Tree shrew bone marrow–derived mesenchymal stem cells express CD81, OCLN, and miR-122, facilitating the entire hepatitis C virus life cycle
Caixia Lu and Yue Feng contributed equally to this study.
Abstract
Hepatitis C virus (HCV) infection is a major cause of chronic liver disease and associated cirrhosis, and hepatocellular carcinoma worldwide. At present, there is no prophylactic vaccine against HCV due to the lack of in vivo and in vitro model systems. Although most recombinants of all major HCV genotypes replicate in Huh-7 cell line and derivatives, these cells are human hepatoma-derived cell line. Therefore, the development of un-tumor-derived cell systems facilitating the entire HCV life cycle is urgently needed. In this study, we aimed to establish a novel tree shrew-derived bone marrow–derived mesenchymal stem cell (BM-MSC) system to reconstruct the HCV life cycle. We transduction cluster of differentiation 81 (CD81), occludin (OCLN), and microRNA-122 (miR-122) into BM-MSCs, then used a well-established HCV, produced from the J6/JFH1-Huh7.5.1 culture system, to infect the cells. We observed that BM-MSCs transduction with CD81/OCLN or CD81/OCLN/miR-122 support HCV RNA replication and infectious virus production. We also found that the addition of exogenous vascular endothelial growth factor (VEGF) can enhance HCV infectivity in BM-MSCs, with HCV virus load up to 105 copies/mL. In conclusion, we identified the minimum essential factors required for HCV replication in tree shrew-derived nonhuman nonhepatic BM-MSCs. Further, we identified that exogenous addition of VEGF, and exogenous expression of CD81, OCLN, and miR-122, facilitates efficient viral replication and production of infectious particles. Our results describe a novel cell system capable of supporting the entire HCV life cycle, which may provide an essential tool for anti-HCV drug discovery, vaccine development, and study of pathogenesis.
Highlights
-
Tree shrew bone marrow-derived mesenchymal stem cells (BM-MSCs) transduction with CD81/OCLN or CD81/OCLN/miR-122 support HCV RNA replication and infectious virus production.
-
The addition of exogenous vascular endothelial growth factor (VEGF) can enhance HCV infectivity in BM-MSCs.
-
The exogenous addition of VEGF, and exogenous expression of CD81, OCLN, and miR-122, facilitates efficient viral replication and production of infectious particles.
1 INTRODUCTION
Hepatitis C virus (HCV) is a single-stranded positive RNA virus of the Flaviviridae family. It is one of the main causes of cirrhosis and hepatocellular carcinoma worldwide.1 More than 70 million people are chronically infected with HCV,2 and ~80% of these develop chronic infections, which can progress to liver cirrhosis and hepatocellular carcinoma. Despite the development of novel direct-acting antiviral agents (DAA), the frequency of chronic carriers has only marginally decreased.3 Furthermore, DAA treatment is expensive and drug resistance is emerging. Vaccination is the most cost-effective strategy to reduce the burden of viral infection globally,4 but there are currently no prophylactic vaccines against HCV. HCV vaccine development has been hampered by a lack of in vivo and in vitro model systems. Natural HCV infection is restricted to humans and chimpanzees, the latter of which are no longer available for research purposes. At present, various recombinants of all major HCV genotypes replicate in Huh-7 cell line and derivatives.5 However, for vaccine development, a cell culture system using tumor-derived cells inevitably increases the risk of contamination of hidden oncogenic elements that might be transferred to vaccine recipients.4 Hence, current HCV cell culture systems are not suitable for vaccine development. Therefore, the development of a new cell system facilitating the entire HCV life cycle is urgently needed.
Studies have reported that cluster of differentiation 81 (CD81), scavenger receptor class B type I (SR-BI), claudin-1, occludin (OCLN), and NPC1L1 are receptors for HCV entry into host cells.6-10 Researchers have also shown that miR-122, a liver-specific miRNA, promotes HCV replication, which was the first demonstrated by the association of miRNAs with viral replication.11 miR-122 promotes HCV infection by binding two binding sites close to the 5′end of the 5′-untranslated region (5′-UTR) of the HCV genomic RNA. Recruitment of Argonaut (Ago)-containing protein complexes masks the viral RNA 5′end and stabilizes it against nucleolytic degradation. At the same time binding the 5′-UTR also stimulates HCV RNA translation directed by the internal ribosome entry site located downstream of miR-122 binding sites.12 The cells which contain these factors can be infected by HCV. Another study showed that human CD81 and OCLN comprise the minimal set of human factors required for HCV uptake into murine cells.13 This led us to hypothesize that nonhepatic cells expressing CD81, OCLN, and miR-122 may have the ability to support HCV replication. To test this hypothesis, we used bone marrow–derived mesenchymal stem cells (BM-MSCs) derived from trees shrew.
The tree shrew (Tupaia belangeri chinensis) is a small animal, and whole-genome analysis demonstrates a close genetic relationship between tupaias and humans.14 It has been documented that tree shrews are susceptible to HCV infection,15-17 and the primary tupaia hepatocytes (PTHs) can also be infected with HCV.18, 19 However, PTHs cannot be passed on, which limits their application. Mesenchymal stem cells (MSCs) are a heterogeneous subset of stromal stem cells that are multipotent and have the capacity to self-renew.20 BM-MSCs allow robust in vitro culture expansion and can be transduction with a variety of genes through transduction with viral vectors. This allows the cells to express the gene efficiently, while maintaining the characteristics of BM-MSCs. Hence, in this study, we used BM-MSCs to establish an HCV cell model, by transfecting CD81, OCLN, and miR-122 into BM-MSCs. We then used a well-established HCVcc, produced from the J6/JFH1-Huh7.5.1cell culture system, to infect cells, aiming to establish a novel permissive cell line for robust propagation of HCVcc by induced expression of CD81, OCLN, and miR-122 in BM-MSCs.
2 MATERIALS AND METHODS
2.1 Animals
The tree shrews used in this study were the first filial generation and were provided by the Institute of Medical Biology, Chinese Academy of Medical Science and Peking Union Medical College in Kunming, China. The tree shrews were healthy and consistent with the group standards for tree shrews (T/CALAS 08-2017 and T/CALAS 09-2017), without visible signs of tumorigenesis or disease, with body weights between 120 and 150 g. All animal procedures were approved by the Ethical Committee for Animal Research in the Institute of Medical Biology, Chinese Academy of Medical Science and Peking Union Medical College.
2.2 Cells
BM-MSCs isolation and culture from femur and tibia of tree shrew were performed as previously described.35 Huh7.5.1 cells were provided by professor Xueshan Xia.
2.3 Virus stock
HCVcc is derived from J6/JFH1 (HCV2a) which was cultured in Huh7.5.1cells. Huh7.5.1 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) high glucose supplemented with 8% (vol/vol) FBS, 100 IU/mL penicillin, and 100 μg/mL streptomycin to 60% confluency. Huh7.5.1 cells were then infected with HCVcc and washed twice with phosphate buffered saline (PBS) after 24 hours. We collected the supernatant after culturing the Huh7.5.1 cells in fresh medium for 4 days, then subjecting the supernatant to sterile filtration with a syringe top filter of pore size 0.2 μm. At the same time, Huh7.5.1 cells were digested at a ratio of 1:1, the supernatant was equally added to the digested cells again and adding fresh medium according to the scale of the culture bottle. Cells were incubated at 37°C with 5% CO2 for 4 days, before harvesting the supernatant and filtering with a syringe top filter of pore size 0.2 μm and storing at −80°C.
2.4 Production of recombinant lentiviruses
Lentivirus stocks carrying human constructs of the two HCV entry factors (CD81 and OCLN) and miR-122 were generated briefly as follows: Lentivirus constructs were transduced into 293T cells using the LipofiterTM method. Transduction cultures were maintained for 6 hours, then the supernatant was removed, before adding fresh medium and harvesting this at 48 and 72 hours. Supernatants were centrifuged at 4°C, 2000 × g, for 10 minutes, and then ultracentrifuged at 4°C, 82700 × g, for 120 minutes. Lentivirus stocks were aliquoted and stored at −80°C.
2.5 Lentiviral transduction
A cell suspension (2 mL) was added at a concentration of ~1 × 106 BM-MSCs cells per well of a 6-well dish. When cells were 60% confluent, they were transduced with lentiviral constructs at MOI = 30, alongside a final concentration of 4 μg/mL polybrene in 1/2 volume. After gently mixing, the cells were incubated at 37°C with 5% CO2 for 4 hours. Cells were then supplemented fresh medium with another 1/2 volume containing 4 µg/mL polybrene. The next day, the old culture medium was replaced with fresh culture medium without polybrene and incubated at 37°C with 5% CO2 for 72 hours.
2.6 Culture of BM-MSCs expressed CD81/OCLN/miR-122 and HCV infection
BM-MSCs were maintained in DMEM/F12 supplemented with 10% fetal bovine serum (FBS) and 100 U/mL penicillin and 100 μg/mL streptomycin. Cultured BM-MSCs were infected with cell culture-grown HCVcc (J6/JFH1) produced from the transduction of Huh7.5.1 cells at MOI = 0.5. Cells were divided into vascular endothelial growth factor positive (VEGF+) and VEGF−(Final Concentration of 50 ng/mL). The infection medium was removed after 12 hours, and cells were washed with 1 mL of PBS five times and PBS from the final wash was collected. Subsequently, 500 µL of fresh medium was added to continue the culturing for 5 days. The culture supernatants were collected on d2, d3, d4, and d5 postinfection for virus load determination using a commercial quantitative reverse transcription polymerase chain reaction (qRT-PCR) kit (Takara; Cat no. 064A).
2.7 The CD81 and OCLN expression analysis
Total RNA was isolated from the cells using the RNAzol (MRC; Cat no. RN 190). Messenger RNA (mRNA) expression of CD81 and OCLN were quantified by Two-step RT-PCR using All-in-OneTM First-Strand cDNA Synthesis Kit (GeneCopoeia; Cat no. AORT-0020) and All-in-OneTM qPCR Mix (GeneCopoeia). The quantitative PCR (qPCR) analysis was performed on a PikoReal PCR system (Thermo Fisher Scientific), with the following protocol: 95°C for 10 minutes, followed by 40 cycles of 95°C for 10 seconds, 60°C for 20 seconds, 72°C for 30 seconds. Solubility curve at 60°C for starting, 95°C for the end. glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA was used as an internal control. The 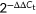 method was used to calculate relative expression. The sequences of CD81, OCLN, and GAPDH-specific primers used for this protocol are listed in Table S1.
method was used to calculate relative expression. The sequences of CD81, OCLN, and GAPDH-specific primers used for this protocol are listed in Table S1.
2.8 The miR-122 expression analysis
RNA was isolated from the cells using miRCURYTM RNA Isolation Kit (Exiqon) in accordance with the manufacturer's instructions. The qRT-PCR analysis of miR-122 using miRCUR YLNATM Universal RT microRNA PCR (Exiqon) was performed on ABI2720. The program was as follows: 95℃, 10 minutes; 95℃, 15 seconds; 60℃, 60 seconds; 45 cycles. U6 was used as an internal control. The 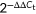 method was used to calculate relative expression.
method was used to calculate relative expression.
2.9 RT-PCR quantification of HCV RNA
Total RNA was isolated from the cellular supernatant using the RNAeasy kit (Qiagen), in accordance with the manufacturer's instructions. RT-PCR primers and Taqman probe are listed in Table S2. The qRT-PCR analysis of HCV RNA using One Step PrimeScript (Takara; Cat no. 064A) was performed on CFX960 (Bio-Rad, CA). The program was as follows: 42°C for 5 minutes, 95°C for 10 seconds, followed by 45 cycles of 95°C for 15 seconds, 58°C for 45 seconds.
2.10 The detection of positive and negative stands of HCV RNA
HCV RNA in the supernatant was extracted with the RNAeasy Kit (Qiagen); the intracellular RNA was extracted with the RNAzol (MRC; Cat. no. RN 190). HCV-positive and -negative stands were detected by reverse transcription reaction and nested PCR using RevertAid First Stand cDNA Synthesis Kit (Thermo Fermentas; Cat. no. K1622) and Dream Tap Green PCR Mater Mix (Thermo Fisher Scientific), respectively, with the following protocol: 25°C for 5 minutes, 42°C for 60 minutes, 85°C for 5 minutes, 4°C for ∞ for reverse transcription reaction. The cDNA was subjected to nested PCR and the protocol was follows: 95°C for 3 minutes, followed by 40 cycles of 95°C for 30 seconds, 62°C for 30 seconds, 72°C for 1 minute, then 72°C for 5 minutes, 4°C for ∞. The sequences of HCV-positive and -negative strand-specific primers used for this protocol were: positive, CGGCAACAAGTATACTCCGCCAACG; and negative, CACTCCCCTGTGAGGAACTACTGTCT. The primers used in the nested PCR are shown in Table S3. The PCR products were tested by electrophoresis on 1.5% agarose gels to identify bands of positive and negative HCV RNA.
2.11 Detection of HCV core by immunofluorescence
Supernatant was collected from BM-MSCs on the third day after HCVcc infection, using 4000 rpm centrifugation for 10 minutes to remove cell debris. Supernatant was then filtered with a syringe top filter, with a pore size 0.2 μm. The naive Huh-7.5.1 cell suspension was seeded at 2 × 105 cells per well of a 96-well dish. When cells were 60% confluent, supernatant from HCV-infected BM-MSCs was added, before incubating cells at 37°C with 5% CO2 for 72 hours. Huh-7.5.1 cells were then fixed in 4% paraformaldehyde and permeabilized. After blocking, HCV-positive cells were visualized by staining with anti-core antibody (Thermo Fisher Scientific; MA1-7366) and Rhodamine Goat Anti-Mouse IgG, and the nuclei were stained with 4′,6-diamidino-2-phenylindole.
2.12 Western blot analysis
Cells for Western blot analysis were lysed in RIPA reagent (Beyotime; Cat no. P0013B) for 10 minutes on ice. Following the pelleting of cell debris, the protein extracts were mixed with sodium dodecyl sulfate (SDS) loading buffer and denatured at 95℃ for 5 minutes. The protein concentration was detected using the BCA assay (Beyotime; Cat no. P0009-1), according to the manufacturer's instructions. We resolved 25 µg protein using 10% (OCLN and β-actin) or 12% (CD81) SDS-polyacrylamide gel electrophoresis. Proteins were then transferred onto polyvinylidene fluoride membranes (Millipore) using semi-dry transfer (Bio-Rad, CA) at 15 V for 65 minutes (OCLN and β-actin) or 35 minutes (CD81). Membranes were blocked with 5% skim milk in TBST and then washed three times on a shaker, at RT, for 5 minutes each time. Membranes were then incubated with antibodies against β-actin (1:5000; Abcam; AB6276), anti-OCLN (1:1000; Abcam; Ab168986), or anti-CD81 (1:500; Genetex; GTX52382). After washing, membranes were incubated for another 1 hour with a secondary anti-rabbit IgG (whole molecule)-Peroxidase antibody (Sigma-Aldrich; A6154) (OCLN and CD81) and anti-mouse IgG (whole molecule)-peroxidase antibody (Sigma-Aldrich; A4416) for β-actin. Protein expression was evaluated using chemiluminescence on a gel imaging system (Bio-Rad, CA).
2.13 Statistical analysis
Significant differences were evaluated using the Student t test. P values of < .05 were considered significant.
3 RESULTS
3.1 HCV replication in BM-MSCs
To establish a novel HCV cell culture system using non-hepatoma-derived cell lines, we first tested the susceptibility of tree shrew-derived BM-MSCs to HCV infection and replication. We did not observe HCV-positive results in BM-MSCs (data not shown). The OCLN, CD81, and miR-122 expression levels in BM-MSCs were quite low compared to the levels in transduced BM-MSCs overexpressing OCLN, CD81, and miR-122 (Figure 1A,B). HCV plus and minus-strand RNA could be detected in all transduction cells on the first day, and the cell supernatant on the third day after HCV inoculation (Figure S1A-D). It is well known that HCV is a small enveloped virus with a single-stranded positive RNA, and therefore the detection of the HCV minus-strand RNA indicated that HCV not only succeeded in cell entry but also replication.
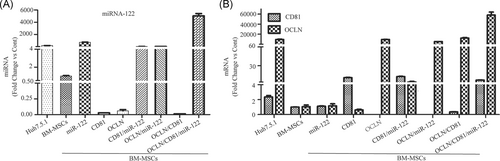
3.2 Infectious virus production in BM-MSCs
To establish a cell line that supported the entire HCV life cycle, OCLN, CD81, and miR-122 were expressed in BM-MSCs via lentiviral transduction. The OCLN, CD81, and miR-122 expression levels of these BM-MSCs transduced with the three molecules simultaneously or separately were higher or similar to Huh7.5.1 cells (Figures 1A,B and 2B). After HCVcc infection, HCV viral load could be detected in the medium of BM-MSCs, which simultaneously or separately expressed OCLN, CD81, and miR-122. Although the viral load in the medium of these cells was still lower than in Huh-7.5.1 cells (Figures 2A and 4D). To test whether infectivity could be transferred to naïve cells, we collected supernatant from BM-MSCs expressing OCLN, CD81, miR-122, CD81/miR-122, OCLN/miR-122, OCLN/CD81, or OCLN/CD81/miR-122 and used it to incubate naïve Huh7.5.1 cells. HCV Core antigen was detected by immunofluorescence after 72 hours of culture. Analysis revealed that the supernatant collected from BM-MSCs overexpressing OCLN/CD81 or OCLN/CD81/miR-122 could express HCV Core antigen after inoculation with Huh-7.5.1 cells (Figure 2C,D). However, the HCV Core antigen was not detected in the supernatant of other cells. These results indicate that BM-MSC cell lines coexpressing OCLN/CD81 or OCLN/CD81/miR-122 can support efficient HCV RNA replication and production of infectious particles.
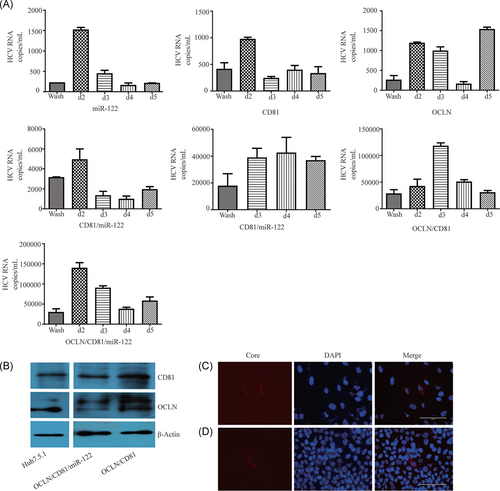
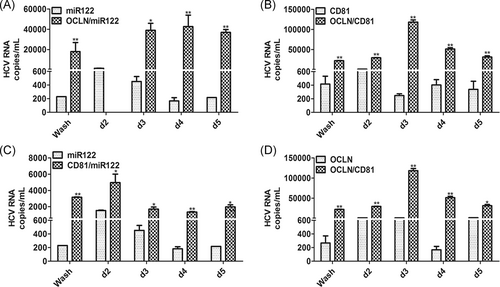
3.3 Effect of OCLN, CD81, and miR-122 on HCV infectivity in BM-MSCs
In this study, we used BM-MSCs derived from tree shrews to test the impact of host factors (OCLN, CD81, and miR-122) on HCV cell entry. The results showed that BM-MSCs expressing OCLN, CD81, and miR-122 supported HCV replication (Figure 2A), and that transduction with OCLN promoted HCVcc infection, compared to transduced with only miR-122 or CD81 (Figure 3A,B). Compared to transduction with only miR-122 or OCLN, the BM-MSCs transduced with CD81 also could promote HCVcc infection (Figure 3C,D). Furthermore, compared to transduction with only OCLN or CD81, transduction with both OCLN and CD81 simultaneously significantly promoted HCV infection after inoculation for d2, d3, d4, and d5 (Figure 3B,D). This confirmed that OCLN and CD81 play a key role in HCV infection in tree shrew-derived BM-MSCs.
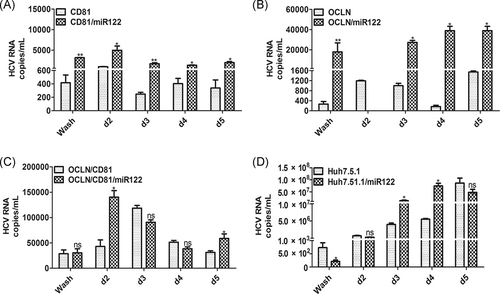
The liver-specific miR-122 is a very important host factor for HCV replication. We introduced miR-122 into BM-MSCs via lentiviral transduction and found that the HCV plus and minus-strand RNA and viral load could be detected in BM-MSCs expressing miR-122 (Figures S1A-D and 2A). Furthermore, the HCV viral load in BM-MSCs expressing OCLN/miR-122, CD81/miR-122, and OCLN/CD81/miR-122 were higher than BM-MSCs only expressing OCLN, CD81, or OCLN/CD81 (Figure 4A-C). At the same time, Huh-7.5.1 overexpressing miR-122 was also able to enhance HCV infectivity to a certain extent (Figure 4D).
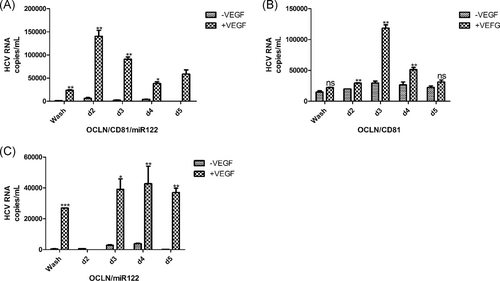
3.4 VEGF increases BM-MSCs infectivity to HCV
Previous studies demonstrated that VEGF regulates hepatocellular tight junction (TJ) integrity and polarity, and significantly reduces OCLN expression at TJs. This promotes basolateral and intracellular pools, and is beneficial to HCV replication in cells.21 To confirm whether BM-MSCs are more susceptible to the HCV after the addition of VEGF, we added 50 ng/mL VEGF to the cells. Our results show that the addition of VEGF to BM-MSCs expressing OCLN/CD81/miR-122, OCLN/miR-122 or OCLN/CD81, increased sensitivity to HCV (Figure 5A-C). The HCV viral load of BM-MSCs expressing OCLN/CD81/miR-122 or OCLN/CD81 increased significantly after infection on d2, d3, and d4 (Figure 5A,B). In BM-MSCs expressing OCLN/miR-122, the HCV viral load increased significantly after infection on d3, d4, and d5 (Figure 5C). The viral loads reached 104-5 copies/mL. However, compared to samples where VEGF was not added, the viral loads were lower (Figure S2).
4 DISCUSSION
The robust in vitro HCV cell culture system uses the HCV JFH1 strain and Huh7-derived cell lines, which were derived from hepatocellular carcinoma. Researchers have used this cell model to investigate the entire life cycle of HCV and develop the anti-HCV drugs. However, for vaccine development, a cell culture system using tumor-derived cells inevitably increases the risk of contamination with hidden oncogenic elements that may be transferred to vaccine recipients.4 The HCV can infect PTHs, but these cannot be subcultured in vitro. For this reason, the development of a new cell system facilitating the entire HCV life cycle is urgently needed. BM-MSCs allow robust in vitro culture expansion, which makes up for the shortcomings of the above cell systems. In this study, we established a novel HCV cell culture system using BM-MSCs derived from trees shrew. We assessed three host factors (CD81, OCLN, and miR-122) and exogenous VEGF for their contribution to the HCV life cycle. We found that CD81 and OCLN were essential for infection, and the expression of miR-122 and addition of exogenous VEGF enhanced replication efficiency.
We obtained OCLN/CD81/miR-122/BM-MSCs that highly expressed CD81, OCLN, and miR-122 via a lentiviral vector, and we observed HCV replication in these cells (Figures 1 and 2), although the level was still lower than that in Huh7.5.1 cells. A possible explanation for this deficiency is that the cell system lacks some host factor that promotes HCV replication. We also observed that HCV can propagate both in OCLN/CD81/miR-122/BM-MSCs and OCLN/CD81/BM-MSCs cells, but the virus load in OCLN/CD81/miR-122/BM-MSCs was higher than that in OCLN/CD81/BM-MSCs (Figure 2A).
HCV cell entry is the first step in infection, and it is well documented that the entry of HCV into hepatocytes is a slow, complex, multistep process,22 which involves both viral (envelope glycoproteins E1/E2) and host factors (cellular receptors and associated factors).23 Studies show that molecules such as CD81, LDLR, SR-BI, OCLN, and claudin-1 are necessary for HCV cell entry.7, 8, 24 CD81 is a tetraspanin family member expressed on a very large panel of cell types and plays an important role in the early stage of normal human hepatocytes infected with HCV,25, 26 and was the first cell entry receptor critical for HCV entry to be identified.27 In our study, we found that BM-MSCs transduced with CD81/miR-122 or OCLN/CD81 had increased HCVcc infection, compared to transduction with miR-122 or OCLN alone (Figure 3C,D). CD81 has also been identified to be an important determinant of HCV-restricted species tropism.22 However, several cell lines expressing ectopic CD81 and SR-BI remained nonpermissive for HCV entry, thus suggesting the existence of other critical entry factors.22 The TJ proteins claudin-1 and OCLN are also identified as cell entry factors allowing virus entry in nonpermissive cell lines.7, 8 Claudin-1 and OCLN are expressed in the hepatocyte apical membranes, which are important mediators of the intercellular junctions between hepatocytes and are essential for the maintenance of the liver tissue. OCLN has been shown to be a critical determinant for the restricted species tropism of HCV with CD81. Downregulation of OCLN decreases both HCV entry and glycoprotein-mediated cell fusion.22 Our studies also showed that transduction of BM-MSCs with OCLN/miR-122 or OCLN/CD81 can promote HCVcc infection, compared to transduction with only miR-122 or CD81 alone (Figure 3A,B). Furthermore, we found that the BM-MSCs simultaneously transduced with CD81 and OCLN exhibited increased HCVcc infection after inoculation at d2, d3, d4, and d5, which was significantly different from that of transduction OCLN or CD81 alone (Figure 3B,D). Our results confirmed that OCLN and CD81 plays a key role in HCV infection in tree shrew-derived BM-MSCs, which is consistent with Ploss A's findings that human CD81 and OCLN comprise the minimal set of human factors required for HCV uptake into murine cells.8, 13
HCV is a hepatophilic virus, partly because it relies on the highly expressed miR-122 in the host liver cells, which binds to two different binding sites at the 5′ end of the viral genome. It protects the viral RNA from degradation by nucleic acid exonucleases and enhances viral replication and translation, thus promoting the transmission of HCV.28 Kato found that the miR-122, SR-BI and ApoE expression in Vero cells facilitated the entire HCV life cycle, identifying miR-122 as an essential factor for HCV replication.4 Other studies also found that transfer of miR-122 into HepG2, Hep3B, Hec1B, and HEK293 cells supports HCV infection.29-31 Our research shows that viral load can be detected after inoculation at d2 and decreases rapidly when BM-MSCs only express miR-122 (Figure 2A). However, we detected higher levels of HCV RNA in BM-MSCs expressed OCLN/miR-122, CD81/miR-122, and OCLN/CD81/miR-122, respectively, after inoculation at d2 (Figure 2A). At the same time, Huh 7.5.1 cells overexpressing miR-122 exhibit enhanced HCV infectivity (Figure 4D). Previous studies have shown that miR-122 can be used as a drug target for anti-HCV therapy. Miravirsen,32 an anti-small RNA drug, was successfully tested in phase II clinical trials. Recently, a second anti-miR-122 drug, RG-101 (N-acetylgalactosamine-bound anti- miR-122 oligonucleotide), has also been tested in clinical trials. Combination of RG-101 with directly acting antiviral drugs greatly reduces HCV viral load.33
Hepatocyte polarity may affect the life cycle of HCV.34 Mee et al21 found that VEGF negatively regulates the hepatocellular TJ integrity and cell polarity. Cell polarity can affect the distribution of HCV co-receptors, therefore, affecting the transmission of HCV in the liver. In this study, we found that adding VEGF significantly improved the infectivity of target cells to HCV and resulted in a higher viral load (Figure 5), compared to the cells without exogenous VEGF (Figure S2). Studies have demonstrated that HCV-induced upregulation of VEGF expression disrupts occludin localization, reduces TJ integrity and promotes viral transmission. Conversely, inhibition of hepatoma-expressed VEGF with the receptor kinase inhibitor Sorafenib or neutralizing anti-VEGF antibodies promotes polarization and inhibition of HCV entry.21
In conclusion, we identified the minimum essential factors required for HCV replication, and the role of exogenous VEGF, in nonhuman nonhepatic tree shrew-derived BM-MSCs. We also identified that exogenous expression of CD81, OCLN, and miR-122 facilitates efficient viral replication and production of infectious particles. Future research will focus on improving the HCV virus load in BM-MSCs, due to that lower than in Huh7.5.1 cells. Even though, our results describe a novel cell system capable of supporting the entire HCV life cycle, which may provide an essential tool for anti-HCV drug discovery, vaccine development and study of pathogenesis.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (No. 31601907 and No. U1702282), the Yunnan Science and Technology Talent and Platform Program (2018HB071), the Yunnan Health Training Project of High-Level Talents (D-2018026), the Kunming Science and Technology Innovation Team (24483), the Yunnan Key Laboratory of Vaccine Research and Development on Severe Infectious Diseases (KF2015-01), the Yunnan Province Major Science and Technology Project (2017ZF007).
AUTHOR CONTRIBUTIONS
JJD and CXL conceived and designed this study. CXL and YF performed the experiments. CXL, JJD, XMSNL, WGW, and XSX analyzed the data. DXK, YYH, and PFT supplied tree shrews. CXL, JJD, and YF wrote the paper, CXL, JJD, and WGW checked and finalized the manuscript.