Clinical and economic impact of treated CMV infection in adult CMV-seropositive patients after allogeneic hematopoietic cell transplantation
Abstract
Objective
Recipients of allogeneic hematopoietic stem cell transplantation (allo-HCT) with positive cytomegalovirus (CMV) serology are at increased risk of morbidity and mortality. The primary objective of this study was to assess the association between treated CMV infection and overall mortality within 1 year after allo-HCT in adult CMV-seropositive Recipients (R+). Secondary objectives included overall 5-year mortality after allo-HCT, risk factors for treated CMV infection, associations between treated CMV infection and allo-HCT complications and medical costs.
Methods
A multicenter retrospective cohort study was conducted in adult CMV-seropositive recipients (R+) who underwent to allo-HCT between 1st January 2010 and 31st December 2014.
Results
Five hundred seventy two CMV-seropositive patients (mean age, 50.2 years) undergoing allo-HCT between 2010 and 2014 were included; 55.9% of donors were CMV seropositive. CMV infection treated with antiviral therapy was reported in 227 patients (39.7%) after transplantation. One-year overall mortality was significantly increased in patients with treated CMV infections (hazard ratio, 1.86; 95% CI, 1.16-3.00; P = .011). Mean medical costs during the first post-HCT year were higher in patients with CMV infection (€46 853 vs €31 318; P < .0001).
Conclusion
In this large cohort of CMV-seropositive patients undergoing allo-HCT, treated CMV infection was significantly associated with an increased 1-year risk of overall mortality, with increased length of stay and with hospitalization cost. The burden of CMV disease in allo-HCT could be reduced in the future by appropriate prophylactic strategies.
1 INTRODUCTION
Human cytomegalovirus (CMV) infection has a high global prevalence. Although, CMV infection is usually asymptomatic or accompanied by mild flu-like symptoms in immunocompetent individuals, CMV is an issue in patients receiving allogeneic hematopoietic stem cell transplantation (allo-HCT), due to severe immunosuppression. In the absence of treatment, CMV infection can progress to CMV end-organ disease resulting in severe sequelae or death. CMV infection has been shown to be associated with graft-versus-host disease (GvHD) occurrence and its treatment with the possible development of infections related to bone marrow toxicity.1
CMV infection is defined as virus isolation or detection of viral proteins or nucleic acid in any body fluid or tissue specimen. CMV reactivation does not always lead to manifest CMV disease; most commonly, it manifests as pneumonia, gastroenteritis, encephalitis, retinitis, nephritis, cystitis, myocarditis, or pancreatitis. Organ involvement is diagnosed with a biopsy or tissue sample.2
In allo-HCT recipients, the risk of CMV infection is high within the first 100 days after transplantation and the majority of cases occur within the 1st year. Before the introduction of antiviral therapies, the incidence of CMV disease in patients receiving an allo-HCT was 25% to 30% for both early and late periods with devastating consequences. Higher incidence rates were reported in patients undergoing allo-HCT who were CMV seropositive at the time of transplantation.3
CMV management remains a major issue after allo-HCT. Current management options for the prevention of CMV infection in allo-HCT recipients include prophylaxis (prevention of the infection) and preemptive therapies (prevention of the disease), which have now decreased the incidence of posttransplant CMV disease to 2% to 11%.4 In the preemptive approach, antiviral therapy is initiated following CMV detection based on weekly blood testing, most commonly using DNA polymerase chain reaction (PCR). Such a preemptive strategy has been adopted in most cancer centers.5 There are limitations with the use of CMV antivirals in prophylaxis due to potential adverse events (neutropenia, dyspnea, diarrhea, renal function impairment, and metabolic disorders) or treatment resistance. In allo-HCT recipients undergoing preemptive treatment (PET), there are additional costs due to antiviral medication and longer hospitalization durations within the first 6 months after transplantation.6-9
Despite its decreased incidence, posttransplantation CMV infection remains an important cause of morbidity and mortality in allo-HCT patients. In the era of viral surveillance based on quantitative PCR, the data on the relationship between early CMV infection and clinical outcomes after allo-HCT are conflicting. The aim of this study was to assess the clinical and economic burden associated with CMV infection in CMV-seropositive allo-HCT recipients in routine clinical practice.
The primary objective of the study was to assess the relationship between treated CMV infection and overall mortality within the 1st year after transplantation in CMV-seropositive allo-HCT recipients. The main secondary objectives included overall survival (OS) up to 5 years posttransplant, risk factors of CMV infection, allo-HCT complications related to CMV treatment, and the economic burden of treated CMV infection within the 1st year after transplantation.
2 PATIENTS AND METHODS
2.1 Study design and patient population
The ECO-CY-STEM study was a multicenter retrospective study that included CMV-seropositive adult recipients who underwent allo-HCT from CMV seropositive or seronegative donors in three participating French centers (Saint-Louis Hospital, Paris; CHRU of Lille; CHU of Nantes) between 2010 and 2014. The three centers are part of the Francophone Society of Bone Marrow Transplantation and Cellular Therapy (SFGM-TC) and were selected based on their high level of allo-HCT practice (together, they represented 18% of all allo-HCT activity in France in 2012).10 These centers are JACIE-accredited (Joint Accreditation Committee of ICST-Europe and EBMT), a European program for accreditation for blood and marrow transplantation.11
Each center identified eligible patients already enrolled in the EBMT registry satisfying the following inclusion criteria: greater than or equal to 18 years old at the date of allo-HCT; CMV-seropositive status; availability of the CMV serostatus of the donor; allo-HCT performed between 1st January 2010 and 31st December 2014. Patients were excluded if they were currently included or had been previously included in a clinical trial for a CMV antiviral drug or if they had a history of CMV disease before transplant.
Patients were followed up for 1 year after allo-HCT; only survival was documented at 5 years.
The study was conducted in accordance with the Declaration of Helsinki and with noninterventional study regulations and guidelines in France. According to applicable laws and regulations, this retrospective study did not require ethics committee review.
2.2 Supportive care and initiation of anti-CMV treatment
The three centers of Paris, Lille, and Nantes practiced the same patient daily care routine as recommended by JACIE11 and annual workshops organized by the SFGM-TC for harmonization of clinical practices.12, 13
Patients were hospitalized in single rooms ventilated with high efficiency particulate air filtration systems. Patients received prophylactic low dose acyclovir until day 100. Monitoring of CMV reactivation or infection was performed at least weekly with quantitative PCR for at least 100 days post transplantation. The criterion for initiating preemptive anti-CMV treatment was a viral load above a prespecified threshold cut-off value specific to each center in the range of 3 to 4 log copies/mL. Documented CMV reactivation or infection after transplantation was treated with therapeutic doses of ganciclovir or foscarnet (depending on cytopenia and kidney function).
Broad spectrum antibiotics were administered for fever during neutropenia and antifungal coverage was added for persistent fever unresponsive to antibiotic therapy. All patients received fluconazole or voriconazole for prophylaxis of fungal infections for 100 days and trimethoprim sulfamethoxazole for prophylaxis of toxoplasmosis and Pneumocystis juroveci after engraftment for 12 months after transplantation.
2.3 Collected data
Study data were derived from existing electronic health records available at each participating center. Data on patient characteristics included demographics, underlying disease, disease status at inclusion, previous allo-HCT, and Karnofsky performance status scores. Data on allo-HCT included transplantation date, CMV serostatus of the donor, type of donor, source of stem cells, and conditioning regimen. Other data included laboratory tests, CMV antiviral treatment and clinical complications associated with CMV infection after allo-HCT (acute and chronic GvHD, viral, bacterial, fungal and parasitic infections, graft failure, relapse of the underlying disease and death).
The status of the patient (alive, lost to follow-up, or dead) was documented 1 and 5 years after transplantation. All hospitalizations lasting more than 1 day within the 1st year after transplantation were documented, including admission and discharge dates, type of hospital stay, reason for admission and treatments of interest.
2.4 Statistical analysis
This was a retrospective study and no formal sample size calculation was performed. We planned to include all patients fulfilling the eligibility criteria identified by the participating centers between 2010 and 2014.
CMV infection was defined by a positive quantitative PCR.14 “Treated CMV infection” was defined as CMV infection treated with antivirals.
For the primary objective, the association between treated CMV infection and overall mortality within the 1st year after transplantation was assessed using a Cox regression model with treated CMV infection as a binary time-dependent covariate. Univariate analysis was performed to select variables (P < .20) to be included in the multivariate analysis.
The association between treated CMV infections and occurrence of allo-HCT complications (relapse of the underlying disease, nonrelapse mortality, acute and chronic GvHD, graft failure, and non-CMV infections) were assessed using Fine and Gray competing risk models with treated CMV infection as a time-dependent covariate and relapse and death considered as competing risks. The risk factors for CMV infection within 1 year were identified using the Fine and Gray model with relapse and death considered as competing risks. A Fine and Gray competing risk model was also used to assess the association between CMV infection as a time-dependent covariate and length of stay with death during hospitalization considered as a competing risk.
Estimates of hospitalization costs were made from a health insurance perspective only. The medical costs were derived from the French case-mix-based payment model (T2A model). The costs of hospital stays were calculated on the basis of the tariff for Diagnosis Related Groups (DRG) and the duration of hospitalization. The DRG tariffs used for cost calculation corresponded to the respective patient treatment dates (DRG tariffs 2010-2016). In this study, only hospitalizations lasting more than 1 day and rehospitalizations in the center of initial treatment were recorded (day hospitalizations, consultations or rehospitalizations in other centers were not considered). The cost analysis did not consider cost of day admission as it was deemed to have a negligible impact on the overall management cost for a CMV infection. It is important to note that drug costs related to the management of CMV were assumed to be included in the DRG tariffs.
To assess the association between occurrence of treated CMV infection and management costs, a 1:1 case-control matching procedure was performed to match randomly patients having experienced CMV infection during the study (cases) with patients who did not experience CMV infection (controls) based on the follow-up duration and demographics (sex and age classes). Costs from the onset of CMV infection were then compared between cases and controls using a paired Wilcoxon test.
The analyses were performed using SAS version 9.4 and later (SAS Institute, Inc, Cary, North Carolina).
3 RESULTS
3.1 Flow chart of the study
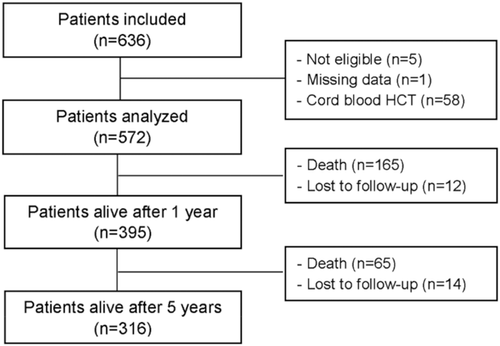
A total of 636 patients were included in three centers, of whom five did not meet inclusion/exclusion criteria (unknown CMV serostatus at transplant, n = 2; history of CMV disease before allo-HCT, n = 2; participation in a clinical trial on CMV infection, n = 1) and one additional patient had numerous missing data (Figure 1). During data review, 58 patients who received cord blood stem cells were also excluded from analysis because they were at considerably higher risk of morbidity and mortality. Finally, 572 patients were analyzed and 395 patients were alive 1 year after transplantation (12 were lost to follow-up and 165 were deceased). From these 395 patients, 316 were alive 5 years after transplantation (14 were lost to follow-up and 65 were deceased). The median follow-up of patients in study after allo-HCT was 905 days.
3.2 Patient characteristics
The mean (SD) age of patients at transplantation was 50.2 (14.0) years (from 18 to 72 years) and the majority were male (54.5%) (Table 1). The main underlying diseases were acute leukemia (33.9%), lymphoid neoplasm (25.9%), and myelodysplastic/myeloproliferative neoplasms (22.6%). A complete remission of the underlying disease was reported in most patients (56.5%), while relapse was reported in 21.6% and stable disease in 16.4% of patients. A previous allo-HCT was reported in 9.3% of patients. A large majority of patients had a Karnofsky score of 100 (71.2%).
| N = 572 | |
|---|---|
| Age at transplantation, y, mean (SD) | 50.2 (14.0) |
| Male sex, n (%) | 312 (54.5) |
| Karnofsky score, n (%) | |
| 60-70 | 15 (3.3) |
| 80-90 | 116 (25.5) |
| 100 | 324 (71.2) |
| Missing | 117 |
| Underlying disease, n (%) | |
| Acute leukemia | 194 (33.9) |
| Lymphoid neoplasm | 148 (25.9) |
| Myelodysplastic/myeloproliferative neoplasms | 129 (22.6) |
| Plasma cell disorder | 46 (8.0) |
| Aplastic anemia | 24 (4.2) |
| Hodgkin lymphoma | 17 (3.0) |
| Chronic myeloid leukemia | 8 (1.4) |
| Hemoglobinopathy | 4 (0.7) |
| Inherited disorders | 1 (0.2) |
| Other | 1 (0.2) |
| Disease status at inclusion, n (%) | |
| Complete remission | 317 (56.5) |
| Relapse/progression | 121 (21.6) |
| Stable disease or untreated | 92 (16.4) |
| Improvement/partial remission | 31 (5.5) |
| Missing | 11 |
| CMV seropositive donor, n (%) | 320 (55.9) |
| Allogeneic donor type, n (%) | |
| Sibling donor | 266 (46.5) |
| Matched unrelated donor | 195 (34.1) |
| Mismatched unrelated donor | 93 (16.3) |
| Mismatched related donor | 18 (3.2) |
| Source of stem cells, n (%) | |
| Peripheral blood | 408 (71.3) |
| Bone marrow | 164 (28.7) |
| Anti-thymocyte globulin, n (%) | 319 (55.8) |
| Total body irradiation, n (%) | 141 (24.7) |
| Conditioning regimen, n (%) | |
| Nonmyeloablative/reduced intensity | 393 (72.5) |
| Myeloablative | 149 (27.5) |
| Missing | 30 |
| Previous allo-HCT, n (%) | 53 (9.3) |
| 1 | 25 (4.4) |
| 2 | 25 (4.4) |
| 3 or 4 | 3 (0.5) |
- Abbreviations: allo-HCT, allogeneic hematopoietic stem cell transplantation.
All recipients were seropositive for CMV and a majority (55.9%) received a graft from a seropositive donor (Table 1). Most patients (46.5%) received a graft from an HLA-matched sibling donor. The source of stem cells was mainly peripheral blood (71.3%) (cord blood was excluded).
The conditioning regimen was myeloablative in 27.5% of recipients (Table 1). Antithymocyte globulin was part of the conditioning regimen in 55.8% patients and total body irradiation was performed in 24.7% of patients.
3.3 Preemptive and curative CMV treatments
Within 1 year after allo-HCT, 227 (39.7%) patients received preemptive or curative antiviral treatment (or both) due to CMV infection episode detected by DNA quantitative PCR. Of these 227 patients, 13 (2.3%) received a curative treatment (two patients received exclusively a curative treatment) (Table 2). Fifteen patients experienced two infection episodes and one patient three infection episodes. Almost all infections (90%) occurred during the first 109 days (range, 3-295) after transplant.
| N = 572 | |
|---|---|
| At least one CMV infection episode (viremia), n (%) | 227 (39.7) |
| Number of episodes, n (%) | |
| 0 | 345 (60.3) |
| 1 | 211 (36.9) |
| 2 | 15 (2.6) |
| 3 | 1 (0.2) |
| At least one CMV disease episode (symptomatic), n (%) | 16 (2.8) |
| Gastrointestinal disease | 7 (43.8) |
| Pneumonia | 1 (6.3) |
| Retinitis | 1 (6.3) |
| Cystitis | 1 (6.3) |
| Other | 1 (6.3) |
| Pneumonia plus other | 1 (6.3) |
| Gastrointestinal disease plus encephalitis plus other | 1 (6.3) |
| Pneumonia plus gastrointestinal disease plus hepatitis | 1 (6.3) |
| Gastrointestinal disease plus hepatitis plus other | 1 (6.3) |
| Graft failure plus other | 1 (6.3) |
| CMV treatments, n (%)b | |
| Patients treated with a preemptive and/or curative treatment | 227 (39.7) |
| Patients treated with a preemptive treatment | 225 (39.3) |
| Patient treated with a curative treatment | 13 (2.3) |
| Patients treated with both preemptive and curative treatments | 11 (1.9) |
| Number of preemptive treatment lines per patient, n (%)a | N = 225 |
| 1 | 123 (54.7) |
| 2 | 50 (22.2) |
| 3 | 30 (13.3) |
| ≥4 | 22 (9.8) |
| Preemptive treatment drugs, n (%) | N = 420a |
| Valganciclovir | 163 (40.0) |
| Ganciclovir | 114 (27.9) |
| Foscarnet | 106 (26.0) |
| Cidofovir | 9 (2.2) |
| Other | 16 (3.9) |
| Missing | 12 |
| Number of curative treatment lines per patient, n (%)a | N = 13 |
| 1 | 8 (61.5) |
| 2 | 2 (15.4) |
| 3 | 3 (23.1) |
| Curative treatment drugs, n (%) | N = 21a |
| Foscarnet | 10 (47.6) |
| Ganciclovir | 6 (28.6) |
| Valganciclovir | 2 (9.5) |
| Cidofovir | 1 (4.8) |
| Other | 2 (9.5) |
- Abbreviation: CMV, cytomegalovirus.
- a One line of treatment = one drug.
- b Preemptive CMV treatment is defined as a CMV antiviral drug initiated when CMV viremia is above a prespecified cut-off value specific to each center. Curative treatment is defined as a CMV antiviral drug initiated when CMV disease episodes are observed.
Sixteen patients developed at least one episode of CMV disease during the 1st year following transplantation (Table 2). The most frequent were gastrointestinal disease (n = 9) and pneumonia (n = 3).
Of the patients who received preemptive CMV treatment, 54.7% received one line of treatment and 45.3% received greater than or equal to 2 lines (Table 2). The most frequent antivirals administered as PETs were valganciclovir (40.0%), ganciclovir (27.9%), and foscarnet (26.0%). The mean ± SD duration of the PETs was 26.5 ± 26.3 days.
Of the 13 patients who received curative CMV treatment, eight received one line, two patients received two lines, and three patients received three lines. The most frequent antivirals administered as curative treatments were foscarnet (10/21) and ganciclovir (6/21). The mean ± SD duration of the curative treatments was 21.7 ± 18.4 days. No patient received prophylactic treatment.
Using the Fine and Gray model, three independent risk factors at baseline for the occurrence of treated CMV infection were identified: CMV seronegativity (hazard ratio [HR] vs seropositive donor: 1.62; 95% confidence interval [CI], 1.22-2.15; P = .0008), graft from a matched donor (HR vs mismatched donor: 0.54; 95% CI, 0.39-0.75; P = .0002) and use of a myeloablative conditioning regimen (HR vs nonmyeloablative regimen: 0.63; 95% CI, 0.44-0.89; P = .0095) (Table 3).
| Baseline variablesb | P value | Modalities | HR | 95% CI |
|---|---|---|---|---|
| Donor CMV serological status | .0008 | Seronegative vs seropositive | 1.62 | 1.22-2.15 |
| Type of donor | .0002 | Matched vs mismatched | 0.54 | 0.39-0.75 |
| Conditioning regimen | .0095 | Myeloablative vs nonmyeloablative | 0.63 | 0.44-0.89 |
| Anti-thymocyte globulin | .39 | Yes vs no | 1.14 | 0.84-1.55 |
| Source of stem cells | .99 | Bone marrow vs peripheral blood | 1.00 | 0.70-1.44 |
- Abbreviations: CI, confidence interval; HR, hazard ratio.
- a Risk factors for occurrence of CMV infection were investigated using Fine and Gray competing risk model with relapse and death as competing risks.
- b Univariate analysis (P < .20 for variables included in multivariate analysis): age (P = .8083), sex (P = .5758), underlying disease (P = .5505), disease status (P = .9799), previous allo-HCT (P = .9586), donor CMV serological status (P < .0001), type of donor (P = .0001), conditioning regimen (P = .0021), antithymocyte globulin (P = .0487), and source of stem cells (P = .2016).
3.4 Treated CMV infections and overall mortality
A total of 395 patients were alive at 1 year and 316 patients at 5 years after allo-HCT transplantation. According to Kaplan-Meier estimations, OS was 70.9% at 1 year and 59.0% at 5 years after transplantation.
The most frequent causes of the 165 deaths at 1 year were relapse or progression of underlying disease (n = 54; 32.7%), GvHD (n = 26; 15.8%) and non-CMV infections (n = 22; 13.6%). CMV infection was the direct cause of the death of two patients (1.2%).
In a multivariate analysis, the 1-year overall mortality was significantly increased in patients with treated CMV infections (HR, 1.86; 95% CI, 1.16-3.00; P = .0105) (Table 4). The other factors that increased 1-year overall mortality significantly were older age at transplantation (global P = .009) and relapse or progression of the underlying disease at allo-HCT (global P < .0001).
| Baseline variables | Global P value | Modalities | HR | 95% CI | P value |
|---|---|---|---|---|---|
| Treated CMV infection (time-dependent) | .0105 | Yes vs no | 1.86 | 1.16-3.00 | .0105 |
| Age (year) at transplantation | .009 | 40-65 vs >65 | 0.62 | 0.39-0.99 | .0456 |
| <40 vs >65 | 0.39 | 0.21-0.71 | .0022 | ||
| Underlying disease | .367 | Lymphoid neoplasm vs acute leukemia | 0.67 | 0.43-1.04 | .0764 |
| MDS/MPN vs acute leukemia | 0.84 | 0.55-1.29 | .431 | ||
| Other vs acute leukemia | 0.85 | 0.47-1.52 | .583 | ||
| Disease status at transplantation | <.0001 | CR vs RR | 0.38 | 0.26-0.52 | <.0001 |
| PR vs RR | 0.42 | 0.18-0.98 | .0449 | ||
| SD or untreated vs RR | 0.54 | 0.33-0.88 | .0145 | ||
| CMV serological status of donor | .539 | CMV seronegative vs seropositive donor | 1.10 | 0.81-1.51 | .539 |
| Previous allo-HCT | .0870 | No vs yes | 1.89 | 0.91-3.90 | .0870 |
- Abbreviations: CI, confidence interval; CR, Complete remission; HR, hazard ratio; MDS/MPN, myelodysplastic/myeloproliferative neoplasms; PR, partial remission; RR, relapse/progression; SD, stable disease.
- a Multivariate Cox regression analysis after univariate analysis (P < .20 for variables included in multivariate analysis): treated CMV infection (P = .0412), age at transplantation (P = .0037), sex (P = .4068), underlying disease (P = .0349), disease status at transplantation (P < .0001), previous allo-HCT (P = .0525), CMV serological status of donor (P = .1775), type of donor (P = .3974), conditioning regimen (P = .7468), and total body irradiation (P = .4941).
3.5 Other clinical complications of allo-HCT
A majority of patients (n = 462; 80.8%) experienced at least one non-CMV infection within the 1st year after transplantation. Most infections were bacterial (n = 658; 58.0%) and viral (n = 383; 32.7%). Fungal (n = 102; 8.7%) and parasitic (n = 7; 0,6%) infections were less frequently observed.
A total of 382 (66.8%) patients experienced at least one episode of acute GvHD. The severity of acute GvHD was grade I and II in 243 cases (70.6%) and grade III to IV in 101 cases (29.4%); this data was missing for 38 patients. Chronic GvHD was reported in 187 (32.7%) patients, and when observed, was extensive and in 98 patients (52.7%) and limited in the remaining 88 patients (47.3%).
A graft failure was observed in four (0.7%) patients and 116 patients (20.3%) experienced a relapse or progression during the 1st year after transplantation.
In a multivariate analysis, occurrence of treated CMV infection was significantly associated with a higher risk of infections other than CMV (HR, 2.34; 95% CI, 1.63-3.37; P < .0001; Supporting Information Table). No association was observed between occurrence of treated CMV infection and acute or chronic GvHD (note that only 90 out of 187 events were included in the model due to missing dates of events). The analysis of the incidence of graft failure was not performed due to the very low incidence of events (n = 4). Treated CMV infection was not significantly associated with a risk of relapse of underlying disease, but was associated with an increased risk of nonrelapse mortality (HR, 2.62; 95% CI, 1.59-4.30; P = .0001).
3.6 Length of stay and cost
At least one CMV infection was experienced by 227 patients within the 1st-year of follow-up after the index procedure (ie, allo-HCT): during the index hospitalization for 74 patients and after the index hospitalization for 153 patients.
The mean ± SD duration of hospital stay during the index hospitalization was 56.7 ± 25.2 days for patients with CMV infections and 36.1 ± 20.3 days for patients without CMV infections. For the 402 patients who were rehospitalized (1248) after the index hospitalization, the mean ± SD duration of hospital stay was 27.2 ± 44.8 days for patients without any CMV infection, 51.1 ± 48.2 days for patients with CMV infection during the index hospitalization and 49.4 ± 45.9 days for patients with CMV infections after the index hospitalization.
The association between CMV infection and hospital stay duration was assessed with the Fine and Gray competing risk model. There was a significant association between CMV infection at index hospitalization and hospital stay duration at allo-HCT (P < .0001) and also between CMV infection at other hospitalization and hospital stay duration (P = .0007).
Mean ± SD hospitalization cost from index (allo-HSCT) hospitalization until the end of the 1st year after transplantation was €61 180 ± 17 111. This cost was significantly higher in patients with CMV infection compared to patients without CMV infection: €66 002 ± 17 424 vs €60 454 ± 16 962, respectively (P = .009).
Mean costs of rehospitalization during the first posttransplant year were significantly higher for patients with CMV infection either during or after the index hospitalization compared to patients without any CMV infection: €27 335 ± 22 208 and €29 517 ± 33 722 vs €15 360 ± 19 190, respectively (P < .001).
A case-control study compared patients having experienced a CMV infection during the study to patients who did not (controls). The hospitalization costs occurring between the CMV infection and last follow-up (and between the match date and last-follow-up for the controls) were calculated. The cohort with a CMV infection incurred a significantly higher mean hospitalization cost (€46 853 ± 41 459) compared to the controls (€31 318 ± 37 913) during the first posttransplant year (P < .0001).
4 DISCUSSION
This retrospective cohort enrolled CMV-seropositive adult patients who underwent allo-HCT between 2010 and 2014 in three French centers. All patients were seropositive for CMV, which is not only a major risk factor for CMV infection after allo-HCT, but is also considered as a surrogate marker of increased mortality.15 CMV infection episodes requiring treatment were observed in 39.7% of patients and almost all episodes occurred during the first trimester. A multivariate analysis confirmed that CMV-seronegative donor and mismatched donor were risk factors for treated CMV infection as reported in other studies.16-18 Recipients of nonmyeloablative stem cells transplantation are usually recognized to have a reduced risk of early CMV disease (but to have an increased risk of late CMV disease).19, 20 In our cohort, patients with a nonmyeloablative conditioning regimen were surprisingly at higher risk of treatment for a CMV infection. In the recent cohort of Sousa et al,17 no significant association of CMV infection with the conditioning regimen was reported.
The 1-year OS was 70.9% in the entire cohort and was consistent with French national data for the 2008 to 2015 period (1-year OS, 70.1%).10 After adjusting for significant risk factors including age, underlying disease, disease status at transplantation, CMV serological status of donor, and previous allo-HCT, overall mortality at 1 year was significantly increased in patients with treated CMV infections (HR, 1.86; 95% CI, 1.16-3.00; P = .011). Comparable results have been reported in recent cohorts. Thus, in the retrospective study of Sousa et al including 305 allo-HCT patients (CMV seropositive, 86.2%), the risk of death was significantly increased in patients with CMV infections (OR, 1.76; 95% CI, 1.07-2.90; P = .025). In the retrospective study of Webb et al,7 the analysis of 310 allo-HCT patients showed a trend for an increased mortality with CMV infection although statistical significance was not achieved (HR, 2.5; 95% CI, 0.9-6.5). In the large retrospective study of Takenaka et al21 including 3539 patients (CMV-seropositive recipients, 68%), CMV infection was associated with a significant increase in overall mortality (HR, 1.30; 95% CI, 1.11 to 1.51; P < .01).
In this multivariate analysis, we did not observe a significant relationship between donor CMV-serostatus and 1-year OS. The role of the CMV serological status of the donor on survival is controversial in CMV-seropositive recipients. Ljungman et al22 analyzed 7018 patients from the EBMT registry who were seropositive for CMV. In the case of an unrelated donor, an improved 5-year survival rate was reported for CMV-seropositive donors (35% vs 27% for a seronegative donor; HR, 0.8; P = .006). This difference in survival was not observed for sibling donors. The authors concluded that the effects of the serological status of the donor were more pronounced in at-risk CMV-seropositive recipients.16 However, HLA-DP-mismatch and CMV reactivation were found to be independent risk factors for GVHD.23
Besides treated CMV infection, older age of recipients and disease status were independent risk factors for mortality within 1 year after allo-HCT as previously observed in other cohorts. Thus, an increased risk of death with age was reported by Sousa et al.17 for patients greater than 38 years (OR, 1.89: 95% CI, 1.14-3.12; P = .137) and by Takenaka et al21 for patients greater than 50 years (OR, 1.57; 95% CI, 1.33-1.86; P < .01). An increased mortality for advanced disease vs standard was also reported by Takenaka et al21 (HR, 1.41; 95% CI, 1.20-1.65; P < .01).
The relationship between treated CMV infection and allo-HCT complications has been assessed in a multivariate analysis. Thus, treated CMV infection during the 1st year after transplantation was significantly associated with a higher risk of infections other than CMV (HR, 2.34; 95% CI, 1.63-3.37; P < .0001). No association was found between treated CMV infection and relapse of underlying disease. This was consistent with the analyses of other cohorts, which did not report an increase in relapse after CMV infection.7, 17, 21, 24 As previously reported in the cohort of Sousa et al,17 we did not observe a relationship between treated CMV infection and acute or chronic GvHD. However, dates of events were missing in half the patients with chronic GvHD and statistical analysis could thus have been underpowered.
The relatively small increase of mean hospitalization costs seen for patients with CMV infection is probably related to their DRG grouping. Indeed, the degree of severity of “diagnosis related group” was already high (Levels 3 or 4) for patients hospitalized for a HCT even without CMV due to the comorbidities of these patients. Thus, 51.7% patients without CMV infection at index hospitalization were at Level 3% and 39.1% at Level 4. As a consequence, hospital revenue, based on DRG tariffs, hardly increases, even though more hospital resources are required for managing patients with CMV infection.
These considerations notwithstanding, our economic analysis suggests that CMV infection contributes to significant higher hospital costs for CMV-seropositive patients. This is in line with other medico-economic analyses performed in France and other countries that show an increase of the financial burden due to CMV infection in allo-HCT patients managed with a preemptive strategy.24 A US study concluded that CMV infection in recipients of allo-HCT led to an additional cost of $58 000-$74 000 per patient within the first 6 months due to antiviral medications and longer hospitalizations.6 In another US study, the relative cost increase was 14% with an estimated incremental cost of $24 892 per transplantation complicated by CMV infection.7 In nine major US cancer centers, the direct costs of CMV infection in allo-HCT patients varied from $24 298 to $57 358 according to center.8 In a French study, the occurrence of more than one CMV infection episode increased the cost of allo-HCT by 25% to 30%.9
There are some limitations in our study. As in any retrospective study, some bias in data reporting and results of analysis cannot be discounted. A careful adjustment to account for confounding variables was performed but some unknown factors could have biased outcomes. Some data were not available for all patients. Therefore, models used to evaluate the association between different parameters should be interpreted with caution. In the economic analysis, only hospitalization costs were analyzed and the costs of day admissions and in-hospital expensive drugs on top of the DRGs were not considered (data on dose and formulation were missing).
The current clinical practice to manage CMV reactivation in CMV-seropositive patients includes prophylaxis of CMV infections, prevention of clinical CMV disease, and treatment of manifest CMV-disease.25, 26 However, due to the hematotoxic and nephrotoxic effects of anti-CMV treatments, European and French clinical guidelines do not recommend prophylaxis to avoid CMV reactivation but rather recommend initiating anti-CMV treatments to prevent CMV disease after a documented CMV reactivation (PET).12, 13, 25, 27, 28 Letermovir received an approval from the European Medicines Agency in January 2018 and was assessed by the French health technology assessment agency (Haute Autorité de Santé) as a treatment with a high clinical benefit and a minor improvement in actual clinical benefit in September 2018.29, 30 Letermovir is an antiviral agent that inhibits the CMV DNA terminase complex (CMV replication), whereas other antiviral drugs such as ganciclovir, valganciclovir, foscarnet, and cidofovir inhibit viral DNA polymerase.31 In a double-blind randomized clinical trial, treatment with letermovir conferred a significantly lower risk of clinically significant CMV infection compared to placebo with a limited toxicity among CMV-seropositive transplant recipients.32, 33 Adverse events with letermovir were mainly of low grade. Furthermore, a recent cost-effectiveness study conducted from the Italian National Health Service concluded that the use of letermovir CMV prophylaxis in adult R+ patients receiving allogenic HSCT compared with a no-prophylaxis strategy, would be cost-effective considering incremental cost-effectiveness thresholds of 40 000 €/QALY (quality-adjusted life year) and of 25 000 €/QALY defined as the additional cost for the payer to save 1-year of life in perfect health.34 Therefore, prophylactic treatment with letermovir could be a useful therapeutic strategy to decrease the burden of CMV infection in high-risk patients.
In conclusion, in this large cohort of 572 CMV-seropositive adults undergoing allo-HCT who were monitored with a PCR-based strategy according to routine clinical practice, treated CMV infections were significantly associated with an increased 1-year risk of non-CMV infections, nonrelapse mortality and overall mortality and with increased length of stay and hospitalization cost. In the era of surveillance and preemptive management, CMV infection after allo-HCT remains a major concern that affects mortality and morbidity.
ACKNOWLEDGMENT
We thank Francis BEAUVAIS (Medical and Scientific Writing, Sèvres, France) for his assistance in writing the manuscript.
CONFLICTS OF INTERESTS
Régis Peffault De Latour received research funding from, consulted for, and received honoraria from Alexion, Pfizer, and Novartis, and received research funding from Amgen. Patrice Chevallier received honoraria from MSD. Sarah Alami and Laurie Levy-Bachelot are employees of MSD France. Ibrahim Yakoub-Agha received honorarium from MSD, Biotest, Astellas and Gilead. Didier Blaise, Thierry Allavoine, Alain Duhamel, Abir Tadmouri, Josefin Blomkvist, Micha Srour, and David Beauvais declared no conflict of interest.
AUTHOR CONTRIBUTIONS
Study conception and design: all authors. Statistical analysis plan: AT, JB, and AD. Statistical analyses: AT and JB. Interpretation of data: all authors. Drafting of the manuscript: RPDL and SA. Critical revisions of the manuscript and final approvement: all authors.




