Risk factors for the development of bronchiolitis obliterans in children with severe adenovirus pneumonia: A retrospective study with dose-response analysis
Abstract
To investigate and analyze the relevant risk factors for bronchiolitis obliterans (BO) in children with severe adenovirus pneumonia, a retrospective study of children with severe adenovirus pneumonia was performed in 34 cases that developing BO and 105 cases not developing BO in Children's hospital of Chongqing Medical University from January 2015 to February 2019. The multivariate logistic regression analysis was used to identify factors which were significantly associated with development of BO after the univariate analysis, and receiver operating characteristic (ROC) curve analysis was performed to find out the cut-off value for the significant relevant factors. A nonlinear dose-response relationship between risk factors and the risk of BO was assessed by restricted cubic spline model. Three factors were independently associated with development of BO, which were length of fever (OR 1.129, 95% CI 1.033-1.234), dyspnea (OR 3.922, 95% CI 1.060-14.514) and invasive mechanical ventilation (OR 6.861, 95% CI 1.854-25.387). The cut-off value of length of fever were 10.5 days. A linear dose-response relationship between length of fever and occurrence of BO was observed (P = .57 for nonlinearity). Children with severe adenovirus pneumonia who have a longer duration of fever (especially more than 10.5 days), have dyspnea or require invasive mechanical ventilation in the acute phase are more likely to develop BO.
Highlights
-
Different with other researchers have reported, we found 24.5% of severe adenovirus pneumonia developed BO.
-
The independent risk factors for BO proved to be length of fever, dyspnea and invasive mechanical ventilation.
-
Children with severe adenovirus pneumonia who have a longer duration of fever, especially more than 10.5 days, are more likely to develop BO.
1 INTRODUCTION
Bronchiolitis obliterans (BO) is an uncommon and severe sequela of chronic obstructive lung disease in children. It implies a chronic necrotizing and fibrosing process affecting the small airways which results in progressive obliteration.1, 2 It is characterized by the presence of signs and symptoms of severe and persistent small airway obstruction.3-5 BO was a chronic obstruction syndrome due to multiple causes. These causes include infections, organ or bone marrow transplants, Steven-Johnson syndrome, connective tissue diseases, toxic inhalation, gastroesophageal reflux, and drug side effects, and et al.
Infection is the most common cause for BO. Adenoviral infection is by far identified the most common cause for post-infectious bronchiolitis obliterans (PBO) in children.6, 7 It has been reported that 47.4% of the adenovirus (AdV) positive children with acute lower respiratory tract infection developed PBO after a 5-year follow-up.8
The prognosis of PBO is overall poor,9 which is related to the late diagnosis, irreversible pulmonary fibrosis, and airway obstruction. There are few studies on the risk factors for BO with severe adenovirus pneumonia. Therefore, It is necessary to identify the risk factors for BO following severe adenovirus pneumonia, to reduce the incidence of BO, take early intervention and improve the prognosis of children.
Our study analyzed the clinical data of 34 patients with severe adenovirus pneumonia who developed BO and 105 patients who did not develop BO, aiming to find out the risk factors for the development of BO with severe adenovirus pneumonia.
1.1 Patients and methods
A retrospective observational study was performed. We included children with severe adenovirus pneumonia hospitalized in Children's hospital of Chongqing Medical University from January 2015 to February 2019. They were between the age of 1 month and 18 years old. We defined children who developed BO as BO group and those who did not develop BO as the control group (Figure 1).
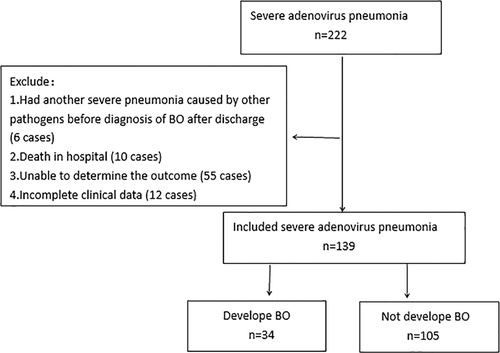
Children were defined of having adenovirus pneumonia if they had a clinical diagnosis of pneumonia with radiological diagnosis of pneumonia made by a pediatric radiologist and positive nasopharyngeal aspiration (NPA) nucleic acid test for AdV. The Chinese Medical Association of Pediatrics case definition of severe pneumonia was used in this study. Children had adenovirus pneumonia with the presence of any one of the following conditions can be diagnosed as severe adenovirus pneumonia: (a) poor general condition, (b) refuse eating or dehydration, (c) disturbance of consciousness, (d) hypoxemia, which include cyanosis, dyspnea (moans, retractions, nasal flaring), respiratory rate increased significantly (>70 times/minute for infant, >50 times/minute for the elder) or pulse oxygen saturation ≤0.92, (e) pulmonary infiltration (multiple lobes involvement or ≥2/3 of the lung), (f) intrapulmonary complications (pleural effusion, pneumothorax, atelectasis, pulmonary necrosis, pulmonary abscess, etc), and (g) extrapulmonary complications (meningitis, pericarditis, endocarditis, osteomyelitis, arthritis, sepsis, hemolytic uremia syndrome, etc).
For diagnosing BO, patients required to fulfill these conditions: had a history of bronchial injury caused by adenoviral infection, persistent or recurrent wheezing or cough, shortness of breath, dyspnea, exercise intolerance, rales for more than 6 weeks and poor response to bronchodilator. High-resolution computed tomography showed alterations suggestive of BO, such as mosaic pattern, bronchiectasis, or atelectasis. Pulmonary function tests demonstrated airflow limitation. Other chronic lung diseases causing wheezing or cough, such as severe asthma, congenital pulmonary dysplasia, and cystic fibrosis were excluded.
Patients with organ and bone marrow transplantation, connective tissue diseases, had diagnosed BO before, had another severe pneumonia caused by other pathogens before diagnosis of BO after discharge, absence of clinical data and those unable to determine the outcome, such as loss of follow-up, death, or diagnosis is not clear were excluded from this study.
NPAs of all subjects were obtained within 24 hours after admission. Indirect immunofluorescence viral testing of nasopharyngeal secretions was performed to identify AdV, respiratory syncytial virus (RSV), influenza, and parainfluenza virus. Polymerase chain reaction was conducted to test AdV and mycoplasma (MP). Sputum culture was completed in all patients. NPA nucleic acid test for AdV of all children in this study showed positive.
1.2 Data collection
Collect data of all children through the medical records system. Data as follows were included sex, age, premature, cesarean delivery, malnutrition, length of fever, length of hospital stay, anemia, laboratory findings, imaging findings, coinfection, complications, treatment, and so on.
Definition: we defined malnutrition as underweight and loss of subcutaneous fat. Define anemia as hemoglobin less than 90 g/L in children aged 1 month to 4 months, less than 100 g/L aged 4 months to 6 months, less than 110 g/L aged 6 months to 6 years, and less than 120 g/L aged 6 years to 18 years. Dyspnea means with the presence of tachypnea (respiratory rate more than 60 times/minute aged 1 month to 2 months, more than 50 times/minute aged 2 months to 12 months, more than 40 times/minute aged 1 year to 5 years, more than 30 times/minute above 5 years old), cyanosis, moans, retractions, or nasal flaring.
1.3 Statistical analysis
Statistical analysis was conducted using SPSS 24.0. The χ2 test was used to compare the rates. The Mann-Whitney U test was used to compare the mean (interquartile range), because the variance did not satisfy normal distribution. Variables that had a P < .05 in the univariate logistic analysis were included in the multivariate logistic regression analysis. Multicollinearity diagnostic tests for these variables were carried out by variance inflation factor (VIF). It is considered that a VIF greater than 10 roughly indicates statistically significant problem of multicollinearity. Multivariate analysis using stepwise forward selection was used to create a logistic proportional hazards model to determine the independent risk factors for BO. The inclusion criteria for factors was P < .05, and the exclusion criteria was P > .1. The sensitivity and specificity of the model were evaluated using the receiver operating characteristic (ROC) curve analysis. ROC curve analysis was performed to find out the cut-off value for the significant relevant factors which were continuous variables. Potential nonlinear dose-response relationship between factors which were continuous variables and the risk of BO were assessed using restricted cubic spline functions with three knots at fixed percentiles of 10%, 50%, and 90% of the distribution.
1.4 Ethics statement
The study protocol was approved by the Ethical Review Committee of Children's Hospital of Chongqing Medical University.
2 RESULTS
2.1 Characteristics of included children with severe adenovirus pneumonia
A total of 222 patients were diagnosed with severe adenovirus pneumonia, and 139 children (99 boys and 40 girls) were included in our study (Figure 1). Mean age of two groups were 15.1 (7.2) months and 20.5 (14.6) months. Table 1 shows the clinical characteristics of the two groups.
| BO group | Non-BO group | ||
|---|---|---|---|
| (n = 34) | (n = 105) | P | |
| Sex (male/female) | 26/8 | 73/32 | .437 |
| Age, mo | 15.1 (7.2) | 20.5 (14.6) | .902 |
| Premature birth | 1 (2.9) | 7 (6.7) | .699 |
| Cesarean | 21 (61.8) | 51 (48.6) | .181 |
| Malnutrition | 3 (8.8) | 6 (5.7) | .811 |
| Anemia | 25 (73.5) | 51 (48.6) | .011 |
| Length of hospital stay, d | 20.0 (12.5) | 10.6 (5.0) | .000 |
| Length of fever, d | 14.0 (5.8) | 8.0 (5.0) | .000 |
| Peak temperature (℃) | 40.0 (0.4) | 38.2 (0.8) | .007 |
| Dyspnea | 30 (88.2) | 60 (57.1) | .001 |
| Laboratory findings | |||
| ALT, U/L | 39.6 (24.4) | 33.9 (20.1) | .045 |
| AST, U/L | 113.2 (74.6) | 66.4 (37.0) | .003 |
| ALB, g/L | 33.1 (13.9) | 38.6 (8.8) | .000 |
| LDH, U/L | 1027.9 (794.4) | 533.2 (258.7) | .000 |
| PCT, ng/mL | 7.1 (6.6) | 1.3 (1.2) | .005 |
| CRP, mg/L | 25.7 (21.2) | 20.0 (18.0) | .009 |
| WBC (×109/L) | 9.3 (6.0) | 11.4 (7.1) | .168 |
| PLT (×109/L) | 317.9 (236.0) | 357.8 (242.0) | .250 |
| Neutrophil proportion | 0.62 (0.19) | 0.56 (0.29) | .158 |
| Lymphocytes proportion | 0.33 (0.20) | 0.39 (0.27) | .113 |
| Imaging findings | |||
| Segmental pulmonary consolidation | 16 (47.1) | 51 (48.6) | .880 |
| Atelectasis | 4 (11.8) | 18 (17.1) | .460 |
| Pleural effusion | 7 (20.6) | 25 (23.8) | .700 |
| Pulmonary necrosis | 0 (0) | 2 (1.9) | 1.000 |
| Co-infection | 31 (91.2) | 81 (77.1) | .072 |
| MP | 3 (8.8) | 6 (5.7) | .811 |
| Bacteria | 27 (79.4) | 59 (56.2) | .015 |
| Virus | 14 (41.2) | 46 (43.8) | .788 |
| Extrapulmonary complications | |||
| Digestive system | 24 (70.6) | 53 (50.5) | .040 |
| Cardiovascular system | 5 (14.7) | 6 (5.7) | .186 |
| Hematologic system | 12 (35.3) | 22 (20.6) | .091 |
| Nervous system | 13 (38.2) | 22 (20.6) | .022 |
| Treatment | |||
| Invasive mechanical ventilation | 13 (38.2) | 4 (3.8) | .000 |
| Length of mechanical ventilation, d | 5.1 (7.8) | 1.5 (0.0) | .000 |
| Use of γ-globulin | 29 (85.2) | 58 (55.2) | .002 |
| Use of glucocorticoids | 16 (47.1) | 36 (34.3) | .181 |
- Note: Data are shown as median (interquartile range) or number (%).
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB, albumin; BO, bronchiolitis obliterans; CRP, C-reaction protein; LDH, lactate dehydrogenase; MP, mycoplasma; PCT, procalcitonin; PLT, platelet; WBC, white blood cell.
Intrapulmonary complications were found in 93 patients (66.9%). Total, 56.1% of patients had respiratory failure, 23.0% pleural effusion, 15.8% atelectasis, 2.9% acute respiratory distress syndrome, 1.4% bronchiectasis, 1.4% pneumothorax, and 0.7% empyema.
Extrapulmonary complications were found in 77.7% of patients. Seventy-seven cases (55.4%) had complications of digestive systems, among them 96.1% had diarrhea and 5.2% had toxic intestinal paralysis. Total 20.9% of patients had neurological symptoms with normal cerebrospinal fluid, 18.0% damage of liver function, 15.8% electrolyte disturbance, 13.7% secondary thrombocytosis, 6.5% pyemia, 5.0% coagulation disorders, 4.3% myocardial damage, 4.3% rash, 3.6% encephalitis, 2.2% conjunctivitis, 1.4% septic shock, 1.4% thrombus of jugular vein which all in patients with extracorporeal membrane oxygenation support, and 0.7% hemophagocytic lymphohistiocytosis.
A total of 80.6% (n = 139) of patients were associated with co-infection. 61.9% (n = 139) of patients had mixed bacterial infection, and there was statistically significant difference between the two groups (P < .05). Haemophilus influenzae, streptococcus pneumoniae, Klebsiella pneumoniae, and Staphylococcus aureus were most common. 43.2% (n = 139) of patients mixed virus infection, 6.5% MP infection, 4.3% bordetella pertussis infection, and 0.7% measles.
2.2 Predictors of BO following severe adenovirus pneumonia
Potential risk factors for development of BO on univariate and multivariate analysis that with statistical significance (P < .05) are shown in Table 2. Fifteen variables with statistical significance after univariate analysis were selected for multicollinearity test. There was no multicollinearity between these variables with VIF all less than 10. When multivariate analysis was performed, the independent risk factors for BO proved to be: length of fever (X1), dyspnea (X2), and invasive mechanical ventilation (X3). Multivariate analysis showed that the predictive probability model for independent risk factors for BO was: Logit(P) = (−3.715) + 0.122 X1 + 1.367 X2 + 1.926 X3, (Table 3).
| Factors | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| P | OR (95% CI) | P | OR (95% CI) | |
| Length of fever, d | .000 | 1.173 (1.083-1.271) | .007 | 1.129 (1.033-1.234) |
| Dyspnea | .001 | 7.750 (2.228-26.956) | .041 | 3.922 (1.060-14.514) |
| Invasive mechanical ventilation | .000 | 15.631 (4.637-52.694) | .004 | 6.861 (1.854-25.387) |
| Length of hospital stay, d | .000 | 1.129 (1.058-1.204) | ||
| Peak temperature (℃) | .010 | 2.746 (1.279-5.895) | ||
| Anemia | .013 | 2.941 (1.254-6.899) | ||
| Co-bacterial infection | .039 | 2.534 (1.050-6.116) | ||
| Extrapulmonary complications | ||||
| Digestive system | .027 | 2.623 (1.118-6.154) | ||
| Nervous system | .047 | 2.335 (1.012-5.390) | ||
| Laboratory findings | ||||
| AST, U/L | .011 | 1.007 (1.001-1.012) | ||
| ALB, g/L | .000 | 0.901 (0.852-0.954) | ||
| LDH, U/L | .001 | 1.001 (1.001-1.002) | ||
| PCT, ng/mL | .013 | 1.143 (1.028-1.270) | ||
| Treatment | ||||
| Length of mechanical ventilation | .024 | 1.103 (1.013-1.201) | ||
| Use of γ-globulin | .002 | 4.884 (1.755-13.597) | ||
- Abbreviations: AST, aspartate aminotransferase; ALB, albumin; BO, bronchiolitis obliterans; CI, confidence interval; LDH, lactate dehydrogenase; OR, odds ratio; PCT, procalcitonin.
| Variable | B | SE | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| X1 | 0.122 | 0.045 | 7.182 | .007 | 1.129 | 1.033-1.234 |
| X2 | 1.367 | 0.668 | 4.19 | .041 | 3.922 | 1.060-14.514 |
| X3 | 1.926 | 0.668 | 8.324 | .004 | 6.861 | 1.854-25.387 |
| Constant | −3.715 | 0.723 | 26.382 | .000 | 0.024 |
- Abbreviations: BO, bronchiolitis obliterans; CI, confidence interval; OR, odds ratio; SE, standard error.
2.3 The value of predictors
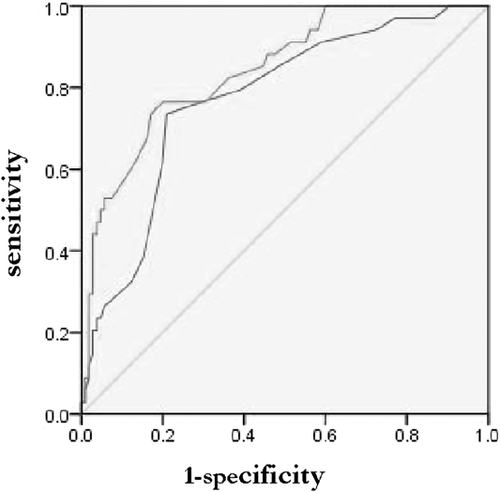
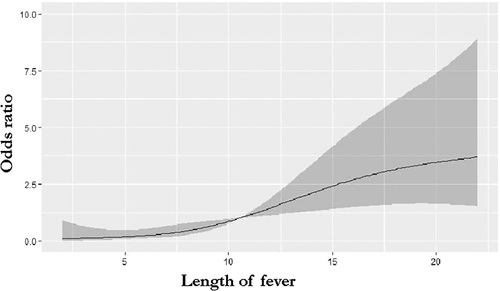
This model was assessed by the ROC curve, which had high diagnostic accuracy by the area of 0. 843 under the curve (95% confidence interval [CI], 0.770-0.917; P < .01) (Figure 2). Length of fever was the only continuous variable in the final model. The cut-off value of fever duration for BO was 10.5 days with a sensitivity and specificity by 0.735 and 0.790 (95% CI, 0.684-0.861; P < .01) (Figure 2). Figure 3 shows that a linear association between length of fever and the risk of BO was found using the restricted cubic splines analysis (P = .57 for nonlinearity).
2.4 Comparisons between two groups divided by duration of fever
We divided these patients into two groups by duration of fever (10.5 days). Total of 47 patients had a duration of fever more than 10.5 days, and they had higher rate of developing BO, longer hospital stay, higher peak temperature, higher incidence of dyspnea and extrapulmonary complications, more use of γ-globulin, and et al (Table 4).
| Longer than 10.5 d | Less than 10.5 d | ||
|---|---|---|---|
| (n = 47) | (n = 92) | P | |
| Developing BO | 25 (53.2) | 9 (9.8) | .000 |
| Length of hospital stay, d | 17.2 (8.0) | 10.7 (5.0) | .000 |
| Peak temperature (℃) | 40.1 (0.5) | 37.9 (0.9) | .000 |
| Dyspnea | 38 (80.9) | 52 (56.5) | .005 |
| Extrapulmonary complications | 43 (91.5) | 65 (70.7) | .005 |
| Invasive mechanical ventilation | 12 (25.5) | 5 (5.4) | .001 |
| Length of mechanical ventilation | 4.1 (6.0) | 1.5 (0.0) | .001 |
| Use of γ-globulin | 40 (85.1) | 47 (51.1) | .000 |
- Note: Data are shown as median (interquartile range) or number (%).
- Abbreviation: BO, bronchiolitis obliterans.
3 DISCUSSION
BO is a severe chronic sequel in children following respiratory viral infection, especially the adenovirus infection.6, 7 In this study, we found 24.5% of severe adenovirus pneumonia developed BO. It's different with other researchers have reported.7, 8, 10
A number of studies have shown that mechanical ventilation is an independent risk factor for BO with adenovirus infection.7-9 Our study discovered that the rate of invasive mechanical ventilation in BO group was significantly higher than that in non-BO group. So it was the same with the rate of intensive care unit (ICU) admission, because all children requiring invasive mechanical ventilation had to be admitted to ICU due to the limitations of our hospital. Previous studies have found that hospital stay longer than 30 days, multiple pulmonary lobes involvement, hypercapnia and hypoxemia are high risk factors for the development of BO after adenovirus infection.6, 7, 10 A prospective study by Castro-Rodriguez et al8 found that children with adenovirus pneumonia who developed BO had higher rate of ICU admission, requiring oxygen therapy, and use of systemic glucocorticoids and beta agonists. We also found that patients in BO group had longer hospital stay, while it was not independent risk factor for BO.
Our study demonstrated that it is more likely to develop BO when the length of fever is longer than 10.5 days. There was a linear association between length of fever and the risk of BO. However, Wu et al10 have showed that the length of fever was not an independent risk factor for BO. Previous studies suggested that the severity of the acute stage of adenovirus pneumonia is related to the appearance of BO.11 All patients in this retrospective analysis had severe pneumonia. The BO group had more use of γ-globulin, more extrapulmonary complications, which may indicate a higher risk of BO in the more severe adenovirus pneumonia.
Colom et al12 created a prediction rule to diagnose PBO in children by BO-Score. Children with typical clinical history, AdV infection, and high-resolution computed tomography with mosaic perfusion are more likely to develop BO. The published model is superior to the model in this study in some ways may be due to the issues related to different inclusion and exclusion criteria or the variables analyzed.
PBO was reported mostly in South America and Asia countries (such as Brazil, Chile, Argentina, Uruguay, South Korea, etc),6, 8, 13 while reported less in mainland China, mainly because of different genes, types, and amount of AdV, the host, immune response, and environmental factors.7, 11 Studies have shown that the occurrence of PBO is related to the high expression of HLA-DR8-DQB1-0302 antigen.14
According to reports,15, 16 3, 5, 7, 21 serotype of adenovirus can lead to BO. The world health organization reported that AdV infection of type 7 accounts for about 20% of severe respiratory diseases caused by AdV infection,17 which is also a high risk factor for long-term complications.18
In addition to the AdV, other pathogens, such as measles, pneumonia MP, RSV, herpes simplex virus, the influenza virus, parainfluenza type 3, human immunodeficiency virus type 1, chlamydia, bordetella pertussis, and cytomegalovirus are associated with BO.15,19-23 In this study, we found mixed infection was not an independent risk factor for BO. Liu et al24 found that there was no significant difference in the clinical characteristics and laboratory findings of patients between single AdV infection and mixed infection.
This study has several limitations. First, the data collected in this study cannot cover all the indicators that may be related to the occurrence of BO, because some indicators can not be measured in our hospital, such as type of AdV. Second, we did not exclude the cases of mixed infection, that could affect outcomes. Further more, it was a single-center study, and the results may not be generalized to other regions.
BO has a poor effect of treatment, and overall has poor prognosis. Colom et al9 found after a 12 year follow-up period, pulmonary function for children with BO remained severely impaired, showing an obstructive pattern with air trapping that slowly improved during childhood. Some children with BO showed improved symptoms, but the absorption of lesions was still not obvious. The study of Sarria et al25 discovered that the quality of life score of PBO children was significantly lower than that of normal children of the same age.
In conclusion, children with severe adenovirus pneumonia who have a longer duration of fever, have dyspnea or require invasive mechanical ventilation in the acute phase are more likely to develop BO. Clinical follow-up is required for children with high risk factors of BO, so as to make early clinical diagnosis, treatment and improve the long-term quality of life for children with BO. Due to the small number of cases in this study, multi-center studies should be conducted to further explore the risk factors of BO in children with severe adenovirus pneumonia in the future.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.




