Molecular epidemiology of hepatitis B virus genotypes circulating in acute hepatitis B patients in the Campania region
Abstract
Fifty-three HBV-DNA-positive patients with symptomatic acute hepatitis B were enrolled from 1999 to 2010 to evaluate molecular and phylogenetic changes in HBV in southern Italy. HBV polymerase region was evaluated by direct sequencing in plasma samples obtained at first observation. Different data sets were aligned and a phylogenetic tree was inferred using PhyML program. Statistical robustness was confirmed with a bootstrap analysis. A Bayesian Markov chain Monte Carlo method and a Bayesian skyline plot were used to estimate the evolution of our samples. The dN/dS rate (ω) was estimated by the maximum likelihood approach to investigate the presence of codons under positive selection. The MacClade program was used to test viral gene out/in flow only among HBV-D3 subgenotype patients with different risk factors. Of the 53 patients, 83% were born in Italy and 17% were foreigners. HBV genotype D was prevalent (64.1%), followed by genotype A (26.4%), E (3.8%), and F (5.7%). The prevalent subgenotype was D3 (70.6%). The Bayesian tree of the 24 D3 subgenotypes showed two main clades both dated 1994; 40% of viral gene flow observed was from intravenous drugs users and heterosexual patients. Phylogenetic analysis of HBV isolates showed that HBV-D3 remains the prevalent genotype, but also subgenotype A2 has become frequent in southern Italy. This may be of clinical relevance in years to come, since patients with HBV-genotype-A chronic infection less frequently than those with genotype D develop HBeAg-negative chronic hepatitis and respond more frequently to alfa-interferon treatment. J. Med. Virol. 86: 1683–1693, 2014. © 2014 Wiley Periodicals, Inc.
Abbreviations
-
- HBV
-
- hepatitis B virus
-
- AST
-
- aspartate aminotransferase
-
- ALT
-
- alanine aminotransferase
-
- HBsAg
-
- hepatitis B surface antigen
-
- anti-HBc IgM
-
- IgM to hepatitis B core antigen
-
- anti-HDV
-
- antibodies to hepatitis D virus
-
- anti-HIV
-
- antibodies to human immunodeficiency virus
-
- PCR
-
- polymerase chain reaction
-
- dN/dS, ω
-
- ratio non-synonymous to synonymous
-
- tMRCA
-
- time of the most recent common ancestor
-
- ML
-
- maximum likelihood
-
- ESS
-
- effective sample size
-
- BSP
-
- Bayesian skyline plot
-
- MCMC
-
- Markov chain Monte Carlo
-
- H0
-
- null hypothesis
-
- E
-
- exponential
INTRODUCTION
The incidence of acute hepatitis B has shown a progressive decline in the last three decades in Italy, from 12 cases per 100,000 inhabitants in 1985 to 1 per 100,000 in 2011 due to socioeconomic improvements, several national campaigns to prevent the diffusion of human immunodeficiency virus (HIV) infection and to the national mass HBV vaccination campaign started in 1991. As a consequence of this mandatory HBV vaccination program involving to date nearly all Italians aged 0–33, the incidence rate per 100,000 inhabitants in 2011 was 0.0% in the age group 0–14, 0.5% in the age group 15–24, 1.2% in the age group 25–34, and 2% in those over 35 years [http://www.iss.it/binary/publ/cont/12_4_web.pdf]. Therefore, in recent years the majority of Italian patients with acute hepatitis B are adults over 35 and show a symptomatic, seldom severe, clinical course, but which in some cases progresses to fulminant liver failure [Da Villa et al., 2007; La Torre et al., 2008; Mele et al., 2008; Stroffolini et al., 2008; Sagnelli et al., 2009].
HBV genotypes, subgenotypes, and HBsAg serotypes are genetically viral populations sharing an evolutionary history. A phylogenetic analysis based on the divergence of the complete genomes revealed that HBV has eight human genotypes from A to H with a sequence divergence larger than 8% of the entire genome [Naumann et al., 1993; Stuyver et al., 2000; Arauz-Ruiz et al., 2002; Kidd-Ljunggren et al., 2002; Miyakawa and Mizokami, 2003; Sumi et al., 2003; Norder et al., 2004; Lyra et al., 2005; Ribeiro et al., 2006; Al Mahtab et al., 2008] and four main HBsAg subtypes ayw, adr, adw, and ayr [Okamoto et al., 1988; Arauz-Ruiz et al., 2002], both genotypes and subtypes showing a distinct distribution in different geographical areas [Schaefer, 2007a,b]. HBV genotypes show a different responsiveness to interferon treatment [Dal Molin et al., 2006; La Torre et al., 2008; Stroffolini et al., 2008], genotype D being the least responsive to alfa-interferon. HBV genotype D has been responsible for decades for nearly 95% of the cases of acute and chronic hepatitis B in Italy [Coppola et al., 2010], a predominance that has certainly reduced the incentive of physicians to determine the HBV genotype, and, therefore, the information available over time has come from sporadic investigations. Little information is at present available on the epidemiological impact of the HBV subgenotypes in Italy, subgenotype D3 being associated with percutaneous transmission (in particular, intravenous drug use) and subgenotype A2 with sexual exposure [Zehender et al., 2008].
Italy has become a land of immigration in the last two decades, mainly from Africa, Eastern Europe, and Eastern Asia and consequently new HBV genotypes have been introduced [Coppola et al., 2010, 2013c]. This phenomenon has been documented well also in other countries, in particular in the USA [Takeda et al., 2006; van Houdt et al., 2007; Deterding et al., 2008; Panessa et al., 2009; Sloan et al., 2009].
The present paper reports the results of a study performed to identify the genetic molecular diversity of the HBV genotype in 53 patients with acute hepatitis B, using both Bayesian and phylodynamic tools.
METHODS
Patients
One-hundred and twenty-five consecutive patients with acute hepatitis B observed from September 1999 to July 2010 at one of the two participating Units of Infectious Diseases in southern Italy, one in Naples, and the other in Caserta, had been evaluated in a previous study performed to identify the lamivudine-resistant HBV strain rtM204V/I in acute hepatitis B [Coppola and Potenza, 2013]. These two Units had been using the same clinical and laboratory approach for years and had cooperated in other clinical investigations [Coppola et al., 2008, 2013b; Sagnelli et al., 2008; 2012]. Of these 125, 53 were included in the present study based on the availability of a plasma sample stored at −40°C and never thawed until used for this investigation.
The 53 patients with acute hepatitis B showed an increase in aspartate aminotransferase (AST) and alanine aminotransferase (ALT) serum levels of at least 10 times the upper normal limit and the presence in serum of hepatitis B surface antigen (HBsAg) and IgM to hepatitis B core antigen (anti-HBc IgM); no patient showed serum antibodies to hepatitis D virus (anti-HDV) or to human immunodeficiency virus (anti-HIV). A major risk factor for the acquisition of HBV infection was identified for 30 patients, 13 patients declared no risk factor and in one case the information was not available. The patients were considered to have developed severe hepatitis if they showed at least one of the following complications: porto-systemic encephalopathy, ascites or a progressive reduction in prothrombin activity to below 35% [Sagnelli et al., 2009].
All procedures used in the study were in accordance with the current international guidelines, with the standards on human experimentation of the Ethics Committee of the Azienda Ospedaliera Universitaria of the Second University of Naples, Italy, and with the Helsinki Declaration of 1975 and revised in 1983. In addition, at the time of the first observation all patients signed their informed consent for the collection and storage of biological samples and for the anonymous use of their data in clinical research, prepared according to the rules of the Ethics Committee of the Azienda Ospedaliera Universitaria of the Second University of Naples.
Routine Methods
HAV, HBV, HCV, HDV, and HIV serum markers were sought using a commercial immunoenzymatic assay [Abbott Laboratories, North Chicago, IL, for HBsAg, anti-HBs, anti-HBc (total and IgM), anti-HAV (total and IgM) and anti-HIV 1 and 2; DiaSorin, Saluggia, VC, Italy, for anti-HDV IgG and anti-HDV IgM; Ortho Diagnostic Systems, Neckargemund, Germany, for anti-HCV]. Liver function tests were performed applying routine methods.
HBV Molecular Methods
Plasma samples of all patients were tested for HBV DNA, HBV genotype, and mutants in the polymerase region. Plasma HBV DNA was sought by real-time polymerase chain reaction (PCR), as previously described [Coppola et al., 2011]. Hepatitis B virus genotypes were determined by phylogenetic analysis of sequences of 400 nt of the pre S–S region. The pre S–S gene was amplified in a nested PCR reaction (outer primers: forward 5′-CCTCATTTTGCGGGTCACC-3′ and reverse 5′-TTTGACATACTTTCCAATCAAT-3′; inner primers: forward 5′-TCACCATATTCTTGGGAAC-3′ and reverse 5′-AGGGTTTAAATGTATACCCA-3′) [Coppola et al., 2010]. The PCR products were then purified using the MinElute PCR purification kit (Qiagen, Qiagen GmbH, Hilden, Germany) according to the manufacturer's instructions; the sequencing reaction was performed using the primer 5′-CTAGGACCCCTGCTCGTGTT-3′ with the Big Dye Terminator v 1.1 Cycle Sequencing Kit (Applied Biosystems, Carlsbad, CA), according to the manufacturer's instructions, and run in an ABI 310 Genetic Analyzer (Applied Biosystems).
The pol region mutants were assessed by direct sequencing of the region by nested PCR using the outer primers F-out-76-5′-CCTGCTGGTTCCAGTTC-3′ and R-out-1178—5′-GGT TGCGTCAGCAAACACTTG-3′ and the inner primers F-in-230—5′-CCTCACAATACCTGCAGAGTCTAGACT-3′ and R-in-997-5′-AAAGCCCAAAAGACCCACAAT-3′. The PCR products were then purified using the MinElute PCR purification kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's instructions; the sequencing reaction was performed using the primers F-in-230 and R-in-997 with the Big Dye Terminator v 1.1 Cycle Sequencing Kit (Applied Biosystems), according to the manufacturer's instructions, and run in an ABI 310 Genetic Analyzer (Applied Biosystems) [Coppola et al., 2013c].
Hepatitis B Virus Data Sets
Two different sequence data sets were analyzed in the present study. The first data set consisted of 128 HBV polymerase gene sequences, of which 53 were isolates and 75 were genotype and subgenotype specific reference sequences. All reference sequences were downloaded from the National Centre for Biotechnology Information [http://www.ncbi.nlm.nih.gov/].
The second data set included only D3 subgenotype isolates (N° = 24) and was used to estimate the evolutionary rate and time of the most recent common ancestor (tMRCA).
Phylogenetic Analysis
All data sets were aligned using CLUSTAL X software [Thompson et al., 1994], then manually edited with the Bioedit software [Hall et al., 1999]. The ModelTest program v 3.7 [Posada and Buckley, 2004] was used to select the simplest evolutionary model that adequately fitted the sequence data. A maximum likelihood (ML) phylogenetic tree was inferred with the PhyML program [http://www.atgc-montpellier.fr/phyml/] using the GTR + I + G nucleotide substitution model for the first data set. The tree was rooted using a midpoint rooting. The statistical robustness and reliability of the branching order within each phylogenetic tree were confirmed with a bootstrap analysis.
Evolutionary Rate Estimate, Time-Scaled Phylogeny, and Phylodynamics
To estimate the evolutionary rate on the second data set both a strict and relaxed clock with an uncorrelated log normal rate distribution were compared. A Bayesian Markov chain Monte Carlo (MCMC) method implemented in the BEAST software package 1.7.4 [Ciccozzi et al., 2011; Zehender et al., 2013] was used. As coalescent priors, three parametric demographic models of population growth (constant size and exponential and logistic growth) and a Bayesian skyline plot (BSP, a piecewise constant non-parametric model) were compared.
The MCMC chains were run for at least 50 million generations, and sampled every 5,000 steps.
Convergence of the MCMC was assessed by calculating the ESS (effective sample size) for each parameter. Only parameter estimates with ESS's of >200 were accepted. Uncertainty in the estimates was indicated by 95% highest posterior density (HPD) intervals, and the best fitting models were selected using a Bayes factor (BF) applying marginal likelihoods implemented in BEAST software [Ciccozzi et al., 2011].
In accordance with [Kass and Raftery, 1995], the strength of the evidence against H0 (Null Hypothesis) was evaluated as follows: 2 ln BF < 2, no evidence; 2–6, weak evidence; 6–10, strong evidence; >10, very strong evidence. A negative value indicated evidence in favor of H0. Only values of ≥6 were considered significant. To reconstruct the time-scaled phylogeny of the HBV-D3 subgenotype (the second data set), a logistic demographic model with relaxed molecular clock was used, assuming the HKY + I + G (Hasegawa, Kashino, Yano, invariable sites, gamma distribution) model of nucleotide substitution (selected by ModelTest). Statistical support for specific clades was obtained by calculating the posterior probability of each monophyletic clade.
Population dynamics was also analyzed in the HBV-D3 isolates, implementing a relaxed molecular clock under the Bayesian skyline plot.
Selection Pressure Analysis
The dN/dS rate (ω; non-synonymous to synonymous) was also estimated by the ML approach implemented in the program HyPhy [Pond and Muse, 2005] using the second data set. Site-specific positive and negative selection were estimated by two different algorithms: the fixed-effects likelihood (FEL), which fits a ω rate to every site and uses the likelihood ratio to test if dN ≠ dS, and the random-effects likelihood (REL), a variant of the Nielsen–Yang approach [Nielsen and Yang, 1998], which assumes that a discrete distribution of rates exists across sites and allows both dS and dN to vary independently from site to site. The two methods have been described in more detail elsewhere [Lo Presti et al., 2011]. In order to select sites under selective pressure and keep our test conservative, a P value of ≤0.1 or a posterior probability of ≥0.9 as relaxed critical values [Kosakovsky Pond and Frost, 2005] were assumed. Part of the analysis was conducted using the web-based interface Datamonkey [http://www.datamonkey.org/]. For the evolutionary analysis, the reference sequences of HBV-D3 complete genome (EU594382; protein polymerase id ACF95157.1) were used to trace the exact position of the amino acids found under positive selection. The HKY + I + G evolutionary model was chosen as the best-fitting model for the ML tree, as the input tree in the program HyPhy and to investigate the presence of codons under positive selection in the second data set.
Viral Gene Flow Analysis
The MacClade version 4 program (Sinauer Associates, Sunderland, MA) was used to test viral gene out/in flow among HBV-D3 subgenotype-infected subjects (second data set) with different risk factors using a modified version of the Slatkin and Maddison test, as already described [Ciccozzi et al., 2013]. A one-character data matrix was obtained from the original data set by assigning to each taxon (viral sequence) in the tree a one-letter code indicating the risk factor of the patient infected with that specific HBV-D3 strain. The putative origin of each ancestral sequence (i.e., internal node) in the tree was inferred by finding the most parsimonious reconstruction (MPR) of the ancestral character. The final tree length, that is, the number of viral gene flow events observed in the genealogy, can be computed easily and compared to the tree-length distribution of 10,000 trees obtained by random joining-splitting (null distribution). Observed genealogies significantly shorter than the random trees indicate the presence of subdivided populations with a restricted gene flow. The viral gene flow among the different risk factors (character states) was traced with the State changes and stasis tool (MacClade software), which counts the number of changes in a tree for each pair-wise character state.
When multiple MPRs were present (as in our data sets), the algorithm calculated the average migration count over all possible MPRs for each pair. The resulting pair-wise migration matrix was then normalized and a randomization test with 10,000 matrices obtained from 10,000 random trees (by random joining-splitting of the original tree) was performed to assess the statistical significance of the migration counts observed.
RESULTS
The demographics and clinical, biochemical, and virological characteristics at baseline of the 53 patients with acute hepatitis B are shown in Table I. The mean age was 36.8 years (standard deviation, SD: ±13.73), males predominated (75.6%) and unsafe sexual intercourse and intravenous drug use were the most frequent risk factors for acquiring HBV infection (Table I).
| Number of patients | 53 |
| Males, N (%) | 40 (75.6) |
| Age, years, M ± SD | 36.85 ± 13.73 |
| Risk factor, No. (%) | |
| Unsafe sexual intercourse | 15 (28.9) |
| Intravenous drug use | 14 (26.9) |
| Surgery, endoscopy, dental care | 6 (11.5) |
| Tattoo/acupuncture | 3 (5.8) |
| Unknown | 14 (26.9) |
| Missing | 1 |
| HBV genotype, No. (%) | |
| D | 34 (64.1) |
| (D1; D2; D3; D4) | (7; 2; 24; 1) |
| A | 14 (26.4) |
| (A2) | (14) |
| F | 2 (3.8) |
| (F1) | (2) |
| E | 3 (5.7) |
| Born in Italy, No. (%) | 44 (83.02) |
| Born abroad, No. (%) | 9 (16.98) |
| Days from onset of symptoms, M ± SD | 32.26 ± 69.14 |
| With severe acute hepatitis B, No. (%) | 5 (9.4) |
| AST (IU/ml), M ± SD | 2000.32 ± 1229.37 |
| ALT (IU/ml), M ± SD | 2768.40 ± 1193.57 |
| Bilirubin (mg/dl), M ± SD | 18.58 ± 10.11 |
| Prothrombin time (%), median (range) | 63.50 (20.1–113) |
| PTL × 103/µl | 225.35 ± 82.70 |
| HBV DNA (IU/ml), M ± SD | 9.34 E* 7 ± 2.9 E 10 |
| Anti-HDV-positive, No. (%) | 0 |
| Anti-HCV, No. (%) | |
| Negative | 47 (88.7) |
| Positive | 6 (11.3) |
| Clinical outcome, No. (%) | |
| Chronic viral hepatitis B | 1 (1.9) |
| Resolution | 49 (96.1) |
| OLT | 1 (1.9) |
| Missing | 2 |
| Seroconversion to anti-HBsAg in 49 pts with resolution, No. (%) | |
| Yes | 31 (63.3) |
| No | 18 (36.7) |
- * E, exponential.
Forty-four (83%) of the patients were born in Italy and nine (17%) in other countries: one in Ghana, one in the Dominican Republic, one in Algeria, one in Tunisia, one in Nigeria, two in Albania, and two in Russia.
Acute hepatitis was severe in five patients (Table I), one of whom developed porto-systemic encephalopathy, two ascites, and two a progressive reduction in the prothrombin activity to below 35%. Of these five, four recovered and one underwent successful orthotopic liver transplantation. Six (11.5%) of the 53 patients had been anti-HCV/HCV RNA-positive for at least 12 months at the time of HBV superinfection, and all six became HCV RNA-negative and remained so throughout the clinical course of acute hepatitis B. The AST, ALT, bilirubin, and HBV DNA serum values and prothrombin time were those characteristic of acute hepatitis B (Table I).
All but two of the 53 patients enrolled were followed up for at least 6 months. Of these 51 patients, 49 (96.1%) recovered and cleared HBV infection, one (1.9%) progressed to chronic hepatitis, and one underwent successful orthotopic liver transplantation. Seroconversion to anti-HBs was observed in 31 (63.3%) of the 49 patients who recovered, whereas 18 patients (12 with HBV genotype D and 6 with genotype A) cleared HBsAg but did not seroconvert to anti-HBs.
Phylogenetic Analysis
The ML tree of the first data set (Fig. 1) shows the presence of different statistically supported clades (bootstrap values > 70%). HBV genotype D was the most common HBV genotype in the 53 patients (34 cases, 64.1%). Of the remaining 19 patients, 14 (26.4%) had HBV genotype A (all A2 subgenotype), 2 (3.8%) HBV genotype E, and 3 (5.7%) HBV genotype F (Table I).
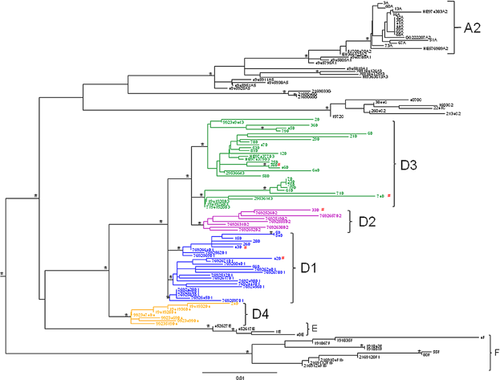
Of the 34 patients with genotype D sequences, 24 (70.6%) had subgenotype D3, 7 (20.6%) subgenotype D1, 2 (5.9%) subgenotype D2, and 1 (2.9%) subgenotype D4 (Table I, Fig. 1). Of these 34 patients 5 were born abroad, 3 with HBV genotype D1, one with D2, and one with D3 (Table II).
| HBV genotype A | HBV genotype D | P-value | |
|---|---|---|---|
| Number of patients | 14 | 34 | |
| Males, No. (%): | 13 (92.9) | 23 (67.6) | 0.06 |
| Age, years, M ± SD | 36.00 ± 10.78 | 37.50 ± 15.75 | 0.764 |
| Risk factor, No. (%) | |||
| Unsafe sexual intercourse | 4 (36.4) | 10 (45.5) | 0.751 |
| Intravenous drug use | 1 (9.1) | 9 (40.9) | 0.064 |
| Surgery, endoscopy, dental care | 6 (54.5) | 0 | 0.0001 |
| Tattoo/acupuncture | 0 | 3 (13.6) | — |
| Unknown | 3 | 12 | — |
| Born in Italy, No. (%) | 13 (92.9) | 29 (85.3) | 0.476 |
| Born abroad, No. (%) | 1 (7.1) | 5 (14.7) | |
| Days from onset of symptoms, M ± SD | 43.57 ± 101.27 | 19.62 ± 8.45 | 0.172 |
| With severe acute hepatitis B, No. (%) | 0 | 4 (11.8) | |
| AST (IU/ml), M ± SD | 1694 ± 2492 | 2144 ± 1389 | 0.482 |
| ALT (IU/ml), M ± SD | 830.50 ± 946.42 | 285.06 ± 1207.07 | 0.139 |
| Bilirubin (mg/dl), M ± SD | 17.15 ± 6.67 | 18.58 ± 10.11 | 0.548 |
| Prothrombin time (%), median (range) | 68.00 (61.0–113) | 60.50 (20.1–77.0) | |
| PTL × 103/µl | 231.08 ± 69.67 | 218.88 ± 90.91 | 0.665 |
| HBV DNA (IU/ml), M ± SD | 1.86 E*8 ± 4.95 E 8 | 6.89 E 7 ± 1.82 E 8 | ns |
| Anti-HDV-positive, No. (%) | 0 | 0 | — |
| Anti-HCV, No. (%) | |||
| Negative | 13 (92.9) | 29 (85.29) | 0.656 |
| Positive | 1 (7.1) | 5 (14.70) | |
| Clinical outcome, No. (%) | |||
| Chronic viral hepatitis B | 0 | 1 (3.03) | 0.35 |
| Resolution | 14 (100) | 31 (93.9) | |
| OLT | 0 | 1 (3.03) | |
| Missing | 0 | 1 | |
| Seroconversion to anti-HBsAg in 45 pts with resolution, No. (%) | |||
| Yes | 8 (57.1) | 19 (61.3) | 0.794 |
| No | 6 (42.9) | 12 (38.7) | |
- * E, exponential.
All five patients with severe acute hepatitis showed subgenotype D3 (Table II).
We also evaluated the demographics and initial clinical, biochemical and virological characteristics of the 34 patients with HBV genotype D and the 14 patients with HBV genotype A (Table II). No significant differences were found regarding age, country of origin or gender, although 13 of the 14 (92.9%) individuals infected by genotype A were males (Table II). Unsafe sexual intercourse and intravenous drug use were the most frequent (86.4%) risk factors for acquiring genotype D, while several risk factors (surgery, endoscopy, dental care, and sexual intercourse with an infected partner) were reported by patients with genotype A (Table II).
Evolutionary Rate Estimate, Time-Scaled Phylogeny, and Phylodynamics
The mean evolutionary rate of the HBV-D3 subgenotype polymerase sequences, the most frequent subgenotype in the patients enrolled, was evaluated on the second data set including 24 isolates with known sampling dates.
A comparison of the strict and relaxed clock models using the BF test showed that the relaxed clock fitted the data significantly better than the strict clock (2 ln BF = 26.45 in favor of the relaxed clock).
A comparison of the coalescent priors showed that the logistic model was better than the other models already described in the methods section (2 ln BF > 6). Under this model, the estimated mean value of the evolutionary rate of the HBV-D3 subgenotype polymerase gene fragment analyzed was 6.002 × 10−4 sub/site/year (95% HPD: 2.81 × 10−5–1.47 × 10−3).
Figure 2 shows the reconstruction of the time-scaled Bayesian tree of the 24 HBV-D3 isolates. The estimation of the time of the tree's root gave a mean value of 23 years ago, corresponding to 1987 (95% HPD: 1870–1997). The Bayesian tree showed two main clades (I and II). Clade I, which included a total of six isolates, had a tMRCA estimation of 16 years, corresponding to the year 1994 (95% HPD: 1941–1999). Interestingly, one of the six sequences from acutely infected subjects, labeled as 61IT@@01, was female; two were intravenous drug users and four belonged to the sexual risk group. Clade II dated back to the year 1994 (95% HPD 1941–1999) and is further divided into two subclades (IIa and IIb). Subclade IIa included two statistically supported clusters. The first, including three isolates, dated back to the year 2002 (95% HPD: 1948–2005) and the second, including three isolates (two female intravenous drug users and one male belonging to the sexual risk group), dated back to the year 1999 (95% HPD: 1939–2001).
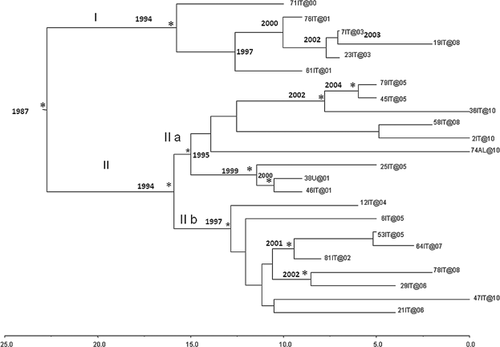
Subclade IIb dated back to the year 1997 and included nine sequences, of which three were included in a cluster dating back to the year 2001 (95% HPD: 1947–2002) and two were included in another cluster dating back to the year 2002 (95% HPD: 1952–2006), both of them statistically supported; the remaining sequences were not statistically significant.
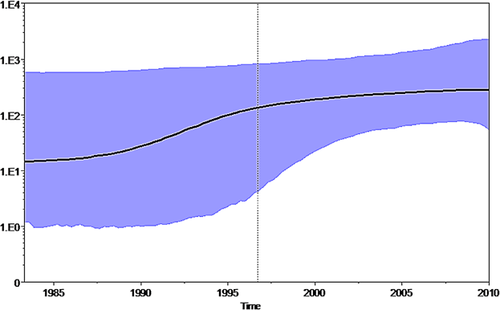
The BSP showed that the effective number of infections grew exponentially between 1987 and 1995 and then the curve reached a plateau approximately around the year 2000 (Fig. 3).
Viral Gene Flow Analysis
HBV-D3 subgenotype sequences were assigned to six risk groups: sexual, intravenous drug use, surgery-endoscopy-dental care, tattoo-acupuncture, unknown and household contact, and the gene flow among these groups were estimated (Fig. 4). The null hypothesis of panmixia (i.e., no population subdivision or complete intermixing of sequences from different geographical areas) was rejected by the randomization test (P < 0.0001).
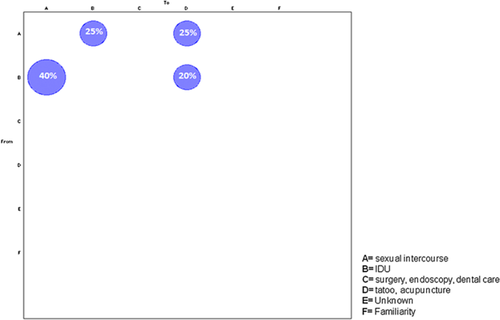
The most frequent (40%) viral gene flow events observed occurred from intravenous drug use to sexual intercourse, 25% of the gene flow was from sexual to intravenous drug use and to tattoo-acupuncture, and 20% was from intravenous drug use to tattoo-acupuncture, suggesting the central role of these risk groups as a potential drive of the HBV-D3 epidemic.
Selection Pressure Analysis
The selection pressure analysis for the polymerase region of the HBV-D3 subgenotype second data set, through computation of the ratio of dN/dS (ω) with Hyphy programs, revealed limited positive selection and abundant negative selection for the polymerase proteins, the dN/dS rate (ω) being around 2.55.
FEL analysis revealed 15 codons under statistically significant negative pressure and REL analysis identified three sites under significant positive selection (Table III). Positive and negative sites were confirmed by FEL and REL analysis and also by Datamonkey (Table III).
| Positively selected sites (ω for sites >1) HYPHY software | 97 (Y,D,H); 165 (L,H,F); 171 (T,A,N,I) |
| Negatively selected sites (ω for sites <1) HYPHY software | H 176; H 240; F 244; H 259; G 275; N 279; K 282; K 284; Y 288; L 290; F 292; G 294; G 301; E 306; K 311 |
The positively selected sites were not located in a specific domain of the polymerase protein, whereas some of the negatively selected sites were located in the reverse transcriptase RNA-dependent DNA polymerase domain (RT-like superfamily, RVT.1) and others were located in the carboxy terminal domain of the viral DNA polymerase (DNA poly-viral-C).
DISCUSSION
The level of endemicity of hepatitis B virus infection in Italy is today low and genotype D infections predominant. However, new HBV strains have been introduced recently as a result of immigration from regions of high endemicity, as shown by two studies regarding the circulation of strains in new cases of HBV infection [Coppola et al., 2010]. The displacement of people from regions where HBV vaccination has not been implemented universally, in combination with poor socioeconomic conditions, can result in pockets of high endemicity in countries where the incidence rates are otherwise low [Palumbo et al., 2008]. This scenario results in an increased risk of infection [Williams et al., 2006; Coppola et al., 2013] and in the introduction of non-D genotypes, which over time could affect the expression pattern of HBV-related diseases and the response to antiviral therapy [Fung and Lok, 2004]. Also, the problem of HBV-positive women in childbearing age or pregnant, which seemed to have been solved in Italy with the introduction of mass vaccination, is now re-emerging, as most of these women are immigrants from countries with a high HBV prevalence [Borgia et al., 2012].
A detailed molecular, phylogenetic, and epidemiological analysis of acute hepatitis B in the Campania region was carried out to evaluate the phylogenetic and evolutionary changes in the epidemiology of acute hepatitis B infection due to the emergence of various HBV genotypes in this area. The majority of the patients analyzed in the present study were infected by HBV genotype D, subgenotype D3 being predominant. Worthy of note is also the observation that 26% of the 53 patients investigated were infected with HBV subgenotype A2, not endemic in the Campania region but prevailing in Africa and India [Zehender et al., 2012]. The recent introduction of HBV genotype A to southern Italy may have clinical implications in few years since among patients with HBV chronic infection those with HBV genotype A more frequently than those with genotype D show a favorable response to alfa-interferon [Liu et al., 2005; Raimondi et al., 2010] and develop less frequently HBeAg-negative chronic hepatitis, a clinical form with a high rate of progression to liver cirrhosis [Schaefer, 2007a,b].
The time-scaled phylogeny reconstruction of the acute HBV genotype D3 sequences showed that the tree root had a tMRCA estimate of 23 years and originated in 1987 (95% HPD: 1870–1997). We also inferred the tMRCA of the internal nodes within the Bayesian tree of the D3 data set. Interestingly, our time-scaled reconstruction supported a relatively recent history of the currently circulating HBV-D3 genotype [Zehender et al., 2012]. The coalescent-based population dynamics analysis [Drummond et al., 2005] of acute HBV-D3 showed that the number of infections starting from 1987 slightly increased until 1995, remained steadily high in 2000 and is still the prevalent HBV subgenotype in acute hepatitis B in Italy [De Maddalena et al., 2007]. A recent phylogeographical reconstruction hypothesized that HBV genotype D originated in India and spread worldwide in the first decades of the 20th century, probably favored by world wars and unsafe medical practices [Zehender et al., 2012]. In the present study, where all the isolates were sampled from acute infections, the tMRCA of the tree root, corresponding also to the beginning of the increase in the number of primary infections, is recent and dates back to less than 30 years ago. Our results suggest that the HBV-D3 subgenotype circulating today in Italy shares a common ancestor that existed in the late 1980s. Interestingly, we know that in those years the decrease in the incidence of acute hepatitis B began, due to a substantial improvement in the socio-economic conditions and to the media and educational campaigns against HIV infection [Sagnelli et al., 2012a,b].
HBV-D3 clades observed in this study may be epidemiological networks originating from the introduction of new strains (still circulating in the population) after the extinction of the majority of strains circulating before. In the HBV-D3 subgenotype sequences, a significant viral gene flow was observed between intravenous drug users and subjects who had acquired HBV by unsafe sexual practices, suggesting specific preventive strategies.
The selection pressure analysis in the acute HBV-D3 data set showed 15 sites under negative selective pressure and three sites under positive selective pressure. The presence of abundant negative selective pressure most likely reflects a high degree of conservation of the polymerase protein, probably necessary for the HBV biological function. The positive selection sites suggest evolutionary “hot spots” possibly involved in the severity of the associated illness. These positive selection sites should be studied more extensively for a better understanding of the correlation between the epidemiological and clinical characteristics of acute hepatitis B.




