evaluate the contributory factors to current CD4/CD8 ratios. The results are shown in Table 2. The independent variables included in the first model were age, sex, time on viral suppression, time from beginning from ART to viral suppression, and nadir CD4 counts. The nadir CD4 counts and the baseline CD4/CD8 ratios were strongly correlated (R = 0.70, P < .001) and were not included in the same models to avoid collinearity. Thus, the second linear regression model included baseline CD4/CD8 ratios instead of nadir CD4 counts. On the first linear model, CD4/CD8 ratios increased 0.019 units for each year on viral suppression (OR = 0.019, P < .001) and 0.001 for each cell/mm3 of nadir CD4 counts (OR = 0.001, P < .001), which were significant predictors of CD4/CD8 ratios. Age, sex, and time from beginning from ART to viral suppression did not predict the CD4/CD8 ratios. In the second model, the baseline CD4/CD8 ratio was the sole predictor of current CD4/CD8 ratios, which increased 1.02 units for each baseline CD4/CD8 ratio unit (OR = 1.02, P < .001). Age, sex, time on viral suppression, and time from beginning from ART to viral suppression did not contribute to current CD4/CD8 ratios.
| Multivariate analysis 1 | Multivariate analysis 2 | ||||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| CD4/CD8 ratio | Age | −0.002 | −0.006-0.002 | .333 | −0.002 | −0.006-0.002 | .316 |
| Sex (male) | −0.054 | −0.146-0.038 | .246 | −0.014 | −0.095-0.067 | .733 | |
| Time on viral suppression | −0.005 | −0.020-0.010 | .533 | 0.000 | −0.014-0.013 | .954 | |
| Time from beginning of ART to viral suppression | 0.019 | 0.003-0.034 | .023 | 0.005 | −0.009-0.019 | .495 | |
| Nadir CD4 counts | 0.001 | 0.001-0.002 | .000 | ||||
| Baseline CD4/CD8 ratio | 1.018 | 0.873-1.164 | .000 | ||||
- Abbreviations: ART, antiretroviral therapy; CI, confidence interval; OD, odds ratio
3.6 Inverted CD4/CD8 ratios
The prevalence of inversion was 63.6% for baseline and 67.9% for current CD4/CD8 ratios. Inverted baseline CD4/CD8 ratios were not associated with age at baseline (OR = 1.05, P = .271, n = 231), nor were the current inverted ratios (OR = 0.99, P = .733, n = 352). Individuals with inverted ratios had significantly reduced CD4 counts, nadir CD4 counts, and baseline CD4/CD8 ratios and increased CD8 counts. Table 3 shows the descriptive data and the comparison between individuals with and without inverted ratios.
| CD4/CD8 <1 (n = 239) | CD4/CD8 ≥1 (n = 113) | P value | |
|---|---|---|---|
| Women | 130 (54.4%) | 44 (39%) | |
| Men | 109 (45.6%) | 69 (61%) | |
| Age | 45 (15) | 47 (15) | .278 |
| ART exposure | 9 (7) | 10 (9) | .281 |
| Time on viral suppression | 6 (7) | 7 (5) | .174 |
| Time from beggining of ART to viral suppression | 1 (2) | 1 (3) | .139 |
| CD4+ T cell counts | 607 (282) | 838 (405) | .000 |
| CD8+ T cell counts | 994 (438) | 632 (346) | .000 |
| Nadir CD4 counts | 223 (203) | 421 (261) | .000 |
| Nadir CD4 counts < 152 | 55 (31.6%) | 4 (6.25%) | |
| Nadir CD4 counts < 265 | 102 (86.2%) | 17 (26.6%) | |
| Nadir CD4 counts < 361 | 150 (63%) | 29 (45.3%) | |
| Baseline CD4/CD8 ratio | 0.230 (0.21) | 0.530 (0.36) | .000 |
| Baseline CD4/CD8 ratio <0.16 | 57 (32.8%) | 1 (1.8%) | |
| Baseline CD4/CD8 ratio <0.28 | 112 (64.4%) | 7 (12.3%) | |
| Baseline CD4/CD8 <0.47 | 151 (86.8%) | 23 (40.4%) | |
| Inverted baseline CD4/CD8 ratio (<1) | 172 (98.9%) | 52 (91.2%) | |
| Comorbidities (overall) | 147 (61.5%) | 71 (62.8%) | |
| Multimorbidity | 72 (30.1%) | 33 (29.2%) |
- Note: The parameters were expressed in absolute numbers and percentage or medians and interquartile ranges. N = 352, except for nadir CD4 counts (n = 238), baseline CD4/CD8 ratio (n = 231), and time with inverted ratios (n = 224, for overall and after commencement of ART). Age and time were measured in years and cell counts and nadir in cells/mm3
- Abbreviation: ART, antiretroviral therapy.
Univariate analyses have shown that men and women had similar chances of having inverted ratios at baseline (OR = 1.33, P = .505). Current inverted ratios, in contrast, were much more common in men than in women (OR = 1.87, P < .01). The median for the overall time with inverted ratios was 6 (IQR: 5) years and after the commencement of ART was 4 (IQR: 5) years. Seventy-seven percent (172) of the individuals with inverted CD4/CD8 ratios at baseline remained with inverted ratios.
The predictors for current inverted CD4/CD8 ratios were evaluated using logistic regression models. Age, sex, time on viral suppression, time from beginning of ART to viral suppression, and nadir CD4 counts or baseline CD4/CD8 ratios were included. The results for these two models are shown in Table 4. The results indicate that lower nadir CD4 counts (first model), and baseline CD4/CD8 ratios (second model) are significant predictors of inverted CD4/CD8 ratios (P < .001). Age, sex, time on viral suppression, and time from beginning from ART to viral suppression did not predict current inverted CD4/CD8 ratios in both models.
| Univariate analysis | Multivariate analysis 1 | Multivariate analysis 2 | ||||
|---|---|---|---|---|---|---|
| OR (CI, 95%) | P value | OR (CI, 95%) | P value | OR (CI, 95%) | P value | |
| Men | 1.87(1.19-2.95) | .007 | 1.553 (0.794-3.037) | .198 | 1.238 (0.584-2.624) | .577 |
| Age | 0.99 (0.97-1.02) | .733 | 1.000 (0.970-1.031) | .999 | 1.000 (0.966-1.036) | .997 |
| ART exposure | 0.97 (0.93-1.02) | .306 | ||||
| Time on viral suppression | 0.92 (0.86-0.98) | .100 | 0.900 (0.802-1.010) | .074 | 1.023 (0.885-1.183) | .759 |
| Time from beginning of ART to viral suppression | 1.06 (0.98-1.14) | .147 | 1.055 (0.925-1.203) | .427 | 1.069 (0.914-1.249) | .404 |
| CD4+ T cell counts | 0.99 (0.99-1.00) | .000 | ||||
| CD8+ T cell counts | 1.00 (1.00-1.00) | .000 | ||||
| Nadir CD4 counts | 0.99 (0.99-0.99) | .000 | 0.993 (0.990-0.995) | |||
| Nadir CD4 counts < 152 | 6.93 (2.40-20.04) | .000 | ||||
| Nadir CD4 counts < 265 | 3.92 (2.08-7.36) | .000 | ||||
| Nadir CD4 counts < 361 | 7.54 (3.92-14.51) | .000 | ||||
| Baseline CD4/CD8 ratio | 0.00 (0.00-0.02) | .000 | 0.003 (0.001-0.018) | .000 | ||
| Baseline CD4/CD8 ratio < 0.16 | 27.28 (3.68-202.10) | .000 | ||||
| Baseline CD4/CD8 ratio < 0.28 | 12.90 (5.52-30.18) | .000 | ||||
| Baseline CD4/CD8 ratio < 0.47 | 9.71 (4.88-19.30) | .000 | ||||
| Inverted baseline CD4/CD8 ratio (<1) | 8.26 (1.56-43.88) | .011 | ||||
| Comorbidities (overall) | 0.945 (0.59-150) | .906 | ||||
| Multimorbidity | 1.11 (0.63-1.95) | .773 | ||||
- Abbreviations: ART, antiretroviral therapy; CI, confidence interval; OD, odds ratio.
3.7 Comorbidities and multimorbidity
Comorbidities included dyslipidemia, diabetes, metabolic syndrome, cancer, and hepatic, renal, pulmonary, neurocognitive, mental, cardiovascular, bone, and thyroid diseases. Dyslipidemia, cardiovascular diseases, and mental disorders are the most common comorbidities among the participants (Table 1). The overall frequency of comorbidities was 61.9% (218), while multimorbidity affected 105 individuals (29.8%).
The descriptive information and comparison between individuals with and without comorbidities are presented in Table 5. Individuals with and without comorbidities showed significantly different age, time on viral suppression, time from begging of ART to viral suppression, and overall time with inverted ratios.
| With comorbidities (n = 218) | Without comorbidities (n = 134) | P value | |
|---|---|---|---|
| Men | 108 (49.5%) | 66 (49.3%) | |
| Women | 110 (50.5%) | 68 (50.7%) | |
| Age | 47 (13) | 39 (17) | .000 |
| Age ≥46 | 125 (57.3%) | 44 (32.8%) | |
| ART exposure | 7 (6) | 6 (5) | .025 |
| Time on viral suppression | 5 (5) | 5 (4) | .038 |
| Time from begging of ART to viral suppression | 1 (2) | 1 (2) | .904 |
| CD4+ T cell counts | 644 (329) | 660.5 (374) | .380 |
| CD8+ T cell counts | 937 (458) | 911.5 (480) | 1.000 |
| Nadir CD4 counts | 244 (219) | 286 (198) | .650 |
| Nadir CD4 counts < .152 | 40 (18.3%) | 19 (14.2%) | |
| Nadir CD4 counts < .265 | 77 (35.3%) | 42 (31.3%) | |
| Nadir CD4 counts < .361 | 108 (49.5%) | 71 (53%) | |
| Baseline CD4/CD8 ratio | 0.27 (0.32) | 0.30 (0.28) | .145 |
| Baseline CD4/CD8 ratio < 0.16 | 43 (19.7%) | 15 (11.2%) | |
| Baseline CD4/CD8 ratio < 0.28 | 75 (34.4%) | 44 (32.8%) | |
| Baseline CD4/CD8 ratio < 0.47 | 102 (46.8%) | 72 (53.7%) | |
| Inverted baseline CD4/CD8 ratio (<1) | 132 (60.6%) | 92 (68.7%) | |
| CD4/CD8 ratio < 0.57 | 55 (25.2%) | 33 (24.6%) | |
| CD4/CD8 ratio < 0.80 | 110 (50.5%) | 66 (49.3%) | |
| Current CD4/CD8 ratio | 0.699 (0.440) | 0.798 (0.570) | .826 |
| Overall time with inverted ratios | 6 (5) | 5 (6) | .043 |
| Time with inverted ratios after beginning ART | 4 (6) | 4 (5) | .149 |
- Note: The parameters were expressed in absolute numbers and percentage or median and interquartile ranges. N = 352, except for nadir CD4 counts (n = 238), baseline CD4/CD8 ratio (n = 231), and time with inverted ratios (n = 224 for overall and after commencement of ART).
- Abbreviation: ART, antiretroviral therapy.
The presence of comorbidities was associated with aging, both in men (OR = 1.061, P < .001) and women (OR = 1.064, P < .001). Women aged over 46 years (OR = 2.78, P < .01) were more likely to have a comorbidity than women younger than 46 years. Men aged over 46 years (OR = 2.83, P < .001) were also more likely to present comorbidities than their younger counterparts.
Age, ART exposure, time on viral suppression, and baseline CD4/CD8 ratios smaller than 0.16 were significant predictors of overall comorbidities on the univariate analyses. To determine whether there was an association between the presence of comorbidities and specific comorbidities with nadir CD4 counts, baseline CD4/CD8 ratios, and current CD4/CD8 ratios, we employed several logistic regression models (observing the including criteria of a minimum of five observation per cell). The results for all the significant models are described in this and the next sections.
Table 6 shows the results of multivariate logistic regression models with the overall presence of comorbidities as the dependent variable. In multivariate logistic regression models, the overall presence of comorbidities was associated with age (P < .001) and baseline CD4/CD8 ratios smaller than 0.16 (OR = 2.68, P < .001) but not with sex (OR = 0.57; P = .064). Aging was consistently associated with the overall presence of comorbidities in all models (P < .001).
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR (CI, 95%) | P value | OR (CI, 95%) | P value | |
| Men | 1.01 (0.66-1.56) | 1.000 | 0.57 (0.32-0.03) | .064 |
| Age, y | 1.06 (1.04-1.08) | .000 | 1.05 (1.02-1.08) | .000 |
| Age ≥46 | 2.7 (1.75-4.31) | .000 | ||
| ART exposure | 1.07 (1.02-1.12) | .008 | 0.99 (0.882-1.13) | .956 |
| Time on viral suppression | 1.07 (1.01-1.14) | .022 | ||
| Time from begging of ART to viral suppression | 1.04 (0.97-1.12) | .226 | ||
| CD4+ T cell counts | 1.00 (0.99-1.00) | .641 | ||
| CD8+ T cell counts | 1.00 (1.00-1.00) | 387 | ||
| Nadir CD4 counts | 0.99 (0.99-1.00) | .166 | ||
| Nadir CD4 counts < 152 | 1.66 (0.89-3.09) | .128 | ||
| Nadir CD4 counts < 265 | 1.63 (0.96-2.74) | .087 | ||
| Nadir CD4 counts < .361 | 1.28 (0.71-2.32) | .447 | ||
| Baseline CD4/CD8 ratio | 0.521 (0.20-1.33) | .172 | ||
| Baseline CD4/CD8 ratio < 0.16 | 2.52 (1.31-4.88) | .006 | 2.93 (1.41-6.12) | .004 |
| Baseline CD4/CD8 ratio < 0.28 | 1.48 (0.87-2.50) | .182 | ||
| Baseline CD4/CD8 ratio < 0.47 | 1.03 (0.56-1.89) | 1.000 | ||
| Inverted baseline CD4/CD8 ratio (<1) | 1.91 (0.42-8.75) | .454 | ||
| CD4/CD8 ratio < 0.57 | 1.03 (0.63-1.70) | 1.000 | ||
| CD4/CD8 ratio < 0.8 | 1.05 (0.69-1.61) | .913 | ||
| Current CD4/CD8 ratio | 0.80 (0.49-1.33) | .399 | ||
| Overall time with inverted ratios | 0.99 (0.92-1.07) | .847 | ||
| Time with inverted ratios after beginning ART | 1.05 (0.98-1.13) | .178 | 0.98(0.862-1.112) | .739 |
- Abbreviations: ART, antiretroviral therapy; CI, confidence interval; OD, odds ratio.
A last multivariate model was built to evaluate the predictors for the overall presence of comorbidities including age, sex, ART exposure, time with inversion after the commencement of ART, and baseline CD4/CD8 ratios below 0.16. Only aging (OR = 1.05, P < .001) and baseline CD4/CD8 ratios below 0.16 predicted the overall presence of comorbidities (OR = 2.93.05, P < .01). Sex (OR = 0.57, P = .064), ART exposure (OR = 0.99, P = .956), and time with inversion after the commencement of ART (OR = 0.98, P = .739) were not predictors of the overall presence of comorbidities.
Multimorbidity was associated with aging (OR = 1.05, P < .001) but not with sex (OR = 0.86, P = .591) nor with the different categories of nadir CD4, baseline and current CD4/CD8 ratios (data not shown). The odds ratio for an individual aged over 46 to have multimorbidity was 1.95 (P < .001).
Renal, bone, pulmonary and liver diseases, metabolic syndrome and cancer, did not fit the criteria to enter a multivariate logistic regression due to low prevalence. However, on the univariate analysis, bone disease (OR = 1.06, P = .004), renal disease (OR = 1.06, P = .006), and metabolic syndrome (OR = 1.03, P = .042) were associated with aging, but not pulmonary (OR = 0.98, P = .567) and liver (OR = 1.01, P = .675) diseases, nor cancer (OR = 1.10, P = .930). Regarding sex, a minimum of five individuals per cell was found only in bone (OR = 0.90, P = 1.000) and renal disease (OR = 1.02, P = 1.000), none were related to sex. CD4/CD8 ratios were not associated with renal (OR = 1.12, P = .862), bone (OR = 1.65, P = .317), pulmonary (OR = 0.50, P = .454) and liver (OR = 2.42, P = .232) diseases, metabolic syndrome (OR = 1.27, P = 0.454) nor cancer (OR = 1.10, P = .930).
Aging was a significant independent predictor of diabetes (OR = 1.04, P = .011), thyroid disease (OR = 1.04, P = .024), and metabolic syndrome (OR = 1.03, P = .042). Age over 46 years significantly increased the chance of developing diabetes (OR = 2.16, P = .025), but neither thyroid disease (OR = 1.67, P = .357) nor metabolic syndrome (OR = 1.80, P = .135) were predicted by age over 46 years. Neither diabetes (OR = 1.20, P = .633), nor thyroid disease (OR = 1.67, P = .491) nor metabolic syndrome (OR = 0.95, P = 1.000) were determined by sex. Multivariate analyses including age, sex, and CD4/CD8 ratios smaller than 0.8, showed that only aging was a significative predictor of diabetes and metabolic syndrome (OR = 1.03, P = .013, and OR = 1.03, P = .036, respectively). In a model with age, sex, and inverted CD4/CD8 ratios, only aging was a determinant of diabetes (OR = 1.03, P = .013) and metabolic syndrome (OR = 1.03, P = .037).
Mental disorders were independently associated with age (OR = 1.02, P = .039) and sex (OR = 0.38, P = .002). When analyzing the influence of age in both sexes, aging was associated with mental disorders only in women (OR = 1.05, P = .010). Current CD4/CD8 ratios greater than 0.8 were independently associated with mental disorders (OR = 2.08, P = .030), but when entered in multivariate models with age in women (OR = 2.04, P = .081) and men (OR = 1.02, P = .274) they were no longer significant.
3.8 Dyslipidemia
We found an association between the incidence of dyslipidemia and aging (OR = 1.04, P < .001), while sex was not a predictor (OR = 1.2, P = .420). The odds for individuals aged over 46 years was 2.2 (χ2 = 11.93, P < .001). In multivariate logistic regression models, aging ((OR = 1.04, P < .001, in all models), nadir CD4 counts below 152 (OR = 2.36, P = .007) and 265 cells/mm3 (OR = 2.05, P = .015), and baseline CD4/CD8 ratios lower than 0.16 (OR = 2.16, P = .019) were predictors of the occurrence of dyslipidemia.
ART exposure and time with inversion after the commencement of ART were added to the previous significant models to investigate their association with the occurrence of dyslipidemia. The first model comprised age (OR = 1.04, P < .01), sex (OR = 0.98, P = .970), ART exposure (OR = 0.96, P = .494), time with inversion after commencement of ART (OR = 1.01, P = .866), and nadir CD4 counts below 265 cells/mm3 (OR = 2.28, P = 0.) (χ2 = 16.31, df = 5, P < .01). A second model evaluated age (OR = 1.04, P = .01), sex (OR = 0.83, P = .638), time on ART (OR = 0.98, P = .701), time with inversion after commencement of ART (OR = 0.99, P = .883), and baseline CD4/CD8 ratios below 0.16 (OR = 2.40, P < .05) (χ2 = 17.17, df = 5, P < .01). Aging was associated with the occurrence of dyslipidemia along with nadir CD4 counts below 265 cells/mm3 in the first model and baseline CD4/CD8 ratios below 0.16 in the second model.
3.9 Cardiovascular diseases
The occurrence of cardiovascular diseases was associated with aging (OR = 1.07, P < .001), but not to sex (P = .193). When age over 46 years was tested, both men (OR = 3.186, P < .05) and women (OR = 3.34, P < .01) on this age range were more likely to have cardiovascular diseases that those below aged below 46 years.
Multivariate regression models were built for prediction of cardiovascular diseases including age, sex, nadir CD4 counts, as well as baseline and current CD4/CD8 ratios. None of the variables tested were determinants of occurrence of cardiovascular diseases, except aging which showed OR = 1.07 and P < .001 in all models.
To investigate other predictors of cardiovascular diseases among the variables collected in this study, we added dyslipidemia and metabolic syndrome to multivariate models. A model including age, sex, and presence of dyslipidemia showed that both aging (OR = 1.06, P < .001) and dyslipidemia (OR = 2.00, P < .05) contributed to the occurrence of cardiovascular diseases, but not sex (P = .164). When metabolic syndrome was tested along with age and sex, aging (OR = 1.07, P < .001) and metabolic syndrome (OR = 14.43, P < .001) were associated with cardiovascular diseases but not sex (P = .200).
3.10 Neurocognitive diseases
Neither age (OR = 1.00, P = .983) nor sex (OR = 0.86, P = .662) were determinants of neurocognitive diseases. Multivariate models showed that nadir CD4 counts below 152 cells/mm3 (P = .040) and current CD4/CD8 ratios below 0.57 (P = .041) successfully predicted the occurrence of neurocognitive diseases. Individuals with nadir CD4 counts below 152 cells/mm3 were 2.4 times more prone to present a neurocognitive disease, and those with current CD4/CD8 ratios below 0.57 were 2.1 times more likely to have these diseases.
4 DISCUSSION
The population of this study was formed by HIV-positive patients on ART for several years and considered successfully treated due to viral suppression and restored CD4+ T cell counts. However, approximately 70% of these individuals have shown CD4/CD8 ratios below 1, and only 23% of the cases with inverted baseline CD4/CD8 ratios have managed to achieve current CD4/CD8 ratios above or equal to 1. This high rate of inversion of CD4/CD8 ratio in treated HIV patients on viral suppression was similar to those observed in previous studies.23, 24 Serrano-Villar et al,25 suggests that the complete immune recovery may involve not only normalization of CD4+ T cell counts but also CD4/CD8 ratios. Therefore, more than three-quarters of our population has incomplete immune recovery.
Emu et al have found that, in ART-treated HIV-positive individuals, CD4/CD8 ratios remained low despite the rise of total CD4+ T cell counts and long-term viral suppression. They have also suggested that the continuous expansion of highly differentiated CD8+ T cells is likely to drive the slow pace of recovery in the number of naive CD4+ and CD8+ T cells.26 We found that CD4/CD8 ratios showed a negative and strong correlation with CD8 counts, and a moderate and positive correlation with CD4 counts, which suggests that the influence of CD8+ T cell expansion on CD4/CD8 ratios was greater.
In noninfected individuals, older individuals have twice the chance of showing an inverted CD4/CD8 ratio than younger ones.27, 28 In this study, we found no association between the CD4/CD8 ratio and age, as well as baseline CD4/C8 ratios and age at baseline. Besides age, sex is known to determine CD4/CD8 ratios.29 Non-HIV infected young and elderly men display lower ratios of CD4/CD8 compared to women.29, 30 Among the participants of our study, men had lower CD4/CD8 ratios than women, in agreement with a previous study with HIV-positive patients.21 However, when corrected by age, time on suppression, and time from the beginning of ART to viral suppression, and baseline CD4/CD8 ratios, sex was no longer a determinant of current CD4/CD8 ratios.
We found that age over 46 years was associated with the overall prevalence of comorbidities and multimorbidity. These results were driven by the high prevalence of metabolic conditions and cardiovascular diseases in participants aged over 46 years. These results may explain the increase in the overall prevalence of comorbidities observed in this study, which was high when compared to previous reports,31, 32 considering our inclusion criteria. The increased median age of our population, and the inclusion of metabolic diseases in the design of this study, may have led to an increased overall frequency of comorbidities. Although we have not compared the prevalence of comorbidities in HIV-positive and uninfected controls, we understand that the normal aging process added to HIV chronic infection is responsible for additional challenges in the management of older HIV patients. Rasmussen et al33 argue that accelerated aging may not be the largest health concern in people living with HIV, but the increased prevalence of severe age-related diseases in this population.
In this study, the overall presence of non-AIDS comorbidities was not related to current decreased CD4/CD8 ratios but associated with very low baseline CD4/CD8 ratios. Previous studies have associated low CD4/CD8 ratios and the occurrence of comorbidities,34, 35 however, there were significant differences in the design of these studies and ours. Our study was more restrictive in terms of current CD4 counts, including only individuals with equal or smaller than 350cells/mm3. On the other hand, we incorporated not only acute events but also chronic and metabolic diseases as comorbidities.
Associations were previously described between CD4/CD8 ratios smaller than 0.435 and 0.8 and cardiovascular diseases21, 36 and between CD4/CD8 ratios below 0.4 and renal disease as well as cancer.35 Aging, instead of CD4/CD8 ratios, explained the manifestation of cardiovascular and renal diseases in our study. This contradiction may be explained by the inclusion of chronic diseases as comorbidities, such as hypertension, in our study rather than only serious events as in the previous studies. Moreover, we found that the occurrence of cardiovascular diseases was associated with dyslipidemia and metabolic syndrome. Dyslipidemia was associated with aging, nadir below 265cells/mm3, and very low baseline CD4/CD8 ratios. A previous study has shown that metabolic syndrome37 and triglyceridemia are associated with lower CD4/CD8 ratios. We have not observed an association between CD4/CD8 ratios and metabolic syndrome, which may also be related to study design differences.
However, we found that neurological diseases were associated with CD4/CD8 ratios below 0.57, very low nadir CD4 counts, and current CD4 counts below 530cells/mm3. Relationships between CD4/CD8 ratios and neurocognitive dysfunction have been described previously. Grauer et al38 found a robust correlation between CD4/CD8 ratios in peripheral blood, as well as in the cerebral spinal fluid, and neurocognitive disorders in patients on ART. Vassallo et al39 showed that inverted CD4/CD8 ratios were associated with immune activation and represented a risk factor for neurocognitive disorders, in treated HIV-positive individuals. In another study, a decrease in CD4/CD8 ratios over time was associated with cognitive impairment regardless of the viral load.40
Neurocognitive disorders are not uncommon in successfully treated HIV-positive individuals, although with reduced levels of impairment and much less often than in those individuals who progressed to AIDS.41, 42 Our findings are consistent with the fact that low-grade inflammation, as in chronic infections and aging, activates the immune cells in the brain, corroborating with neuroinflammation and the onset of neurological diseases, including cognitive loss.43 Even though these findings suggest that a CD4/CD8 ratio could be predictive of neurocognitive dysfunction, further studies with a greater number of subjects, and specifically designed to investigate the role of CD4/CD8 ratios in the advent of neurocognitive diseases, are necessary.
5 CONCLUSION
Current CD4/CD8 ratios were determined by baseline CD4/CD8 ratios and nadir CD4 counts. Despite the high rates of inverted CD4/CD8 ratios and prevalence of comorbidities, no association between them was found. The most prevalent comorbidities occurred more often in older individuals, even though aging alone did not explain the rate of all individual comorbidities analyzed in this study. Low CD4/CD8 ratios were linked to the presence of neurocognitive disorders, suggesting that persistent T cell dysfunction contributes to neurocognitive decline. This result also implies that low CD4/CD8 ratios may indicative of an increased risk of developing such disorders.
ACKNOWLEDGMENTS
This study was partly funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; financial code 001. PROEX number 88882.182150/2018-01) and by the Programa Institucional de Bolsas de Iniciação Científica do Hospital Universitário de Santa Maria (PROIC-HUSM). We would also like to thank the HUSM Archive department personnel.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
DFP designed the study, collected, entered, analyzed and interpreted the data, and wrote the manuscript. JMB, LLDS, MHJ, ED, and ACF collected and entered data into the database. JLGDS collected and entered data into the database and revised the manuscript. JFPR and AVS interpreted the data. MRCS and DBRL read and approved the final manuscript.
ETHICS STATEMENT
This study was approved by the Ethics Committee of the Federal University of Santa Maria (protocol number 65777517.0.0000.5346).




