The role of baseline HIV-1 RNA, drug resistance, and regimen type as determinants of response to first-line antiretroviral therapy
Abstract
The factors influencing virological response to first-line combined antiretroviral therapy (cART) in an Italian cohort of HIV-1-infected patients were examined. Eligible patients were those enrolled in a national prospective observational cohort (Antiretroviral Resistance Cohort Analysis), starting first-line cART between 2001 and 2011 and who had at least one follow-up of HIV-1 RNA. The primary endpoint was virological success, defined as the first viral load <50 copies/ml. Time to events were analyzed by Kaplan–Meier analysis and Cox proportional hazard model. One thousand three hundred five patients met the study inclusion criteria. In a multivariable model adjusting for transmission mode, presence of transmitted drug resistance, baseline CD4+ cell count, viral subtype, and type of NRTI backbone employed, independent predictors of virological success were higher baseline viral load (≥500,000 vs. <100,000 HR 0.52; P < 0.001), a weighted genotypic susceptibility score (wGSS) <3 (HR 0.58; P = 0.003), male sex (HR 0.76 P = 0.001), and type of initial third drug employed (integrase inhibitor vs. boosted protease inhibitors HR 3.23; P < 0.001). In the subset with HIV-1 RNA >100,000 copies/ml, virologic success was only associated with the use of integrase inhibitors in the first cART regimen. Independent predictors of immunological success were baseline CD4+ cell count and wGSS <3. High baseline HIV-1 RNA, predicted activity of the first-line regimen based on genotypic resistance testing, gender, and use of new agents were found to predict time to achieve virological success. The type of initial nucleoside analog backbone was not found to predict virological response. J. Med. Virol. 86: 1648–1655, 2014. © 2014 Wiley Periodicals, Inc.
INTRODUCTION
The benefits of combination antiretroviral therapy (cART) in the management of HIV-1 disease are well established [Egger et al., 2002; May et al., 2007; Lima et al., 2007; Antiretroviral Therapy Cohort Collaboration, 2008]. Virological suppression is the primary target for therapy because it is a reliable predictor of immunologic response and clinical outcome [Ghani et al., 2001; Lundgren et al., 2002; Anastos et al., 2004]. By contrast, incomplete suppression of viral replication allows for the emergence of drug resistance and ultimately treatment failure [Hermankova et al., 2001; Barbour et al., 2002]. Thus, quantification of HIV-1 RNA is the standard method for monitoring response to cART [Marschner et al., 1998; Thiébaut et al., 2000] and patients should achieve complete virological suppression and maintain it thereafter for as long as possible [Panel on Antiretroviral Guidelines for Adults and Adolescents, ]. Several studies have suggested that baseline plasma HIV-1 RNA levels ≥100,000 copies/ml are associated with increased mortality independently from CD4 cell counts [Wood et al., 2003, 2006]. However, there is no conclusive evidence about the impact of high-level viral load on virological response to first-line cART and even less is known about the role of specific drug regimens in this setting. Indeed, few randomized clinical trials provided a simultaneous head-to-head efficacy comparison of NNRTI-, PI-, and new agents-based regimens in treatment-naïve HIV-1-infected patients with high levels of viremia.
Recently, a randomized trial comparing the efficacy of efavirenz and rilpivirine-containing single tablet regimens in treatment-naïve patients showed a superior virological efficacy for the efavirenz-based regimen only when baseline viral load was >500,000 copies/ml [Cohen et al., 2012; Santoro et al., 2013]. A role for high pre-therapy viral load on virological outcome has been also reported [Liu and Shafer, 2006].
The aim of this study was to examine at the same time the influence of virus and treatment-related factors on the virological and immunological outcome, in a cohort of HIV-1-infected patients initiating first-line cART.
MATERIALS AND METHODS
Patients and Study Design
This is a retrospective, longitudinal, multicenter study on cART naïve HIV-1-infected patients aged >16 years enrolled in the Antiretroviral Resistance Cohort Analysis (ARCA) database. (http://www.hivarca.net). At the time of this study, data from more than 26,000 patients were available in the whole cohort. Patients are enrolled in the ARCA database after signing an informed consent to provide their anonymized data for academic not-for-profit studies. Data include demographics, hepatitis B and C status, AIDS-defining events, antiretroviral treatment, viral load, CD4+ cell counts, HIV-1 subtype, and HIV-1 sequences used for resistance and tropism testing.
Patient's Selection Criteria
Eligible patients were HIV-1 infected, antiretroviral-naïve adults who initiated a first-line cART containing either one non-nucleoside reverse transcriptase inhibitors (NNRTI) or a ritonavir-boosted protease inhibitor (bPI) or an integrase inhibitor (INI) between January 1, 2001 and December 31, 2011 and who had at least one available follow-up HIV-1 RNA determination. Treatment modifications during follow-up were ignored.
Baseline Drug Resistance Interpretation
The weighted genotypic susceptibility score (wGSS) of the first-line regimens was computed according to the Rega 8.0.1 genotypic resistance interpretation available on hivdb.stanford.edu [Van Laethem et al., 2002; Vercauteren et al., 2013]. Pre-cART reverse transcriptase and protease sequences were available for 1,052 subjects (80.6%). Each combination regimen was then given a wGSS based on the sum of the weighted scores coded for the individual drugs included in the regimen.
Study Endpoints
The primary endpoint was time to HIV-1 RNA suppression, defined as achieving a HIV-1 RNA <50 copies/ml. Another endpoint was the time to immunologic success, defined as the first achieved CD4+ cell count >500 cells/µl in the subgroup with pre-therapy CD4 count <500 cells/µl.
As this is an observational study, each treating clinician prescribed the first-line of cART according to local guidelines, although within internationally approved rules.
Statistical Analysis
Baseline demographic and clinical characteristics were summarized using median (interquartile range) or frequencies (percentages). The Kaplan–Meier approach was used to estimate the time to the study endpoints with the different first-line regimens. Multivariable Cox proportional hazard models were used to adjust for potential confounders and results were expressed as hazard ratio (HR) with 95% confidence intervals (CI). Variables with a P-value lower than or equal to 0.20 in the univariate analysis were entered in the multivariate models. Statistical significance was assessed at a level of 0.05 (two-tailed). Statistical analyses were performed using SPSS v.20 (IBM Corp., Armonk, NY).
RESULTS
Patients Baseline Characteristics and Treatments Employed
The study sample was based on 1,305 eligible HIV-1-infected individuals. Baseline demographic and clinical characteristics are reported in Table I. Of note, 628 (48.1%) patients had a baseline viral load >100,000 copies/ml, including 192 (14.7%) with >500,000 copies/ml.
| Demographic and clinical characteristics | N (%) or median (range) |
|---|---|
| Males | 975 (74.7) |
| Age (years) | 39 (17–82) |
| Nadir of CD4 cell count (cells/mm3) | 212 (0–1,275) |
| Baseline CD4 cell count (cells/mm3) | 225 (0–1,275) |
| Viral load (copies/ml) | |
| <100,000 | 677 (51.9) |
| ≥100,000 and ≤500,000 | 436 (24.9) |
| >500,000 | 192 (23.2) |
| Mode of transmission | |
| Heterosexual | 616 (47.2) |
| Homosexual | 333 (25.5) |
| Intravenous drug abuse | 181 (13.9) |
| Not available | 153 (11.7) |
| Other | 22 (1.7) |
| Subtype | |
| Non B | 246 (18.9) |
| B | 806 (61.8) |
| Unknown | 253 (19.3) |
| First-line HAART regimen | |
| 2 NRTI plus 1 bPI | 754 (57.8) |
| 2 NRTI + NNRTI | 457 (35.0) |
| 2 NRTI + new class agent | 40 (3.1) |
| Other | 49 (4.1) |
| Type of NRTI backbone drugs | |
| TDF/FTC | 610 (46.7) |
| ABC/3TC | 182 (13.9) |
| ZDV/3TC | 339 (26.0) |
| Other | 174 (13.3) |
| wGSS | |
| <3 | 53 (4.1) |
| ≥3 | 999 (76.6) |
| Not available | 253 (19.3) |
- Percentages are calculated on a total denominator of 1,305 patients, except where otherwise indicated.
- NRTI, nucleoside reverse transcriptase inhibitors; NNRTI, non-nucleoside transcriptase inhibitors; bPI, boosted PI; TDF, tenofovir; FTC, emtricitabine; ABC, abacavir; 3TC, lamivudine; ZDV, zidovudine; wGSS, weighted genotypic susceptibility score.
Boosted PIs were used more frequently than NNRTI (57.8% vs. 35.0%) and there were only 40 (3.1%) regimens consisting of two NRTI plus a new class agent.
Overall, 819 patients started therapy with a bPI, (saquinavir 16, indinavir 16, amprenavir 2, fosamprenavir 54, lopinavir 557, atazanavir 116, darunavir 57, tipranavir 1), and 483 patients initiated therapy with NNRTI (nevirapine 98 and efavirenz 385). Forty patients started therapy with an agent in the new anti-HIV-1 class (raltegravir 18, maraviroc 1, and enfuvirtide 21). Other combinations of three or more anti-HIV-1 drugs were prescribed in 54 patients.
The wGSS was <3 in 53 patients (4.1%), ≥3 for 999 (76.6%) subjects and not available for the remaining 253 (19.3%) due to missing protease and reverse transcriptase sequences.
Virologic Success and Its Predictors
Figure 1 shows the cumulative Kaplan–Meier estimated proportion with virological success for the complete patients set stratified by baseline viral load levels. The 1-year estimated probability of virological success was 85.2% (95% CI 82.3–87.8) among the 677 individuals with baseline HIV-1 RNA <100,000 copies/ml, 81.8% (95% CI 77.8–85.5) for the 436 patients with baseline HIV-1 RNA between 100,000 and 499,999 copies/ml, and 80.9% (95% CI 74.8–86.4) in the 192 patients with baseline HIV-1 RNA ≥500,000 copies/ml.

Table II shows the results of univariate and multivariate Cox regression models analyzing predictors of time to virological success. Independent predictors of virological success were lower baseline viral load (P < 0.001), wGSS >3 or higher versus <3 (P = 0.003), female sex (P = 0.001), and type of third drug employed in the first-line regimen (P < 0.001), while the type of NRTI backbone type was not associated with virological response.
| Univariate | Multivariate* | |||
|---|---|---|---|---|
| HR (95 % CI) | P-value | HR (95% CI) | P-value | |
| Baseline HIV RNA (copies/ml) | ||||
| <100,000 | 1.00 (Ref) | <0.001 | 1.00 | <0.001 |
| 100,000–499,999 | 0.73 (0.64–0.83) | 0.76 (0.65–0.88) | ||
| ≥500,000 | 0.67 (0.56–0.79) | 0.52 (0.42–0.64) | ||
| wGSS | ||||
| ≥3 | 1.00 (Ref) | 0.05 | 1.00 (Ref) | 0.003 |
| <3 | 0.74 (0.54–1.00) | 0.58 (0.40–0.83) | ||
| NRTI backbone of initial regimen | ||||
| TDF/FTC | 1.00 (Ref) | 0.021 | 1.00 (Ref) | 0.73 |
| ABC/3TC | 1.17 (0.98–1.39) | 1.07 (0.88–1.30) | ||
| ZDV/3TC | 0.91 (0.79–1.04) | 1.04 (0.84–1.28) | ||
| Other | 0.86 (0.71–1.03) | 1.16 (0.88–1.54) | ||
| Gender | ||||
| Male vs. female | 0.84 (0.73–0.96) | 0.008 | 0.76 (0.64–0.90) | 0.001 |
| 3rd drug of initial regimen | ||||
| bPI | 1.00 (Ref) | 0.038 | 1.00 | <0.001 |
| NNRTI | 0.96 (0.85–1.09) | 0.98 (0.83–1.14) | ||
| INI | 2.02 (1.23–3.31) | 3.23 (1.84–5.68) | ||
| Other | 1.02 (0.80–1.32) | 1.32 (0.96–1.82) | ||
- Time to achieve HIV RNA <50 copies/ml. Univariate and multivariate Cox regression (N = 1,305).
- Ref, reference category for interpretation of odds-ratios (OR); wGSS, weighted genotypic susceptibility score; NRTI, nucleoside reverse transcriptase inhibitors; TDF/FTC, tenofovir/emtricitabine; ABC/3TC, abacavir/lamivudine; ZDV/3TC, zidovudine/lamivudine; bPI, boosted protease inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitors; INI, integrase inhibitor.
- * Variables were mutually adjusted in the multivariate model that also included transmission mode, presence of transmitted drug resistance, baseline CD4+ and viral subtype.
In a secondary analysis of the subgroup of patients with HIV-1 RNA >100,000 copies/ml only (n = 628), the impact of HIV-1 RNA levels on virological success was not significant (P = 0.40) since no differences were observed comparing the different strata with more than 100,000 copies/ml. Only the type of third drug remained significantly associated with virological success with a similar effect as registered in the complete patients set (INI vs. bPI HR 3.23 [95% CI 1.84–5.68]; P < 0.001).
Immunologic Success and Its Predictors
Time to immunologic success (achieving a CD4 count of ≥500 cells/µl) was explored in the subgroup of patients with pre-therapy CD4 count <500 cells/µl (n = 1,262).
Figure 2 shows the Kaplan–Meier curves estimating the time to achieve immunological success for the entire cohort stratified by pre-therapy CD4+ cell count. The 1-year estimated probability of immunological success was 11.6% (95% CI 9.1–14.8) for patients with CD4+ cell count lower than 200; 48.2% (95% CI 43.5–53.3) with CD4+ cell count between 200 and 349; and 86.6% (95% CI 80.5–91.5) in patients with CD4+ cell count greater than or equal to 350.
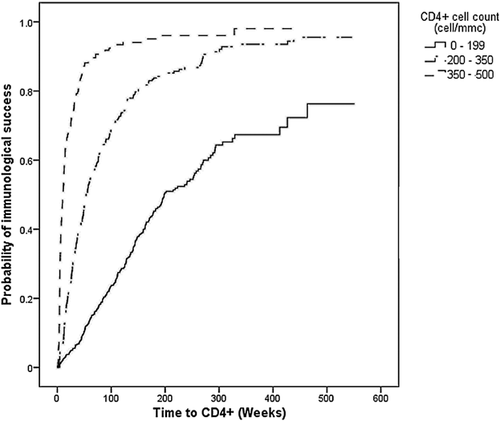
Table III shows the results of the Cox regression analysis regarding the factors associated with immunological success. Independent predictors of immunological success were higher baseline CD4+ cell count (P < 0.001), a regimen's wGSS >3 (P < 0.001), and the mode of HIV-1 transmission (P < 0.001).
| Clinical characteristics | Univariate | Multivariate* | ||
|---|---|---|---|---|
| HR (95 % CI) | P-value | HR (95% CI) | P-value | |
| Baseline CD4 cell count | ||||
| <200 | 1.00 (Ref) | <0.001 | 1.00 (Ref) | <0.001 |
| 200–349 | 3.18 (2.67–3.79) | 3.30 (2.74–3.97) | ||
| 350–499 | 9.94 (8.17–12.08) | 11.2 (9.07–13.83) | ||
| wGSS | ||||
| ≥3 | 1.00 (Ref) | <0.001 | 1.00 (Ref) | 0.032 |
| <3 | 0.54 (0.44–0.66) | 0.64 (0.42–0.96) | ||
| Mode of transmission | ||||
| Heterosexual | 1.00 (Ref) | <0.001 | 1.00 (Ref) | 0.001 |
| Homosexual | 1.37 (1.16–1.62) | 1.33 (1.12–1.59) | ||
| IDU | 0.76 (0.60–0.95) | 0.80 (0.63–1.02) | ||
| Other | 0.61 (0.34–1.12) | 0.98 (0.53–1.79) | ||
| NRTI backbone of initial regimen | ||||
| TDF/FTC | 1.00 | 0.009 | 1.00 | 0.38 |
| ABC/3TC | 1.11 (0.89–1.37) | 1.21 (0.96–1.51) | ||
| ZDV/3TC | 0.84 (0.70–0.99) | 1.03 (0.82–1.28) | ||
| Other | 0.76 (0.61–0.95) | 1.11 (0.84–1.47) | ||
| 3rd drug of initial regimen | ||||
| bPI | 1.00 | 0.034 | 1.00 | 0.009 |
| NNRTI | 1.20 (1.03–1.39) | 0.91 (0.77–1.08) | ||
| INI | 1.81 (0.96–3.39) | 1.72 (0.90–3.30) | ||
| Other | 1.22 (0.90–1.66) | 1.53 (1.10–2.13) | ||
- Time to achieve CD4+ cell counts >500 cells/µl: n = 1,250 with baseline CD4 <500 cells/µl.
- Ref, reference category for interpretation of odds-ratio (OR); wGSS, weighted genotypic susceptibility score; IDU, intravenous drug abuser; TDF/FTC, tenofovir/emtricitabine; ABC/3TC, abacavir/lamivudine; ZDV/3TC, zidovudine/lamivudine; bPI, boosted protease inhibitors; NNRTI, non-nucleoside analog reverse transcriptase inhibitor; INI, integrase inhibitor.
- * Variables were mutually adjusted. Other characteristics which were assessed to be added into the model were HIV transmission mode, age, calendar year, viral subtype, and transmitted drug resistance.
The type of third drug in the regimen was also significantly associated with immunological success (P = 0.034). The only significant effect was determined comparing “other” drugs with bPI, while a trend versus a higher probability of immunological success was observed comparing INI with bPI. Also, the NRTI backbone abacavir/lamivudine showed a trend toward a higher hazard of achieving immunological success as compared to tenofovir/emtricitabine.
DISCUSSION
The present study, involving 1,305 treatment-naïve patients infected with HIV who started cART in a large national cohort study, confirmed that the initial HIV-1 RNA >100,000 copies/ml led to a reduced hazard of virological success when compared with HIV-1 RNA <100,000 copies/ml. The adjusted analysis showed a decreasing HR of virological response by increasing viral load stratum above this threshold, suggesting that the phenomenon of reduced response with increasing viral load may represent a continuum. This finding in a clinical setting of naïve patients confirms other observations that high baseline viral loads are a relevant predictor of virological response [Wood et al., 2003, 2006; Santoro et al., 2013]. The results obtained in this study confirm that this holds true after adjusting for different viral subtypes and a number of different cART types.
Raltegravir-based regimens appear to be at least as effective as regimens based on bPIs or NNRTIs, low number of patients enrolled in this arm decreases the impact. A recent mixed treatment comparison meta-analysis indicated that the rate at which HIV-1 RNA levels fell to levels below 50 copies/ml was the greatest with raltegravir followed by NNRTI and next by PIs [Vieira et al., 2011]. The use of INI-based regimens is an independent predictor of virological success in patients with baseline viral load >100,000 copies/ml, despite the few patients included in this group. This finding warrants further, prospective studies to explore the potential relevance of INIs in treating individuals presenting with particularly high HIV-1 RNA levels.
Interestingly, the type of NRTI backbone included in the initial cART regimen was not associated with the virological response. This lack of association was confirmed in the subgroup with baseline viral load >100,000 copies/ml. These findings are in contrast with observations from clinical trials in treatment-naïve subjects, indicating inferior virological response with zidovudine/lamivudine as compared with tenofovir/emtricitabine when combined with efavirenz [Gallant et al., 2006] and, in subsets of patients with baseline HIV-1 RNA >100,000 copies/ml, of abacavir/lamivudine as compared with tenofovir/emtricitabine when combined with efavirenz or atazanavir/ritonavir [Sax et al., 2011]. Discrepancies can be justified by the different design and nature of the studies, by the definition of virological response, ignoring treatment changes in this study, and by differences in third drugs employed in the regimens [Currier et al., 2010; Squires et al., 2011].
Gender differences in response rates were also observed. Previous observations highlighted a higher propensity to cART interruption in female patients. The reasons for the higher discontinuation rate appear complex but may include poorer adherence, pregnancy, and higher incidence of adverse events in women than in men [Gras et al., 2007; Ortego et al., 2012].
The activity of the initial cART regimen, as estimated by the interpretation of pre-treatment genotypic resistance results using a wGSS was an independent predictor of virological success, with a greater chance of reaching HIV-1 RNA <50 copies/ml if wGSS was ≥3, that is if the regimen was fully active. The relevance of baseline drug resistance in predicting virological response has been established by previous observations [Sax et al., 2011]. This study confirms the independent relevance of pre-therapy resistance and viral load on virological outcome of cART and also shows that baseline susceptibility is associated with the probability of immunologic response. Genotypic resistance testing prior to cART initiation has become the standard of care and is always recommended to guide cART, both in antiretroviral-naïve and experienced patients [Panel on Antiretroviral Guidelines for Adults and Adolescents, ]. As expected, a higher rate of immunological success (i.e., CD4+ ≥500 cells/µl) was observed in patients with higher baseline CD4+ [Rizzardini et al., 2006]. The time to immunological success was also associated with the HIV-1 transmission risk group: in particular, it was significantly shorter in male homosexuals as compared to the heterosexual group. A recent meta-analysis suggested that men have a marginally higher probability than women of maintaining ≥90% adherence to cART; however, this difference was more significant in studies with higher proportions of men having sex with men [Ortego et al., 2012], which may justify our observation.
Among the NRTIs backbones, we confirmed ABC/3TC recipients experienced a trend toward greater CD4+ cells/µl recovery compared with tenofovir/emtricitabine [Sax et al., 2011], although this association was borderline significant.
This study has some limitations that are inherent in all observational evaluations of HIV-1 treatment outcomes, such as confounding by indication, information on HLA-B*5701 status, and missing adherence levels.
In conclusion, in this Italian nationwide observational database, high baseline viral load, wGSS, gender, as a possible surrogate of adherence, and use of INIs were the strongest independent predictors of time to achieve virological success, while type of cART backbone seems to predict immunological success rather than virological success in clinical practice. This information may be valuable when starting a first-line antiretroviral treatment in clinical practice, particularly in cases with higher baseline viral load.
Acknowledgements
Andrea Giacometti (ANCONA - Clinica di Malattie Infettive), Luca Butini (ANCONA - Immunologia Clinica), Romana del Gobbo (ANCONA - Malattie Infettive), Patrizia Bagnarelli (ANCONA - Virologia), Danilo Tacconi (AREZZO - Malattie Infettive), Giovanni Corbelli (ASCOLI PICENO - Malattie Infettive), Stefania Zanussi (AVIANO - Centro di Riferimento Oncologico), Stefania Zanussi (AVIANO - Laboratorio Centro di Riferimento Oncologico), Laura Monno (BARI - Clinica Malattie Infettive Università), Grazia Punzi (BARI - Virologia), Franco Maggiolo (BERGAMO - Malattie Infettive), Annapaola Callegaro (BERGAMO - Microbiologia e Virologia), Leonardo Calza (BOLOGNA - Malattie Infettive S. Orsola), Maria Carla Re (BOLOGNA - UO Microbiologia, Lab. Retrovirus), Raffaele Pristerà (BOLZANO - Malattie Infettive), Paola Turconi (BRESCIA - Fleming Labs), Antonella Mandas (CAGLIARI - Centro S.I.D.A., Policlinico Universitario), Alessandra Pozzo (CAMPOBASSO - Malattie Infettive Cardarelli), Nuccia Simeone (CASERTA - Malattie Infettive AO S. Sebastiano e S. Anna), Sauro Tini (CITTA' DI CASTELLO - Medicina Generale), Alessia Zoncada (CREMONA - Malattie Infettive), Elisabetta Paolini (CREMONA - Servizio Immunoematologia e Medcina Trasfusionale), Giorgio Amadio (FERMO - Malattie Infettive), Laura Sighinolfi (FERRARA - Malattie Infettive AOU S. Anna), Giuliano Zuccati (FIRENZE - Centro MTS), Massimo Morfini (FIRENZE - Ematologia CAREGGI), Roberto Manetti (FIRENZE - Immunoallergologia CAREGGI), Paola Corsi (FIRENZE - Malattie Infettive CAREGGI), Luisa Galli (FIRENZE - Malattie Infettive Pediatria Meyer), Massimo Di Pietro (FIRENZE - Malattie Infettive SM Annunziata), Filippo Bartalesi (FIRENZE - Malattie Infettive Università), Grazia Colao (FIRENZE - Virologia CAREGGI), Andrea Tosti (FOLIGNO - Malattie Infettive / SERT), Antonio Di Biagio (GENOVA - Clinica Malattie Infettive, IRCCS AOU S. Martino, IST di Genova), Maurizio Setti (GENOVA - Clinica Medica Immunologia, IRCCS AOU S. Martino, IST di Genova), Bianca Bruzzone (GENOVA - Laboratorio di Igiene, IRCCS AOU S. Martino, IST di Genova), Giovanni Penco (GENOVA - Malattie Infettive Ospedali Galliera), Michele Trezzi (GROSSETO - Malattie Infettive), Paolo Bonfanti (LECCO - Malattie Infettive), Riccardo Pardelli (LIVORNO - Malattie Infettive), Irene Arcidiacono (LODI - Malattie Infettive), Alberto Degiuli (LODI - Virologia Lodi), Michele De Gennaro (LUCCA - Malattie Infettive), Alessandro Chiodera (MACERATA - Malattie Infettive), Alfredo Scalzini (MANTOVA - Malattie Infettive Ospedale 'C. Poma'), Loredana Palvarini (MANTOVA - Virologia), Paolo Almi (MASSA - Malattie Infettive), Giovanni Todaro (MESSINA - Malattie Infettive), Paola Cicconi (MILANO - Clinica di Malattie Infettive Ospedale S. Paolo), Stefano Rusconi (MILANO - Dipart. Scienze Cliniche, Sez. Malattie Infettive - Università degli Studi), Maria Rita Gismondo (MILANO - Laboratorio Microbiologia Ospedale L. Sacco (Dipart. Scienze Cliniche, Sez. Malattie Infettive)), Maria Rita Gismondo (MILANO - Laboratorio Microbiologia Ospedale L. Sacco (Prima Divisione Malattie Infettive)), Valeria Micheli (MILANO - Laboratorio Microbiologia Ospedale L. Sacco (Seconda Divisione Malattie Infettive)), Maria Luisa Biondi (MILANO - Laboratorio di diagnostica molecolare infettivologica AO S. Paolo), Nicola Gianotti (MILANO - Malattie Infettive San Raffaele), Amedeo Capetti (MILANO - Prima Divisione Malattie Infettive Ospedale L. Sacco), Paola Meraviglia (MILANO - Seconda Divisione Malattie Infettive Ospedale L. Sacco), Enzo Boeri (MILANO - Virologia HSR), Cristina Mussini (MODENA - Clinica Malattie Infettive), Monica Pecorari (MODENA - Virologia), Alessandro Soria (MONZA - Malattie Infettive), Sergio Malandrin (MONZA - UO Microbiologia AO S. Gerardo), Maurizio Santirocchi (NARNI - SERT), Diego Brustia (NOVARA - Malattie Infettive AO Maggiore), Paolo Ravanini (NOVARA - Virologia), Federico Dal Bello (PADOVA - Virologia), Nino Romano (PALERMO - Centro Riferimento AIDS Università), Maurizio Mineo (PALERMO - Malattie Infettive Azienda Policlinico), Salvatrice Mancuso (PALERMO - Servizio Riferimento Regionale Diagnosi AIDS), Carlo Calzetti (PARMA - Divisione Malattie Infettive ed Epatologia Azienda Ospedaliera), Renato Maserati (PAVIA - Ambulatorio Clinica Malattie Infettive S. Matteo), Gaetano Filice (PAVIA - Clinica Malattie Infettive e Tropicali), Fausto Baldanti (PAVIA - Virologia S. Matteo), Daniela Francisci (PERUGIA - Malattie Infettive), Giustino Parruti (PESCARA - Malattie Infettive), Ennio Polilli (PESCARA - Virologia Pescara), Daria Sacchini (PIACENZA - Malattie Infettive), Chiara Martinelli (PISA - Malattie Infettive), Rita Consolini (PISA - Pediatria I Università), Linda Vatteroni (PISA - Virologia), Angela Vivarelli (PISTOIA - Malattie Infettive), Alessandro Nerli (PRATO - Malattie Infettive), Lucia Lenzi (PRATO - Virologia), Giacomo Magnani (REGGIO EMILIA - Malattie Infettive), Patrizia Ortolani (RIMINI - Malattie Infettive RIMINI), Massimo Andreoni (ROMA - Malattie Infettive Tor Vergata), Guido Palamara (ROMA - IRCCS S. Gallicano), Caterina Fimiani (ROMA - Immunologia Clinica Umberto I), Lucia Palmisano (ROMA - Istituto Superiore di Sanità), Simona Di Gaimbenedetto (ROMA - Istituto di Clinica Malattie Infettive Cattolica), Manuela Colafigli (ROMA - Laboratorio virologia Cattolica), Vincenzo Vullo (ROMA - Malattie Infettive e Tropicali La Sapienza - Umberto I), Ombretta Turriziani (ROMA - Medicina Sperimentale e Patologia - Sezione Virologia - La Sapienza), Marco Montano (ROMA - Virologia per Malattie Infettive Tor Vergata), Cataldo Senatore (SALERNO - Laboratorio Biologia Molecolare AOU Salerno), Cataldo Senatore (SALERNO - Malattie Infettive Ospedali Riuniti S. Giovanni e Ruggi), Chiara Dentone (SAN REMO - Malattie Infettive), Angela Gonnelli (SIENA - Malattie Infettive), Andrea De Luca (SIENA - Malattie Infettive 2), Maurizio Zazzi (SIENA - Virologia), Michele Palumbo (TERNI - Malattie Infettive), Valeria Ghisetti (TORINO - Laboratorio di Virologia, Ospedale Amedeo di Savoia), Stefano Bonora (TORINO - Malattie Infettive Amedeo di Savoia), Palma Delle Foglie (TRENTO - Malattie Infettive), Cristina Rossi (TREVISO - Malattie Infettive), Paolo Grossi (VARESE - Clinica Malattie Infettive e Tropicali), Elena Seminari (VARESE - Virologia), Federica Poletti (VERBANIA - Malattie Infettive VERBANIA), Vincenzo Mondino (VERBANIA - Virologia), Marina Malena (VERONA - Centro di Medicina Preventiva-ULSS 20).




