Comparison of three human papillomavirus DNA detection methods: Next generation sequencing, multiplex-PCR and nested-PCR followed by Sanger based sequencing
Abstract
To compare the diagnostic performance for HPV infection using three laboratorial techniques. Ninty-five cervicovaginal samples were randomly selected; each was tested for HPV DNA and genotypes using 3 methods in parallel: Multiplex-PCR, the Nested PCR followed by Sanger sequencing, and the Next_Gen Sequencing (NGS) with two assays (NGS-A1, NGS-A2). The study was approved by the Brazilian National IRB (CONEP protocol 16,800). The prevalence of HPV by the NGS assays was higher than that using the Multiplex-PCR (64.2% vs. 45.2%, respectively; P = 0.001) and the Nested-PCR (64.2% vs. 49.5 %, respectively; P = 0.003). NGS also showed better performance in detecting high-risk HPV (HR-HPV) and HPV16. There was a weak interobservers agreement between the results of Multiplex-PCR and Nested-PCR in relation to NGS for the diagnosis of HPV infection, and a moderate correlation for HR-HPV detection. Both NGS assays showed a strong correlation for detection of HPVs (k = 0.86), HR-HPVs (k = 0.91), HPV16 (k = 0.92) and HPV18 (k = 0.91). NGS is more sensitive than the traditional Sanger sequencing and the Multiplex PCR to genotype HPVs, with promising ability to detect multiple infections, and may have the potential to establish an alternative method for the diagnosis and genotyping of HPV. J. Med. Virol. 88:888–894, 2016. © 2015 Wiley Periodicals, Inc.
BACKGROUND
Human papillomaviruses (HPVs) are a globally distributed and heterogeneous Family of double-stranded DNA viruses from the taxonomic family Papillomaviridae [de Villiers et al., 2004]. Among the over 170 HPV genotypes described, ∼40 types infect the human cervicovaginal mucosa. They vary greatly in the magnitude of risk and clinical manifestations. The high-risk HPV types play a key role in cervical cancer (CC), responsible for over 90% of all cases, and in a subset of head and neck cancer [Bruni et al., 2010; Chen et al., 2015]. Some cutaneous HPV types, that have tropism for the skin, are suspected to play a role in skin cancer. More important than to diagnose HPV infection, is to define its genotype, what allows to estimate more accurately the risk of carcinogenesis [de Sanjose et al., 2010].
To date there is no in vitro culture method for HPV, neither a robust serotype response, therefore the diagnosis of HPV infection is based solely on molecular methods for detection of HPV DNA and their sequences. The HPV has a circular double stranded DNA genome of approximately 8000 bp in length, divided into three main parts: early region (E), late region (L), and a noncoding region (upstream regulatory region, URR). There is also a noncoding small region with high variability between E2 and L2 ORFs. All known HPVs have a very similar genetic content organization, and the variations greater than 10% in the nucleotides from L1 region define, in taxonomy, the HPV genotypes [de Villiers et al., 2004].
Several laboratory tests are currently available for HPV-DNA detection. The Multiplex-PCR for HPV-DNA has been used in several countries for screening and prevention of cervical cancer associated with cervicovaginal cytology [Shim et al., 2010; Souho and Bennani, 2014]. It is based on the PCR technique using specific primers for some HPV types (grouped by risk of carcinogenesis). Although most of the kits available today are not restricted to patent properties anymore, the manufacturers maintain the exact sequence of their primers as an industrial secret. In general, four primers are simultaneously used to amplify different genes sizes for each risk group/type: HPV 16, HPV 18, high-risk HPV (except 16 and 18) and HPV6/11. Multiplex-PCR for HPV has a higher sensitivity than HC and it is able to detect cumulative infection by HPV genotypes of different groups. Since it is a method widely used in medical practice, it has a lower relative cost but their use in scientific research is limited by not being able to specify the HPV genotypes or variants, besides the limitations inherent in non sequencing the HPV-DNA [Shim et al., 2010].
The most traditional technique in scientific research, considered the gold standard for many years, is the PCR using the consensus primers MY09 and MY11 (forward and reverse) followed by Sanger based genetic sequencing [Gravitt and Manos, 1992] or liquid hybridization techniques with type-specific probes [de Villiers, 2013]. This classical technique was described by Manos and Bauer [Bauer et al., 1991] in 1991 and involves an unique PCR, which amplifies a 450 bp fragments of L1 region of the HPV genome followed by type-specific probe hybridization or Sanger sequencing. Lately Roda Husman et al. [de Roda Husman et al., 1995] described another pair of primers (GP5+ / GP6+) that amplifies a fragment of 140 to 150 bp in L1 region, superimposed on the MY primers. Studies comparing these techniques reported that the combination of them increases the accuracy of the test [Husnjak et al., 2000; Fuessel Haws et al., 2004]. The so-called Nested-PCR has a higher sensitivity and consists of two consecutive PCR reactions (in two tubes). In the first round the MY primers are used, and in the second round, the GP primers, and then analyzed in electrophoresis and Sanger sequencing. This technique is very sensitive and has been used in scientific research. However it may fail to detect cumulative infections by multiple HPV genotypes.
Finally, a new technique has been recently described with promising results. The Next_Gen Sequencing (NGS) has been considered a transformer advent of modern genomics [Schuster, 2008]. NGS is not based on the Sanger method, and can sequence DNA at unprecedented speed, opening up new applications in biomedical research. This sequencing system can deliver data output ranging from 300 kilobases up to 1 terabase in a single run. Its main difference is the use of primers tagged with initial sequences of specific nucleotides for each individual sample, which has been called primers with “barcodes“ or labeled primers [Sun et al., 2014]. Multiple primers may be used in the same PCR reaction, having multiple target DNA sequences (multi-species if necessary). The result is a massive parallel sequencing and DNA fragments, all having in common the initial sequence specific for each sample (barcode), and analyzed molecule to molecule. NGS allows the characterization of cumulative infections and microbiomes. A single sequencer may generate NGS reading of millions of DNA sequences in 24 hr, more than hundreds of Sanger type sequencers in the same time. NGS is not yet widely available, and its accuracy for the diagnosis of HPV has yet to be confirmed [Yi et al., 2014]. The objective of this study is to compare the diagnostic performance for HPV infection (in qualitative and quantitative terms) of three laboratory techniques: Multiplex-PCR, Nested-PCR followed by Sanger sequening, and NGS Sequencing.
METHODS
The study was designed to compare three laboratory methods for HPV DNA detection and genotyping in cervical secretion samples. We used a convenience sample, taken from an original study [Fonseca et al., 2015] designed to evaluate the prevalence of cervicovaginal HPV infection in a population-based sample of native Amazonian women, without previous diagnosis of HPV infection but in high risk of cervical cancer. The study was approved by the National IRB (National Committee of Ethics in Research, Brasília-DF, protocol 16,800). Ninety five samples were randomly selected. The samples were processed in a blinded fashion.
All 95 samples were analyzed twice by NGS, following the same procedures (described below). So, each sample was analyzed four times blindly and in parallel, once in the Multiplex-PCR, once in the Nested PCR followed by Sanger based sequencing, and twice in NGS (a total of four assays). Samples were numbered rather than identified for confidentiality guarantee. Positive and negative controls were used in all assays.
Specimen Collection and DNA Extraction
In the original study [Fonseca et al., 2015], the participants underwent a gynecological examination including speculum examination with collection of cervical cells, using a cervical brush, which was inserted into cervical channel (1 to 1,5 cm deep) and twisted three times. The samples were eluted into specimen transport medium (STM®; Qiagen, CA) and DNA was isolated using QIAamp DNA® kits (Qiagen, CA) following the recommended protocol. All samples were processed in a BioSafety Cabinet in a laboratory physically separated from where the PCR amplification was performed.
Multiplex-PCR Procedures
A 3 uL aliquot of the recovered DNA sample was used in Multiplex-PCR. We used the HPV4a ACE Screnning® kit (Seegene, Seoul, South Korea) according to the manufacturer's recommendations. The PCR system consisted of 4 uL of primer solution (5x HPV4A ACE AM), 3 uL of 8-MOP buffer, and 10 uL 2X Multiplex Master Mix (containing the polymerase), with a final volume of 20 uL. The Eppendorf thermocycler–Gradient® Mastercycler (Eppendorf AG, Hamburg, Germany) was programmed: initial denaturation at 94°C for 15 min, followed by 40 cycles of 30 sec at 94°C, 90 sec at 60°C and 90 sec at 72°C, with a final extension at 72°C for 10 min. A 5 uL aliquot of the PCR products were then subjected to electrophoresis on 2.0% agarose gel stained with ethidium bromide (1.0 ug/ml) for twenty minutes. The definition of HPV genotypes analysis was performed according to the size of the amplicon (visual inspection), as described in Table I. The multiplex PCR assay enabled the detection of 27 mucosal HPV-genotypes: i) high risk (HR)-HPV types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68; ii) possibly HR-HPV types: 26, 53, 67, 70, 73, 82; low risk (LR)-HPV types: 6, 11.
| Results in Multiplex-PCR | Size of the amplicon (bp) |
|---|---|
| Intern control (human DNA) | 1000 |
| HPV 16 (only) | 588 |
| Other HR-HPVa | 465 |
| HPV 6/11 | 302 |
| HPV 18 (only) | 230 |
- a Other HR-HPV: 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73 e 82. According to the manufacturer, the primers used for HR-HPV may detect 3 other HPV types by cross reaction (39, 52 e 68).
Nested-PCR and Sanger-Based Sequencing Procedures
Two successive rounds of PCR were performed as described by Roda Husman et al. The system used for the first round PCR was composed of a 5 μL aliquot of the recovered DNA solution; 5 μL of buffer 10X; 1.5 μL of MgCl2 50 mM; 5 μL of the forward primer MY11; 5 μL of the reverse primer MY09; 1.0 μL of dNTP 10 mM; 0.5 μL Taq polymerase 5 U/μL and 27 μL of Milli-Q water up to a final volume of 50 μL. The Eppendorf thermocycler equipment–Gradient® Mastercycler was programmed to run the protocol describe in Table II.
| Stages | Temperature | Time (sec) | Number of cycles |
|---|---|---|---|
| Initial DNA denaturation | 95 °C | 60 | – |
| Denaturation | 95 °C | 60 | 40 |
| Anealling | 55 °C | 60 | |
| DNA elongation | 72 °C | 60 | |
| Final elongation | 72 °C | 300 | – |
| Hold | 4 °C | – | – |
All samples were taken to the second round PCR. The system used for the second round of PCR was composed of 1 μL of the first round PCR product (amplified DNA sample); 5 μL of buffer 10X; 1.5 μL of MgCl2 50 mM; 5 μL of the primer GP5+; 5 μL of the GP6+ primer; 1.0 μL of dNTP 10 mM; 0.5 μL of Taq polymerase 5U/μL and 31 μL of MiliQ water to final volume of 50 μL. The same thermocycler protocol was used. The nested PCR products were analyzed by agarose gel stained with 2.0% ethidium bromide (1.0 μg/ml) for 20 min. The corresponding amplicon was 150 bp.
The positive samples were purified with Exo-SAP and subjected to automated DNA sequencing (MegaBace 1000®, Biociences Amersham, UK) by the Sanger technique. We used the reaction system: 5.0 μL of the amplified DNA; 4.0 μL of pre-mix DyEnamic ET Terminator Kit® (GE Healthcare, United Kingdom); 1.0 μL of primer GP5+ 50 pmol/uL. We used the following protocol: 95°C for 25 sec, 95°C for 15 sec, 50°C for 20 sec, 60°C for 1 min repeated for 30 cycles of amplification. The precipitation was performed by adding 1 μL of ammonium acetate and 40 μL of absolute etanol to the sequencing PCR product, followed by stirring and incubating for 20 min at room temperature, protected from light. The plate was centrifuged at 4,000 rpm for 40 min in a refrigerated centrifuge 5804R (Eppendorf®) and the supernatant was discarded. Capillary polyacrylamide gel electrophoresis was performed based on the standard methodology of the manufacturer (3 KV Injection was used for 80 sec, and the run was processed to 6 KV for 200 min at 44°C). The analyzes were performed on bioinformatics software MEGA 6® (Arizona). For alignment of the sequences we used CLUSTAL W® BIOEDIT (Clustal, Dublin, Ireland). For confirmation and identification of the type of HPV, the sequences were compared to those deposited at the World Database Nucleotides–GeneBank® using the BLAST/BLASTN® program (www.ncbi.nlm.nih.gov). The sequence homologies with e-value less than e−10 were considered valid.
Next Generation Sequencing Procedures
NGS primer design and sample sources
Since the spectrum of HPV types present in the population studied was unknown, we utilized a novel next-gen sequencing assay developed in the Burk Laboratory (Albert Einstein College of Medicine, New York) by targetting two regions within the L1 ORF. For each assay, several degenerate primers specific to different HPV genera, species or types were pooled and a unique 8 bp Hamming DNA barcode was appended to the 5' terminal end of each primer. Each barcode was at least 2 bp different from all other primers [Smith et al., 2012] (Fig. 1).
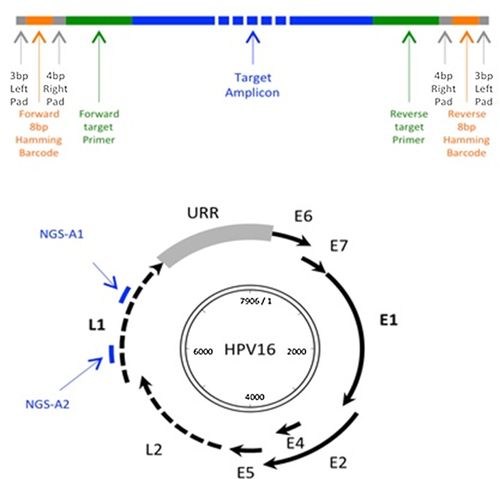
Next-Gen PCR Amplification and Sequencing
An aliquot of each DNA was amplified using 8 bp barcoded oligonucleotide primers for each NGS assay. For all samples, a unique barcode was introduced to the PCR amplicon by the forward and reverse primers. In brief, 1 μL DNA sample in a 25 μL reaction with an equal amount of AmpliTaq Gold DNA Polymerase (Life Technologies, CA) and HotStart-IT FideliTaq DNA Polymerase (Affymetrix, CA). The PCR conditions included an initial 5 min denaturation at 95°C followed by 15 cycles at 95°C for 1 min, 55°C for 1 min, and 68°C for 1 min, 25 cycles at 95°C for 1 min, 60°C for 1 min, and 68°C for 1 min, and a final extension at 68°C for 10 min. The annealing temperatures of the assay fapR were adjusted to 57°C for the first 15 cycles and 62°C for the second 25 cycles.
Successful amplification of the predicted HPV fragment was estimated for each amplicon by relative band intensity in an ethidium gel compared to a control; barcoded PCR products from all samples were pooled adjusting for DNA concentration of the PCR produtcs by adding less for samples with abudant PCR product. If no amplicon was observed by ethidium bromide staining, 20 μL of reaction was added into the pooled mixture. The PCR product mixtures were purified with QIAquick Gel Extraction Kit (Qiagen, CA); an aliquot of purified DNA amplicons was prepared using the Illumina TruSeq DNA Sample Preparation Kit and sequenced on an Illumina HiSeq (Illumina Inc., San Diego, CA) at the Albert Einstein Epigenomics Shared Facility using 150bp paired-end reads.
Bioinformatics Pipeline and Taxonomic Classification
The paired-end short Illumina reads were demultiplexed according to their sample-specific barcodes using Novobarcode v1.00 (http://www.novocraft.com/), filtered for low quality reads (Q20) and short length (50 bp) using prinseq-lite v0.20.4 [Schmieder and Edwards, 2011], and merged to single reads using FLASh v1.2.7 [Magoc and Salzberg, 2011]. The single-end reads that failed to merge were appended. These sequences were then subjected to cull the chimeras using UChime [Edgar et al., 2011] in USEARCH v 7.0.1001 [Edgar, 2010] against a “gold” PV reference database. All reads passing the QC filter were then clustered into 95% identity operation taxonomic units (OTUs) and assigned an HPV taxonomy using USEARCH and a training PV dataset. A table of counts for the number of times each HPV type was observed in each sample was created; the taxonomy was classified at the type level.
Cutoff values were set to remove “low frequency clusters”. A sample was considered negative if the total number of HPV reads was less than 1,000. Within each sample, for a given HPV type to be considered present, the number of reads had to be greater than 1% of the total number of HPV reads in the sample, and greater than 1% of the maximum read number of this type in another sample.
Data Analysis Methods
Descriptive analysis was used to the results of each laboratory technique. To compare proportions, we used the χ2 test or Fisher's exact test in the case of small samples. The interobserver agreement was performed by the Cohen's kappa index. The results of the Multiplex-PCR, the Nested-PCR and the NGS assay 1 were compared with the results of NGS assay 2. The issues analyzed were positivity for HPV, for high-risk HPV (HR-HPV), HPV16, HPV18, detection of cumulative infection of HPV types, and diversity of HPV types detected in the sample. We assume the HR-HPV types as: 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73 and 82, according to WHO International Agency for Research on Cancer [Cogliano et al., 2005].
RESULTS
There was no loss of samples, and all four assays were performed for each individual sample. The HPV detection varied substantially between laboratory methods. The prevalence of HPV in the NGS test was higher than in the Multiplex-PCR (64.2% vs. 45.2%, respectively; P = 0.001) and also higher than the Nested-PCR (64.2% vs. 49.5 %, respectively; P = 0.003). The same could be seen for HR-HPV detection. There was no difference between the prevalence of HPV and HR-HPV when the two NGS assays were compared. The NGS test also showed better performance to detect HPV16 when compared to Nested-PCR and Multiplex-PCR (12.6% vs. 7.2% vs. 6.8%, respectively, P < 0.05). However, there was no significant difference in the prevalence of HPV 18 between the laboratory methods (Table III).
| Laboratory methods | |||||||
|---|---|---|---|---|---|---|---|
| HPV results | Multiplex-PCR | Multiplex vs NGS (2 assay combined) (P value) | Nested-PCR | Nested vs NGS (2 assay combined) (P value) | NGS-A1 | NGS-A1 vs NGS-A2 (P value) | NGS-A2 |
| HPV detection (%) | 45.2% | 0.001 | 49.5% | 0.003 | 62.1% | ns | 64.2% |
| HR-HPV detection (%) | 25.0% | <0.001 | 26.2% | 0.001 | 42.1% | ns | 44.2% |
| HPV16 detection (%) | 6.8% | 0.03 | 7.2% | 0.04 | 11.6% | ns | 12.6% |
| HPV18 detection (%) | 5.2% | ns | 5.8% | ns | 7.4% | ns | 7.4% |
| Diversity of HPV types detected (n) | – | – | 12 | – | 37 | – | 35 |
| Cumulative HPV types detection (%)a | 23.8% | 0.002 | 0 | – | 33.5% | ns | 35.6% |
| HPV types detectedb | – | 31, 16, 18, 58, 62, 6, 81, 33, 45, 84, 59, 85 | 16, 31, 58, 6, 18, 62, 66, 45, 53, 81, 30, 68, 71, 39, 54, 67, 89, 34, 56, 59, 61, 86, 13, 40, 51, 52, 70, 72, 74, 90, 87, 84, 103, 102, 91, 121, 108 | – | 16, 31, 6, 58, 62, 18, 45, 66, 81, 53, 59, 68, 30, 39, 71, 67, 89, 90, 34, 54, 56, 61, 86, 13, 40, 51, 70, 84, 74, 72, 103, 91, 87, 121, 108 | ||
| HPV genera detectedb | Alpha9, Alpha7, Alpha3, Alpha10, Alpha6 | Alpha9, Alpha7, Alpha6, Alpha3, Alpha14, Alpha10, Alpha11, Gamma, Alpha5, Alpha1, Alpha8, Alpha13 | Alpha9, Alpha7, Alpha6, Alpha3, Alpha14, Alpha10, Alpha11, Gamma, Alpha13, Alpha8, Alpha1, Alpha5 | ||||
- NGS-A1, next generation sequencing assay 1; NGS-A2, next generation sequencing assay 2; ns, not significative (P > 0.05).
- a Frequence of samples with at least two types of HPV detected.
- b In decreasing order of frequency.
The most prevalent types of HPV in NGS tests were HPV 16 and HPV 31, and the same was observed in the Nested-PCR, but in reverse order. The NGS assays also showed advantages in the detection of cumulative infection by HPV types. While one-third of the samples had more than one HPV type detected by NGS assays, cumulative HPV types were detected in only one quarter of the samples by Multiplex-PCR. Two patients had nine HPV types in cervicovaginal secretion by NGS assay. The Nested-PCR followed by Sanger sequencing does not detect cumulative infections. The diversity of HPV types detected was higher in NGS assays (types 35 and 37), compared to 12 types of HPV in Nested-PCR (Table III).
There was a weak interobserver agreement between the results of Multiplex-PCR and Nested-PCR in relation to NGS (assay 2) for the diagnosis of HPV infection, and a moderate correlation for the diagnosis of HR-HPV, HPV 16 and HPV 18 infection (Table IV). The NGS-A1 showed a strong correlation with NGS-A2 for the detection of HPV (k = 0.86), of HR-HPV (k = 0.91), of HPV 16 (k = 0.92) and of HPV 18 (k = 0.91).
| HPVs | HR-HPVs | HPV16 | HPV18 | |
|---|---|---|---|---|
| Interobserver agreement | NGS-A2 | |||
| Multiplex-PCR | k = 0.38 | k = 0.55 | k = 0.62 | k = 0.66 |
| Nested-PCR + Sanger | k = 0.44 | k = 0.66 | k = 0.68 | k = 0.70 |
| NGS-A1 | k = 0.86 | k = 0.91 | k = 0.92 | k = 0.91 |
- NGS-A1, next generation sequencing assay 1; NGS-A2, next generation sequencing assay 2; HR-HPV, high risk HPV; SBS, Sanger based sequencing.
DISCUSSION
Next_Gen Sequencing (also known as massive parallel sequencing) has been considered a transformer advent in genetic research [Schuster, 2008], due to its ability to increase characterization of genomes, transcriptomes, epigenomes and microbiomes [Smith et al., 2012; Harari et al., 2014]. As a relatively new method, validation of this method for the detection of HPV are necessary. In our study, we demonstrated that the NGS had a superior performance in HPV detection and genotyping when compared to the most commonly used method in clinical practice (Multiplex-PCR) and also to the traditional method widely used in scientific research, Sanger based sequencing.
The NGS assays increased the HPV detection (all types) up to 40% when compared to other methods. Regarding the HR-HPV detection, the performance of the NGS assays was even higher, nearly doubling the positivity. The higher sensitivity of NGS may be partially explained by its higher ability to detect cumulative infections. The Sanger sequencing generates a unique sequence for each positive sample, probably the most abundant HPV type, tending to neglect other co-infective types, less abundant in that sample. It is noteworthy that there were samples in this study with detection of nine types of HPV by NGS, and only one of these types was detected by the traditional method (Sanger). The difference in the HPV types diversity detected by the laboratory methods highlights this point. Another methodological aspect which may contribute to increased sensitivity of NGS is that its definition of HPV positivity is not based on visual inspection of the electrophoresis gel, a common practice in the Sanger sequencing. As described above, all positive and negative samples in the first amplification step were reassessed in the NGS sequencing system. The visual inspection of NGS electrophoresis served only to determine the size of the aliquot of each sample to be used in sequencing. These evidences suggest that many types of HPV, with epidemiological importance, may not have been detected in classical epidemiological studies using the traditional methods of molecular biology.
It is well known that the size of the amplicon obtained in each PCR can influence the sensitivity of the assay. The smaller the amplicon, the greater the sensitivity. Although the first reaction of the Nested-PCR has used MY primers (which generate a 450 bp amplicon), the primer used in Sanger based sequencing was the GP5+, which generates an amplicon from 140 to 150 bp, similar to the amplicons generated by the two NGS assays (150 bp). However, the amplicons generated in the PCR-Multiplex were substantially larger (up to 500 bp), which may have influenced the lower sensitivity of this test.
Another contribution of this study was the comparison of two assays of NGS, performed in parallel and in a blinded fashion. There was no significant difference in HPV prevalence (and types), and the assays showed a high interobserver agreement for the main analysed outcomes. In our study, most samples with disagreement among the NGS assays were related to the occurrence of HPV with very low readings count (low load viral infections). The high correlation between the assays confirms the robustness of the methodological procedures of NGS for the diagnosis and genotyping of HPV. All DNA sequences resulting from each NGS assay (approximately 1.5 million sequences for each assay) were sequenced together after mixing of all amplicon aliquots in a single sequencing reaction. The use of barcoded primers was the advent that allowed the distinction of the origin of each sequence unambiguously [Sun et al., 2014]. An interesting point related to NGS is that a single sequencing reaction generates millions of genetic sequences, and consequently, a huge amount of information, making its interpretation a limiting step of this process. This analytical “bottleneck“ requires training and expertise in bioinformatics, in addition to a clear desing of the procedures involved in interpretation of the results and taxonomic assignment [Schuster, 2008]. This feature has not been so importante so far, since the Sanger sequencing usually provides unique sequences. Therefore, special attention was given in our study to the detailed description of the analytical procedures of bioinformatics used in this assay.
We conclude that NGS is a sensitive method for detection and genotyping the HPV in cervicovaginal secretion than traditional Sanger sequencing and Multiplex PCR, with promising ability to detect cumulative infection of HPV types and/or variants, opening new perspectives for comprehensive studies on the epidemiology of HPV, particularly for monitoring the effects of vaccination against HPV. NGS has the potential to establish an alternative method for the diagnosis and genotyping of HPV. However, NGS availability and cost may still represent barriers for its wide use in clinical practice and scientific studies.
ACKNOWLEDGMENT
We thank Dr. Robert David Burk for giving technical, financial and logistical support for this study.




