Antiviral response is not sustained after cessation of lamivudine treatment in chronic hepatitis B patients: A 10-year follow-up study
Abstract
Although the ideal end point for antiviral treatment in patients with chronic hepatitis B (CHB) is loss of HBsAg, the typical clinical end points are HBeAg seroconversion in HBeAg-positive patients and long-term DNA suppression in HBeAg-negative patients. We evaluated the long-term antiviral response after cessation of lamivudine treatment in CHB patients. A total of 157 patients who had discontinued lamivudine between 1997 and 2014 were enrolled (97 HBeAg-positive and 60 HBeAg-negative CHB patients). The long-term durability of the antiviral response (viralogical relapse; HBV DNA ≥104 copies/ml) and the clinical course of these patients were analyzed retrospectively. In HBeAg-positive patients, the mean follow-up period after discontinuation was 72.3 months. The cumulative probabilities of virological relapse at 1, 12, 24, 48, 60, 96, and 120 months were 10.3%, 40.2%, 55.6%, 62.8%, 65.9%, 67.0%, and 67.0%, respectively. In HBeAg-negative patients, the cumulative probabilities of a virological relapse at 1, 12, 24, 48, 60, 96, and 120 months were 25.0%, 35.0%, 41.7%, 43.3%, 43.3%, 46.7%, and 48.3%, respectively. Younger age (HR 1.732, 95%CI: 1.058–2.835, P = 0.02) was predictive of non-virological relapse in HBeAg-positive patients. And achievement of undetectable HBV DNA level within 3 months of treatment discontinuation was associated with decreased rate of virological relapse (HR 0.159, 95%CI: 0.069–0.367 P < 0.01) in HBeAg-negative patients. Despite meeting the requirements for treatment discontinuation, approximately half of the CHB patients treated with lamivudine relapsed. Thus, the antiviral response is not reliably sustained after lamivudine treatment cessation. J. Med. Virol. 89:849–856, 2017. © 2016 Wiley Periodicals, Inc.
Abbreviations
-
- ALT
-
- Alanine aminotransferase
-
- AST
-
- Aspartate aminotransferase
-
- CHB
-
- Chronic hepatitis B
-
- LAM
-
- Lamivudine
-
- NUC
-
- Nucleos(t)ide
INTRODUCTION
Hepatitis B virus (HBV) infection is a major health problem that results in 1 million deaths each year due to liver cirrhosis and hepatocellular carcinoma (HCC) [Chen, 1993; Lee, 1997; Lee et al., 1999]. After the introduction of nucleos(t)ide analogs (NAs), antiviral therapy has been shown to be effective for reducing liver damage, thus preventing hepatic decompensation and HCC [Lok et al., 2003; Liaw, 2009]. The primary goal in chronic hepatitis B (CHB) management is sustained viral suppression or, ideally, improving or eliminating hepatic inflammation, thereby preventing liver cirrhosis and/or HCC [Lok and McMahon, 2009; European Association for the Study of the Liver, 2012; Liaw et al., 2012]. In this regard, it has been presumed that patients with CHB require life-long oral antiviral therapy. However, there are several problems with long-term NA treatment, including declining adherence, development of drug resistance, and financial burden [Ridruejo and Silva, 2012].
Although current guidelines have consistent recommendations for the initiation of antiviral therapy, there is no clear consensus on the timing of cessation. Current guidelines of the American Association for the Study of Liver Disease (AASLD) and the European Association for the Study of Liver Disease (EASL) suggest that the treatment for patients with HBeAg-positive CHB be stopped after HBeAg seroconversion and the HBV DNA level has become undetectable, followed by an additional 6–12 months of consolidation therapy [Lok and McMahon, 2009; European Association for the Study of the Liver, 2012]. On the other hand, for HBeAg-negative CHB patients, AASLD and EASL guidelines recommend long-term NA therapy until HBsAg seroclearance has been achieved. International guidelines have suggested HBsAg seroclearance as an ideal endpoint of NA therapy [Lok and McMahon, 2009; European Association for the Study of the Liver, 2012]. However, HBsAg seroclearance occurs in only 1–3% of patients on NA therapy per year [Dienstag et al., 1999; Chang et al., 2006; Lai et al., 2007; Lee et al., 2010]. Consequently, the Asian Pacific Association for the Study of the Liver (APASL) guidelines suggest that the discontinuation of NA therapy in patients with HBeAg-negative CHB be considered after at least 2 years of treatment if the HBV DNA level remains undetectable after three tests conducted 6 months apart [Liaw et al., 2012]. Even though the guidelines for CHB management provide suggestions regarding the discontinuation of NA therapy, the controversy is over the safe timing of such discontinuation. Several studies pertaining to the discontinuation of lamivudine therapy have been reported. However, the long-term clinical course of patients who discontinued lamivudine therapy more than 5 years prior is not well known. We performed a comprehensive investigation into the long-term clinical course of both HBeAg-positive and -negative patients after antiviral treatment cessation.
METHODS
Patients
The medical records of 240 consecutive and previously treatment-naïve CHB patients at Korea University Guro Hospital who received at least 12 months of lamivudine monotherapy and discontinued this treatment between January 1997 and April 2014 were reviewed. Among these patients, 157 discontinued lamivudine treatment according to guideline recommendations and were further analyzed (Fig. 1). All patients included in the study had documented hepatitis B infection for greater than 6 months along with abnormal ALT and elevated HBV DNA levels (HBV DNA ≥105 copies for HBeAg-positive patients and HBV DNA ≥104 copies for HBeAg-negative patients). Patients were excluded if any of the following criteria were met: (i) co-infection with hepatitis C virus or human immunodeficiency virus; (ii) history of decompensated liver cirrhosis; or (iii) history of hepatocellular carcinoma (HCC). This study was performed in accordance with the declaration of Helsinki.
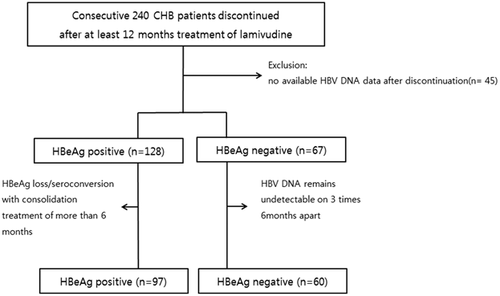
Study Design
In this study, we selected patients who stopped lamivudine antiviral therapy in accordance with the AASLD 2009, KASL 2011, and APASL 2012 recommendations. In brief, lamivudine was stopped in HBeAg-positive patients when HBeAg seroconversion was achieved after more than 6 months of consolidation treatment. In HBeAg-negative patients, lamivudine discontinuation was considered after at least 2 years of treatment if the HBV DNA level remained undetectable on three tests conducted 6 months apart. The patients were followed-up at 1–2 month intervals for 6 months after discontinuation of lamivudine and at 3–6-month intervals thereafter. Serological biomarkers were monitored at every visit, including HBV DNA level by real-time PCR (CobasTaqMan, Roche Diagnostics, Branchburg, NJ, the lower detection limit of 70 copies/ml), HBV serological tests, and ALT level. Serum HBsAg was quantified by microparticle chemiluscent assay (Abbott Laboratories, Abbott Park, IL). Samples were diluted at 1:100, whereas sample with serum HBsAg levels of >1,455 copies/ml was diluted to 1:500 and 1:1,000 to bring the reading into the calibration curve range (1 IU/ml = 5.82 copies/ml). HBsAg level could be checked in practice since 2010. Therefore, we could use data of HBsAg level in some patients because it was not available to use blood samples in that time.
Outcome Measures
Virological relapse was defined as an increase in serum HBV DNA level to greater than 104 copies/ml on two consecutive tests at least 1 month apart after lamivudine discontinuation. A hepatitis flare was defined as an elevation in ALT level greater than five times the upper limit of normal after discontinuation of lamivudine.
Statistical Analysis
Statistical analysis was performed using SPSS software (for Windows, version 20.0, SPSS Inc., Chicago, IL). The differences in patient clinical variables were evaluated using the Chi-square test and the independent t-test. The cumulative rate of virological relapse was calculated by the Kaplan–Meier method, and the log-rank test was used to compare the curves. Multivariate analyses of the potential predictor variables were performed using Cox's proportional hazard model. Results were expressed as mean ± standard deviation, median (25th, 75th percentiles), and a P-value < 0.05 was considered to be statistically significant.
RESULTS
Patient Characteristics
The baselines clinical characteristics of the 157 patients at the time of lamivudine therapy initiation are summarized in Table I. Ninety-seven patients were positive for HBeAg and 60 patients were negative for HBeAg. Several of the baseline characteristics were not significantly different between HBeAg-positive and -negative patients, namely age, serum ALT, presence of liver cirrhosis, total duration of lamivudine treatment, and length of follow-up. Serum level of HBV DNA in HBeAg-negative patients was lower than that of HBeAg-positive patients. Before the initiation of lamivudine therapy, liver cirrhosis was observed in 29 (18.4%) patients, none of whom had decompensated liver cirrhosis.
| All (n = 157) | HBeAg-positive (n = 97) | HBeAg-negative (n = 60) | P-value | |
|---|---|---|---|---|
| Characteristics at the initiation of LAM | ||||
| Sex (male), n (%) | 118 (75.1) | 69 (71.1) | 49 (81.6) | 0.13 |
| Age (years)a | 36.5 (±11.2) | 34.8 (±10.8) | 39.2 (±11.4) | 0.01 |
| AST (IU/L)a | 218.6 (±213.2) | 231.2 (±236.8) | 198.5 (±168.7) | 0.35 |
| ALT (IU/L)a | 332.6 (±292.0) | 354.6 (±329.9) | 297.4 (±216.2) | 0.23 |
| Total bilirubin (mg/dl)a | 1.6 (±2.9) | 1.8 (±3.6) | 1.3 (±3.3) | 0.29 |
| HBV DNA (log10 copies/ml)b | 7.72 (6.83–8.11) | 7.95 (7.15–8.38) | 7.24 (6.36–7.74) | <0.01 |
| Liver cirrhosis, n (%) | 29 (18.4) | 15 (15.4) | 14 (23.3) | 0.21 |
| Characteristics during and after LAM treatment | ||||
| Treatment duration (months)b | 26.0 (19.0–39.5) | 25.0 (18.5–25.0) | 26.5 (21.5–36.7) | 0.88 |
| Follow-up period after discontinuation (months)b | 69.0 (26.5–106.0) | 71.0 (27.0–107.0) | 67.0 (23.3–96.0) | 0.70 |
| Duration of consolidation (months)b | 15.0 (11.0–24.0) | 12.0 (9.0–18.5) | 22.0 (15.0–27.25) | 0.01 |
| ≥12 months, n (%) | 112 (71.3) | 57 (58.7) | 55 (91.6) | |
| ≥24 months, n (%) | 44 (28.0) | 18 (18.5) | 26 (43.3) | |
- MALT, alanine aminotransferase; AST, aspartate aminotransferase.
- a Mean ± standard deviation.
- b Data are given as median (25th, 75th percentiles).
Virologic Relapse After Lamivudine Discontinuation
Ninety-five of 157 patients developed virologic relapse by 24 months (Interquartile range [IQR], 4–150) after lamivudine discontinuation. The median duration of lamivudine treatment was 26.0 months, with a median post-discontinuation follow-up period of 69.0 months. The cumulative probability of virologic relapse at 1, 12, 24, 48, 60, 96, and 120 months was 15.9%, 38.2%, 50.8%, 55.4%, 57.3%, 59.2%, and 60.5%, respectively.
HBeAg-Positive Patients After Lamivudine Discontinuation
The 97 HBeAg-positive patients initiated treatment at a mean age of 34.8 years. The median durations of lamivudine treatment and consolidation therapy were 25.0 and 12.0 months, respectively. The median follow-up period after discontinuation was 71.0 months (IQR, 27.0–107.0). In total, 66 of 97 patients (68.0%) relapsed. The cumulative probability of virologic relapse at 1, 12, 24, 48, 60, 96, and 120 months was 10.3%, 40.2%, 55.6%, 62.8%, 65.9%, 67.0%, and 67.0%, respectively (Fig. 2A). It was noted that patients in the relapse group were significantly older than their non-relapsing counterparts (36.7 vs. 30.7, P < 0.05) (Supplementary Data S1). There was no difference in baseline HBV DNA levels among the relapse and non-relapse groups (7.79 vs. 7.75 copies/ml, P = 0.53). The median duration of lamivudine treatment was 26.0 and 24.0 months in the relapse and non-relapse groups, respectively (P = 0.94). There was no statistically significant difference in the median duration of the consolidation period after HBeAg seroconversion, which was 12.0 months in both the relapse group and the non-relapsing group (P = 0.88). In HBeAg positive patients, 73 (75.2%) achieved undetectable HBV DNA within 3 months after treatment. Among them, 48 patients (65.8%) developed virologic relapse.
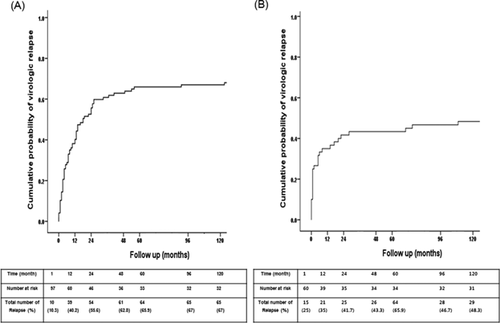
HBeAg-Negative Patients After Lamivudine Discontinuation
In the 60 HBeAg-negative patients, the median duration of lamivudine treatment was 26.5 months, with a mean follow-up period of 67.0 months (IQR, 23.3–96.0). Virologic relapse occurred in 29 (48.3%) of the 60 patients who satisfied lamivudine discontinuation criteria. The cumulative probability of virologic relapse at 1, 12, 24, 48, 96, and 120 months was 25.0%, 35.0%, 41.7%, 43.3%, 43.3%, 46.7%, and 48.3%, respectively (Fig. 2B). There was no difference in baseline HBV DNA levels among the relapse and non-relapse groups (7.20 vs. 7.42 copies/ml, P = 0.33) (Supplementary Data S2). The median time to virologic relapse was 13.6 months. The median duration of lamivudine treatment was 26.0 months in the relapse group and 24.0 months in the non-relapse group (P = 0.44). In HBeAg negative patients, 41 (68.3%) achieved undetectable HBV DNA within three months after treatment. Among them, 12 patients (29.3%) developed virologic relapse.
Factors Associated With Virologic Relapse
Univariate analysis showed that older age (HR = 1.732, 95%CI: 1.058–2.835, P = 0.029) was the only clinical factor that was independently associated with virologic relapse in HBeAg-positive patients.
In 60 HBeAg-negative patients, male sex (HR = 0.364, 95%CI: 0.165–0.803, P = 0.012) and maintenance of undetectable HBV DNA level within 3 months after treatment discontinuation (HR = 0.148, 95%CI: 0.0.065–0.0.336, P < 0.001) were inversely associated with virologic relapse in univariate analysis. However, in multivariate analysis, a maintenance of undetectable HBV DNA level within 3 months after discontinuation was the sole inversely associated with virologic relapse (HR 0.159, 95%CI: 0.069–0.367 P < 0.01) (Table II).
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P-value | HR | 95%CI | P-value | |
| HBeAg-positive patients | ||||||
| Sex (male) | 1.220 | 0.703–2.119 | 0.480 | |||
| Initial age (years) | 1.732 | 1.058–2.835 | 0.029 | |||
| Baseline ALT (IU/L) | 1.139 | 0.656–1.979 | 0.643 | |||
| Liver cirrhosis | 1.023 | 0.522–2.005 | 0.948 | |||
| Baseline HBV DNA (≥7 log10 copies/ml) | 1.212 | 0.660–2.224 | 0.535 | |||
| Total treatment duration | 0.997 | 0.585–1.700 | 0.992 | |||
| Consolidation duration ≥12 months | 0.861 | 0.525–1.411 | 0.553 | |||
| Consolidation duration ≥24 months | 0.877 | 0.478–1.609 | 0.672 | |||
| Undetectable HBV DNA within 3 months after treatment | 0.844 | 0.491–1.452 | 0.540 | |||
| HBeAg-negative patients | ||||||
| Sex (male) | 0.364 | 0.165–0.803 | 0.012 | 0.666 | 0.433–1.022 | 0.063 |
| Initial age (years) | 1.358 | 0.579–3.181 | 0.482 | |||
| Baseline ALT (IU/L) | 1.096 | 0.468–2.568 | 0.833 | |||
| Liver cirrhosis | 1.017 | 0.434–2.383 | 0.969 | |||
| Baseline HBV DNA (≥7 log copies/ml) | 2.854 | 0.678–12.010 | 0.153 | |||
| Total treatment duration | 0.573 | 0.233–1.408 | 0.225 | |||
| Undetectable HBV DNA within 3 months after treatment discontinuation | 0.148 | 0.065–0.336 | <0.001 | 0.159 | 0.069–0.367 | <0.001 |
- ALT, alanine aminotransferase.
Clinical Course After Lamivudine Discontinuation
Among the total 157 patients, hepatitis flare was observed in 11 (21.6%) HBeAg-positive patients and in 6 (10.0%) HBeAg-negative patients (Table III). With regard to relapse, 52 (33.1%) patients (37 HBeAg-positive and 15 HBeAg-negative) required retreatment with oral NAs due to virologic relapse and/or ALT elevation. Among 52 patients, lamivudine-resistant mutation was observed in eight patients (seven HBeAg-positive and one HBeAg-negative) at the time of retreatment. HCC developed in five patients (two HBeAg-positive and three HBeAg-negative) at a median of 69.0 months after discontinuation of lamivudine.
| Outcomes | All (n = 157) | HBeAg-positive (n = 97) | HBeAg-negative (n = 60) |
|---|---|---|---|
| HBsAg loss n, (%) | 17 (10.8) | 6 (6.1) | 11 (18.3) |
| Retreatment with antiviral agent n, (%) | 52 (33.1) | 37 (38.1) | 15 (25.0) |
| LAM/ADV/ETV/TDF | 26/4/19/2 | 19/4/12/2 | 8/0/7/0 |
| Time to retreatment after LAM discontinuation (months)a | 11.5 (5.3, 27.8) | 12.0 (5.5, 31.5) | 8.0 (4.0, 26.0) |
| LAM resistance n, (%) | 8 (5.1) | 7 (7.2) | 1 (1.7) |
| HCC development n, (%) | 5 (3.1) | 2 (2.0) | 3 (5.0) |
| Hepatitis flare n, (%) | 27 (17.1) | 21 (21.6) | 6 (10.0) |
| Decompensated LC development | 0 | 0 | 0 |
- ADV, adefovir; ETV, entecavir; HCC, hepatocellular carcinoma; LAM, lamivudine; LC, liver cirrhosis; TDF, tenofovirean.
- a Data are given as median (25th, 75th percentiles).
When the clinical course was analyzed in the context of virologic relapse, 26 (27.3%) of the 95 relapse patients, compared with one (1.6%) of 62 non-relapse patients, experienced hepatitis flare. In addition, HCC developed in three (3.1%) of the relapse patients versus two (3.2%) of the non-relapse patients. Among the 62 non-relapse patients, HBsAg clearance was achieved in six HBeAg-positive patients (6.1%) and 11 HBeAg-negative patients (18.3%).
Additionally, we evaluated the clinical course after discontinuation in patients with cirrhosis. Hepatitis flare was observed in eight (27.5%) of 29 patients with cirrhosis and 19 (14.8%) of 128 patients without cirrhosis (P = 0.101). HCC developed in one (3.4%) patient with cirrhosis and four (3.1%) patients without cirrhosis (P = 1.00). Twelve (41.3%) of the 29 patients with cirrhosis were retreated with oral NAs. Overall, no significant differences were observed in the clinical course when accounting for the presence of cirrhosis.
DISCUSSION
We explored the long-term durability of the viral response after lamivudine treatment in both HBeAg-positive and -negative patients. We showed a cumulative probability of virologic relapse of 67.0% in HBeAg-positive and 48.3% in HBeAg-negative patients during a median follow-up period of 69.0 months. In other words, half of patients with HBeAg-positive or -negative CHB relapsed after treatment discontinuation. Moreover, more than 50% of relapses occurred in the first year after discontinuation of the antiviral agent.
Previous studies have reported contradictory results on the therapeutic durability of NAs in HBeAg-positive patients. In a study of 78 HBeAg-positive Korean patients who had stopped lamivudine treatment, the cumulative virologic relapse rate was 15.9% and 30.2% at 1 and 5 years, respectively [Lee et al., 2010]. However, this result was likely an underestimate, as virologic relapse was defined as a detectable HBV DNA level, with 140,000 copies/ml as the lower limit of detection. One prospective study also showed a sustained therapeutic response in 125 Chinese HBeAg-positive patients, with cumulative virologic relapse (defined as serum HBV DNA ≥104 copies/ml) rates of 23.4% and 29.4% after 12 and 48 months, respectively [Wang et al., 2010]. Other studies performed in Western countries have reported durable HBeAg seroconversion in 80–90% of cases [Dienstag et al., 2003; Poynard and Chutaputti, 2008]. In recent reports, the cumulative virologic relapse rate was found to be 45.2% over a median of 28–30 months in 102 Korean HBeAg-positive patients and 67% during a median of 26 months of follow-up in 132 Dutch patients [Reijnders et al., 2010; Jin et al., 2012]. These high rates of virologic relapse were consistent with that reported in our study.
However, few studies exist in the literature pertaining to therapeutic durability after discontinuation of antiviral agents in HBeAg-negative patients. Most of the earlier studies reported that the antiviral response was not sustained in HBeAg-negative patients. In a small study, the rate of virologic and/or biochemical relapse was reported to be as high as 86% in the 15 HBeAg-negative patients studied [Santantonio et al., 2000]. Recent studies have also reported disappointing results regarding therapeutic durability in HBeAg-negative patients. In a long-term prospective study of 50 Korean HBeAg-negative patients, the cumulative virologic relapse rate was 43.1% during a median follow-up period of 36 months [Paik et al., 2010]. Similarly, a recent retrospective study has reported that the cumulative virologic relapse rate (defined as serum HBV DNA ≥104 copies/ml) was 56.1% during a median of 60 months of follow-up [Liu et al., 2011].
Despite these findings, there have been few reports on the risk of relapse during follow-up periods longer than 4 years after antiviral discontinuation. Additionally, previous studies have clearly cited the APASL guidelines for discontinuation of antiviral agents. We presented long-term follow-up data on the risk of virologic relapse and showed that about half of the patients with HBeAg-negative CHB did not attain durability after discontinuation of lamivudine.
The reason for the discrepancies in virologic relapse rate appears to be dependent on the definition of virologic relapse. Among previous studies, these definitions included the re-appearance of HBeAg, re-emergence of HBV DNA, and an elevated ALT level. In addition, the detection limits for HBV DNA level and, therefore, the definition of viremia also varied, ranging from 1,000 copies/ml in some studies to as high as 100,000 copies/ml [Lu et al., 2008; Kim et al., 2009; Yeh et al., 2009; Kuo et al., 2010; Reijnders et al., 2010; Wong et al., 2010]. Moreover, the contradictory results appear to depend on the HBV genotype; most of the CHB patients in Korean studies had genotype C [Cho et al., 2009]. Different results were obtained in studies conducted in Asian versus Western countries [Song et al., 2000; van Nunen et al., 2003; Wong et al., 2004; Wu et al., 2008; Kim et al., 2009].
Current international guidelines suggest that discontinuation of NAs be considered after HBeAg seroconversion and 6–12 months of consolidation therapy [Lok and McMahon, 2009; European Association for the Study of the Liver, 2012]. In our study, however, consolidation treatment for longer than 12 months had no effect on the risk of virologic relapse. However, more studies are required to determine the optimal duration of consolidation therapy in patients with HBeAg seroconversion.
Several factors have been identified to predict the risk of relapse after lamivudine discontinuation in HBeAg-positive patients. In previous studies, high level of pre-treatment HBV DNA and low level of ALT were independent predictive factors [Dienstag et al., 2003; van Nunen et al., 2003]. However, the impact of pre-treatment HBV DNA and ALT levels on the risk of relapse was not verified in our study. As noted above, more than half of virologic relapses occurred within 12 months of lamivudine cessation. Our findings suggest that virologic relapse can be predicted by the presence of HBV DNA within 3 months of therapy discontinuation. Therefore, we suggest that close monitoring of HBV DNA is necessary to detect virologic relapse during the early stage of antiviral discontinuation.
The age at which lamivudine treatment was initiated was also a risk factor of virologic relapse. This result was similar to previous studies that showed younger age to be associated with reduced risk of relapse. Other studies have suggested that this result was due to younger patients having a more competent immune response [Brunetto et al., 2002; Liu et al., 2011; Ha et al., 2012].
And recent studies have reported that HBsAg quantification is an important predicting factor of virologic relapse after discontinuation of antiviral agents [Werle-Lapostolle et al., 2004; Chan et al., 2007]. We attempted to analyze the data of HBsAg level. But, it was not available to check the level of HBsAg of all patients in that time. Then, we could use the data of HBsAg level in 30 HBeAg positive patients and 23 HBeAg negative patients excluding to patients who were checked the HBsAg level after retreatment. Although not statistically significant, our study showed that level of HBsAg in relapse group was slightly higher compared to non-relapse group (Supplementary Data S1 and S2).
We also comprehensively evaluated the long-term clinical course, including HBsAg loss, hepatitis flare, and development of HCC. HBsAg loss results in stable remission of the disease. Our study reported a higher rate of clearance of surface antigen than in previous studies [Dienstag et al., 1999; Chang et al., 2006; Lai et al., 2007; Lee et al., 2010]. This result might be drawn because our study was a long-term follow-up. Moreover, all patients with HBsAg loss maintained virologic response. These results indicate that HBsAg loss is probably a suitable end point of antiviral treatment. In previous studies, hepatitis flare occurred in 17–19% of patients during a median of 6 months after discontinuation of therapy [Dienstag et al., 1999; Honkoop et al., 2000; Schalm et al., 2000; Lee et al., 2003; Liu et al., 2004]. In our study, hepatitis flare was observed in about 17% of patients over a median of 69 months of follow-up. Moreover, among the patients with liver cirrhosis, there was no decompensation during the follow-up period.
HCC is a dreaded complication of chronic HBV infection. The rate of HCC development was not associated with virologic relapse in our study (3.1% vs. 3.2%). A previous study has reported that even a low level of HBV DNA (<103 copies/ml) was linked to HCC development [Fung et al., 2007]. Therefore, long-term maximal viral suppression with antiviral therapy is desirable to minimize the risk of HCC development, even in patients with low viral titer. Limitations in the mechanism of current antiviral agents prevent them from eliminating the virus, which persists in hepatocytes in the form of covalently closed circular DNA (cccDNA) [Wong et al., 2004], thus perpetuating the risk of viral reactivation.
This study had several limitations. First, due to its retrospective nature, we could not control for clinical variables; however, we attempted to avoid selection bias. For example, in our study, the baseline median serum HBV DNA level was different in the HBeAg-positive and -negative patients. In general, mean serum HBV DNA level was significantly lower in HBeAg-negative patients, which is consistent with the results from previous studies [Niitsuma et al., 1997; Chu et al., 2002]. Second, virologic relapse rates among individual antiviral agents were not compared, prohibiting analysis of the superiority of the newer medications, entecavir, and tenofovir, to lamivudine in terms of relapse rate. However, a recent study analyzing the durability of virologic response and HBeAg seroconversion in patients treated with these agents (entecavir and tenofovir) showed a low rate of sustained virologic remission (7%) [Fong et al., 2015]. Further studies with larger cohorts are needed. Fourth, LMV monotherapy in CHB patients is no longer recommended according to the current guidelines, so the results of this study will not affect the decision of the current treatment direction. However, despite these limitations, it should be noted that this study constitutes one of the largest cohorts in the literature reporting outcomes more than 5 years post-therapy and this study offers a current guideline regarding the discontinuation of antiviral agents when they are not very effective and when longer treatment duration may be needed.
In conclusion, our data suggest high rates of virologic relapse in patients who stopped lamivudine treatment according to current guidelines. Even with long-term consolidation after HBeAg seroconversion, most HBeAg-positive CHB patients showed virologic relapse. Furthermore, half of patients with HBeAg-negative CHB relapsed after cessation of treatment. We suggest that the induction of HBeAg seroconversion and the suppression of HBV DNA that occurs in patients treated with lamivudine are not maintained after discontinuation of the medication.
AUTHORS' CONTRIBUTIONS
Seong Hee Kang, and Keunhee Kang analyzed the clinical data, performed the research, wrote the paper; Young Sun Lee, Tae Suk Kim, Yang Jae Yoo, Sang Jun Suh, Eileen L. Yoon, Young Kul Jung, Ji Hoon Kim, Yeon Seok Seo, Hyung Joon Yim, and Kwan Soo Byun participated in the research; Jong EunYeon was instrumental in developing, coordinating the research project, reviewed the manuscript.




