Prevalence and genetic diversity of human sapovirus associated with sporadic acute gastroenteritis in South China from 2013 to 2017
Abstract
Human sapovirus (SaV) is an important viral agent for acute diarrhea worldwide, but timely prevalence data of human SaV in South China are still lacking. In this study, a 4-year surveillance was conducted to characterize the prevalence and genetic characteristics of the circulating SaV associated with sporadic diarrhea in South China. From November 2013 to October 2017, 569 fecal samples from patients with acute diarrhea were collected. SaV was detected in 11 samples with a positive rate of 1.93%. Three human genogroups of GI, GII, and GIV were identified, including five GI.1 strains, three GI.2 strains, one GI.3 strain, one GII.8 strain, and one GIV strain. Furthermore, multiple alignments of complete capsid protein VP1 genes of five local GI.1 strains and other available GI.1 strains in GenBank were performed. Average pairwise identities were calculated at 95.33% and 99.36% at nucleotide and amino acid levels, and only six variable amino acid sites were found during its 36-years’ evolution process. GI.1 strains could be further phylogenetically divided into four clusters with an approximate temporal evolution pattern, and local strains belonged to Cluster-d with other four strains from China and Japan. In summary, SaV was identified as an etiological agent responsible for sporadic gastroenteritis in Guangzhou with a low prevalence rate as in other Chinese cities, but its high genetic diversity suggested the necessity of continuous SaV surveillance in the future.
Highlight
-
A four-year surveillance of human sapovirus was conducted in South China.
-
The sapovirus positive rate was detected as 1.93% by the RT-PCR method.
-
Three sapovirus genogroups of GI, GII and GIV were all identified.
-
Only six variable amino acid sites were verified on GI.1 capsid protein VP1.
-
GI.1 sapovirus could be divided into four clusters based on its capsid protein VP1.
1 INTRODUCTION
Human sapovirus (SaV) belongs to the common viral agent of acute diarrhea globally, especially after the successful introduction of rotavirus vaccines.1, 2 In China, a domestic Jennerian vaccine (the Lanzhou lamb rotavirus vaccine) has been licensed since 2000, and at the end of 2014, over 60 million doses had been distributed to children.3 SaV can cause disease in people of all age groups, but most infections occurred in children.4 The clinical symptoms of SaV gastroenteritis are similar as those infected by human noroviruses, mainly including diarrhea and vomiting. SaV infection is often caused by the fecal–oral pathway through person-to-person contact or contaminated food and water.5-7 SaVs could cause outbreaks, which often occur in semi-closed settings, such as schools, hospitals, restaurants, and cruises.8-12 However, there are no available vaccines for the control and prevention of SaV infections as of this writing.
SaVs belong to the Sapovirus genus within the Caliciviridae family. It has a positive-sense, single-stranded RNA genome, which is approximately 7.3 to 7.5 kb in size. The SaV genome contains two or three open reading frames (ORFs).13 ORF1 encodes a large polyprotein containing the nonstructural proteins followed by the major capsid protein VP1, and ORF2 is predicted to encode the minor structural protein VP2, which is an alkaline protein.14, 15 In several human and bat variants, the third ORF (ORF3) has been predicted, but its function is still unknown.16
SaVs could be classified into five genogroups (GI-GV) based on complete capsid protein VP1 sequences,17 and recently nine additional genogroups (GVI-GXIV) were proposed.18 GI, GII, GIV, and GV could infect humans, and other genogroups could infect pigs, mink, dogs, sea lions, and rodents.1, 19 Human SaV GI and GII could be further divided into seven genotypes,17 and recently a new genotype GII.8 was reported.20 GIV has only a single genotype GIV.1, and GV could be subdivided into two genotypes; GV.1 and GV.2.17 A series of studies revealed high genetic diversity of human SaVs in patients suffering from acute gastroenteritis.9, 21-23
The importance of SaVs as the cause of acute gastroenteritis has drawn more attention in recent years. SaV infection is also one of the major public health concerns that cause acute diarrhea in China. Monitoring conducted in different cities revealed low positive rates, which ranged from 0.5% to 3.7%.22, 24 However, prevalence data of human SaV in South China in recent years are still lacking. In this study, we conducted a 4-year monitoring of human SaV associated with sporadic gastroenteritis in Guangzhou, aiming to identify the prevalence of SaV infections and their genetic diversity.
2 MATERIALS AND METHODS
2.1 Specimen collection
From November 2013 to October 2017, a total of 569 fecal specimens were collected from patients with acute gastroenteritis in the sentinel hospital, consisting of 177 children under 5 years and 392 older children and adults with their age ranging from 6 to 88 years.25, 26 The collections of this study were approved by the Research Ethics Committee at the Third Affiliated Hospital of Sun Yat-Sen University and the Institutional Review Board at the Chinese CDC for the protection of human subjects. Informed patient/guardian consent was obtained from all patients or their guardian prior to sample collection. All specimens were stored at −80°C until detection.
2.2 Viral RNA extraction and sapovirus detection
All samples were 10% (w/v) diluted using phosphate-buffered saline (PBS) buffer (pH 7.3, diethylpyrocarbonate-treated). After centrifugation at 10 000 g for 1 minute, 140 μL supernatant was transferred for viral RNA extraction using a QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Complementary DNA (cDNA) was first synthesized from viral RNA by using random primers and PrimeScript II 1st Strand cDNA Synthesis Kit (Takara, Dalian, China). The primer set of SLV5317/SLV5749 targeting a 434-bp region (nt position 5083–5516; accession number X86560) was used to detect human SaV with the ExTaq PCR Kit (Takara).27 Besides, a reverse primer SaVI-5R (5′-ACYAACCAACTCATTGGA-3′, nt position 6848-6865; accession number X86560) was designed for amplifying the VP1 encoding regions of GI SaV. Combined with the detection primer SLV5317, the 1783bp VP1 encoding region (nt position 5083-6865; accession number X86560) was amplified using viral RNA as a template with the one-step RT-PCR kit (Takara). In each run, RNase-free distilled water was used as a negative control and a SaV-positive stool sample was used as a positive control. All tests were conducted in different rooms to avoid cross-contamination.
2.3 Sequencing and phylogenetic analyses
All positive amplicons were directly sequenced on an automated sequencer (ABI 3730XL DNA Analyzer; Applied Biosystems) after gel purification, and nucleotide sequences of SaV strains had been submitted to the GenBank database under accession numbers MH477427-MH477437. Nucleotide sequences were first edited with the BioEdit Sequence Alignment Editor software (v.7.0.1). BLASTN was used to compare the detected sequences to homology sequences in GenBank (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Nucleotide sequences or derived amino acid sequences detected in this study with reference sequences were aligned using the ClustalX v1.83 with the default parameters.28 The consensus logos were generated by WebLogo (http://weblogo.threeplusone.com/create.cgi).29 All nucleotide and amino acid sequences of GI.1 strains were also compared to investigate their differences using DNASTAR Lasergene MegAlign program (DNASTAR Inc, WI). Phylogenetic analysis of aligned sequences was performed in MEGA v7.0 software.30 We inferred the maximum-likelihood trees using best nucleotide substitution models for the dataset based with the lowest Bayesian information criterion scores. The reliability of the phylogenetic trees was assessed by bootstrap sampling of 1000 replicates.
3 RESULTS
3.1 Prevalence of human SaV in Guangzhou
From November 2013 to October 2017, 569 fecal specimens were collected from patients with acute gastroenteritis. Using the reverse transcription polymerase chain reaction (RT-PCR) detection method, only 11 human SaV-positive samples (11 of 569; 1.93%) were identified. The number of positive samples detected each year from 2013 to 2017 were two, four, four, zero, and one, respectively (Table 1). Besides, four positive samples (4 of 177; 2.26%) were detected from children under 5 years, and seven were from the older children and adults (7 of 392; 1.79%).
| Year | Samples collected | Positive samples | Positive samples of different genotypes | ||||
|---|---|---|---|---|---|---|---|
| GI.1 | GI.2 | GI.3 | GII.8 | GIV | |||
| 2013 | 62 | 2 (3.23%) | 2 | ||||
| 2014 | 219 | 4 (1.83%) | 1 | 1 | 1 | 1 | |
| 2015 | 136 | 4 (2.94%) | 2 | 1 | 1 | ||
| 2016 | 91 | 0 (0.00%) | |||||
| 2017 | 61 | 1 (1.64%) | 1 | ||||
| Total | 569 | 11 (1.93%) | 5 | 3 | 1 | 1 | 1 |
3.2 Genotype distribution of SaV strains in Guangzhou
To verify the genotypes of the detected SaV strains, the amplified partial VP1 encoding regions by detection primers were all sequenced. Three human SaV genogroups of GI, GII, and GIV were identified by comparing with reference strains (Figure 1). Nine positive strains belonged to the GI genogroup, of which five strains were GI.1, three were GI.2, and one was GI.3. SaV-positive samples collected from children under 5 years (n = 4) all belonged to GI.1, and the remaining GI.1 sample was collected from a 70-year old female. Three GI.2 samples were all from female patients with their ages ranging from 29 to 56, and the GI.3 samples belonged to a male patient aged 39-years old. Only one strain was identified as GIV that was collected from a female adult aged 78-years old. Besides, one GII SaV strain collected from a female adult aged 28-year old was identified that belonged to GII.8.
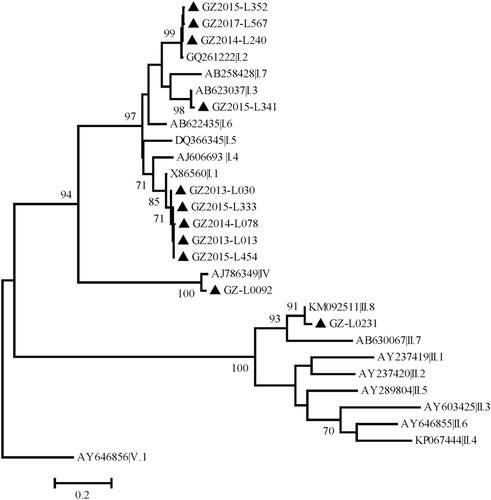
Phylogenetic tree of human sapovirus (SaV) detected in Guangzhou based on the 419 bp-partial capsid protein. The dendrogram was constructed by the maximum likelihood method with the Kimura two-parameter model in MEGA v7.0, and a discrete γ-distribution was used to model evolutionary rate differences among sites (five categories). Numbers at the nodes indicate supporting bootstrap values (%) for 1000 resampled datasets; only values greater than 70% are shown. The scale bar represents the unit for the expected number of substitutions per site. Local NoV strains are designated by location, year, and identification number (indicated by black triangles)
3.3 Nucleotide sequence analysis of capsid protein VP1 of GI.1 SaV strains
To further characterize the genetic diversity of GI.1 SaV strains, full-length VP1-encoding region of local strains was amplified and sequenced for analyses of their genetic diversity. Pairwise similarities between capsid protein VP1 of Guangzhou GI.1 strains were 98.6%-99.9% and 99.8%-100% at the nucleotide and amino acid levels, respectively. BLAST analyses showed only 26 strains with query cover >99% and the identity value >92%, and two Chinese strains (KT327081/Zhejiang1/2014, KX980412/Hunan/2016) showed the highest homology (identity value =99%).
Besides, multiple alignments of VP1 sequences of Guangzhou strains and other available GI.1 strains in GenBank were then conducted (Table S1). The average pairwise identity was calculated as 95.33% (91.90%-100%) at the nucleotide level, but 99.36% (96.60%-100%) at the amino acid level. GI.1 capsid protein showed conservation at the amino acid level during its evolutionary process from 1982 to 2017. Although there are 336 nonconserved nucleotide sites among these sequences, only 31 nonconserved amino acid sites were created, of which only six were identified as variable amino acid sites (8, 100, 333, 382, 524, 557; Figure S1).
Phylogenetic trees were inferred with Guangzhou GI.1 strains and other available sequences, and most strains could be divided into four clusters, GI.1-a to GI.1-d, with an approximate temporal evolution pattern (Figure 2). Besides, Cluster GI.1-a included seven strains, most of which were collected from 1982 to 2001; Cluster GI.1-b included seven strains, which were from Japan in 2008/2009; and Cluster GI.1-c included four strains collected from 1998 to 2007. All Guangzhou strains were clustered as GI.1-d with other two Chinese strains (2014/2016) and two Japanese strains (2001/2008). Four clusters all showed high intracluster nucleotide similarity values of 94.80%, 99.56%, 98.62%, and 98.51% and amino acid values of 98.96%, 99.75%, 99.80%, and 99.71%.
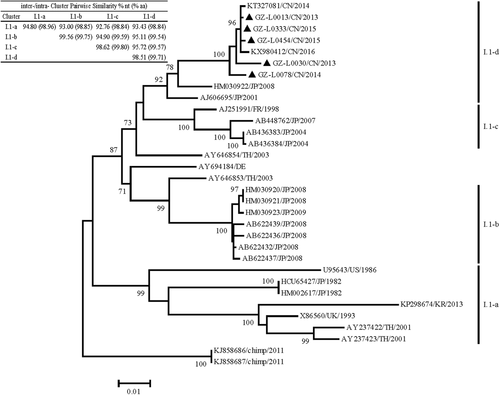
Phylogenetic analysis based on nucleotide sequences of the GI.1 SaV 1686bp-capsid protein VP1. The evolutionary history was inferred by using the maximum likelihood method based on the Tamura-Nei (+G) model in MEGA v7.0. Bootstrap values higher than 70% are shown at the corresponding branches. The scale bar represents the unit for the expected number of substitutions per site. Local SaV strains are designated by location, year, and identification number (indicated by black triangles). SaV, sapovirus
4 DISCUSSION
In addition to noroviruses, SaV is another important pathogen for acute gastroenteritis in many countries.31 In this study, we performed 4-year monitoring of viral agents for acute diarrhea in South China, of which human SaV was identified with a prevalent rate of 1.93%.
In China, SaV detection rates were generally a little more than 1% when using the primer set targeting the 5′ end of the VP1 gene, except for one that reached 3.73%.22, 32, 33 However, when using the primer set for the partial RdRp region, its positive rate was even lower (0.5%; 2 of 436).24 Globally, the low detection rate of SaV has also been reported in many countries. In India, SaV was detected at the rate of 2.7% (21 of 778) for symptomatic patients and 1.9% (4 of 207) for asymptomatic control group from 2007 to 2011.34 In Thailand, only six (0.7%) samples were detected SaV-positive out of 889 fecal specimens from 2012 to 2014.23 However, high rates of SaV infections more than 10% were reported in Latin America and Spain.35 For example, SaV could be detected in 17.3% (57 of 330) of diarrheic children in Nicaragua between 2009 and 2010.31 In Brazil, 18.6% of fecal samples and 36.3% of nasopharyngeal swab samples were detected SaV-positive from 102 hospitalized children with symptoms of acute gastroenteritis.36 More importantly, it was reported that 11.4% (9 of 79) diarrheal deaths of children were caused by SaV infection in South Africa.37
SaV often showed high genetic diversity during its surveillance, and three of the four human SaV genogroups were detected in this study. During a SaV surveillance in South Africa, a total of 14 genotypes were discovered, including all four human genogroups.21 Although no SaV genotype has always been prevalent as a predominant genotype in the human population like GII.4 noroviruses, GI.1 and GI.2 are the most reported SaV genotypes.22, 32, 33 In China, surveillances in Chongqing and Nanjing showed GI.1 and GI.2 as the most detected genotypes.32, 33 Over the past few years, GI.1 SaV was identified as the predominant strain in the neighboring countries, such as Thailand and Japan.38, 39 On the other hand, GI.2 SaV has been reported to cause infections worldwide, recently. In the study conducted in Shanghai, 33 of 43 SaV samples were genotypes as GI.2.22 Especially in Europe, increased prevalence of GI.2 caused 58% of SaV-associated outbreaks in The Netherlands, 27% in Sweden, 40% in Slovenia, and 100% in Hungary, which showed time-ordered genetic changes in its VP1 region during the surveillance.40 In addition, GII.8 SaV is a recently identified genotype, which was also detected in our study. This genotype was detected only in the United States, Peru, and South Africa.20, 41 Moreover, water environmental monitoring is also an important way to discover this new genotype.42 Extensive detection of the GII.8 genotype suggests that it may have a ubiquitous circulation pattern in different regions.
We also performed the phylogenetic analyses on GI.1 SaVs to clarify its genetic diversity based on their capsid protein VP1, and it could be divided into four clusters from 1982 to the present. It exhibits time-dependent evolutionary process, which is similar to GI.2 SaV and GII.4 norovirus.40 However, some newly detected strains (such as KP298674/KR/2013) may also be assigned to previous clusters. This may be due to its infrequent prevalence or inconsistent levels of prevalence in different regions globally, which caused inconsistent herd immune status. On the other hand, phylogenetic trees of GI.1 strains constructed at the nucleotide and amino acid levels have similar topologies (Figure 2, Figure S2). However, the homology at the amino acid level (99.45%) is higher than that at the nucleotide level (95.2%), and only six variable amino acid sites (8, 100, 333, 382, 524, and 557) were verified. And those differences may be related to the error-prone RNase activity or herd immunity.43, 44 However, difficulties in functional and immunogenic validation may hinder the understanding of SaV, which should be solved in the next work.
However, the limitation of this study was the small number of SaV positive samples, which was related to the low prevalence of this virus. The result of this study is consistent with the SaV prevalence reported in other cities of China. This may indicate that SaV has indeed had a low prevalence in China in recent years, and the long-term surveillance is still required to prevent a sudden increase in epidemics like in other countries. On the other hand, the low SaV detection rate is also related to its detection method. The broad-spectrum and highly sensitive method will be helpful to SaV detection. In addition, with the reduction of the cost of next-generation sequencing technologies, the detection of nontargeted pathogens based on metagenomics is its future direction.
In summary, our study described the prevalence of human SaV in patients associated with sporadic acute gastroenteritis in South China. Although SaV prevalence is not as high as noroviruses, its abundant genetic diversity suggested the necessity of continuous surveillance to identify changes in predominant variants as well as the emergence of new variants in the future.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (31701717, 31872912), the National Key Research and Development Program of China (2017YFC1601200), Pearl River S&T Nova Program of Guangzhou (201610010020), GDAS’ Project of Science and Technology Development (2019GDASYL-0104008), and the Key Research and Development Program of Guangdong Province (2019B020209001).




