Pre-vaccination prevalence of infections with 25 non-high-risk human papillomavirus types among 1,000 Slovenian women in cervical cancer screening
Abstract
Cervical infections with non-high-risk human papillomavirus (non-HR-HPV) types have been associated with genital warts and a fraction of atypical squamous cells of undetermined significance and low-grade squamous intraepithelial lesions. The pre-vaccination prevalence of cervical infections with 25 non-HR-HPV types has been estimated, regardless of and without the coexistence of infection with HR-HPV types among Slovenian women 20–64 years old in cervical cancer screening, overall and according to age and cytology result. One thousand cervical specimens selected randomly from 4,455 specimens collected in 2010 in the Slovenian HPV prevalence survey were tested with Linear Array HPV Genotyping Test. Prevalence of cervical infections with any of the 25 non-HR-HPV types was 10.0% (95% CI: 8.1–11.9%) and with exclusively non-HR-HPV types 4.5% (95% CI: 3.2–5.8%). Prevalence of infections with any non-HR-HPV types among women with normal cytology was 8.8%, with atypical squamous cells of undetermined significance 30.4%, with low-grade squamous intraepithelial lesions 60.0%, and with high-grade squamous intraepithelial lesions 7.7%. Non-HR-HPV types without coexisting HR-HPV types were found in 4.0% of women with normal cytology, 26.1% with atypical squamous cells of undetermined significance, 6.7% with low-grade squamous intraepithelial lesions, and none with high-grade squamous intraepithelial lesion. Non-HR-HPV type cervical infections without coexisting HR-HPV infections were common among Slovenian women in cervical cancer screening with atypical squamous cells of undetermined significance, while rare in those with low-grade squamous intraepithelial lesions or worse. J. Med. Virol. 86: 1772–1779, 2014. © 2014 Wiley Periodicals, Inc.
INTRODUCTION
Over 40 human papillomaviruses (HPV) from alpha genus are the most common sexually transmitted infections. The categorization of alpha-HPV types into different risk categories is extremely challenging, especially for weakly carcinogenic and rare HPV types, and because multiple HPV types coexist often within the cervical epithelium [Schiffman et al., 2009; Castle et al., 2010]. As a consequence, certain HPV types have been moved frequently from one risk category to another in the last two decades [Schiffman et al., 2009; Castle et al., 2010].
The following 12 HPV types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59 are considered to be carcinogenic (class I), as defined by the International Agency for Research on Cancer (IARC) in 2009 and referred usually as high-risk (HR) HPV (HR-HPV) [Bouvard et al., 2009]. Infections with other alpha-HPV types (in this study referred to as non-HR-HPV types) are responsible also for substantial morbidity and invoke costs associated with the treatment of clinically relevant lesions including genital warts and laryngeal papillomas and triage and monitoring of women with atypical squamous cells of undetermined significance and low-grade squamous intraepithelial lesions [Holowaty et al., 1999; Institute of Oncology Ljubljana, 2011].
Information on HPV infection prevalence and HPV type distribution from large studies in Europe has been accumulating, mostly on HR-HPV types, and only more recently also on a limited number of non-HR-HPV types [Clifford et al., 2005a; De Vuyst et al., 2009; Nielsen et al., 2012].
In 2009, free of charge vaccination of 11–12 years old girls with quadrivalent HPV vaccine was introduced into the Slovenian National Vaccination Program in the absence of reliable baseline data on the prevalence of infection with HR-HPV and non-HR-HPV types among Slovenian women. To provide baseline data for monitoring the impact of Slovenian HPV vaccination program and the development of future cervical cancer screening strategies after the introduction of HPV vaccination, the pre-vaccination prevalence of cervical infections with any HR-HPV type has been estimated in 4,431 Slovenian women in cervical cancer screening in 2010 (Slovenian HPV prevalence survey) [Učakar et al., 2012].
To complement information on pre-vaccination prevalence of HR-HPV types and to assess the importance of cervical infection with non-HR-HPV types among 20–64 years old women in cervical cancer screening, the prevalence of cervical infections with 25 non-HR-HPV types (6, 11, 26, 34sub (former HPV-64), 40, 42, 44sub (former HPV-55), 53, 54, 61, 62, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 82sub (former IS39), 83, 84, and 89) has been estimated, regardless of and without the coexistence of HR-HPV types, overall and according to age and cytology result in 1,000 women randomly selected from the participants of the Slovenian HPV prevalence survey.
MATERIALS AND METHODS
In the cross-sectional study, the Slovenian HPV prevalence survey, 4,514 women 20–64 years old and eligible for cervical cancer screening according to the criteria of the National Cervical Cancer Screening Programme (NCCSP) [Institute of Oncology Ljubljana, 2011] were enrolled consecutively through 22 outpatient gynaecology services all over the country into a convenience sample between December 2009 and August 2010. Exclusion criteria were: attendance after an atypical/abnormal cytology result, history of cervical intraepithelial neoplasia of any grade, treatment for cervical disease in the preceding year, hysterectomy, and menstruating or pregnancy at presentation. After obtaining informed consent, gynecological examination including visualization of cervix and collection of cervical smear specimen for cytological examination was performed according to the NCCSP guidelines [Institute of Oncology Ljubljana, 2011]. Specimens were examined under routine screening conditions in certified cytological laboratories used normally by participating sites by cytologists who were not aware of the results of HPV testing. A second specimen was obtained for HPV DNA testing. The complete protocol of HPV testing, genotyping and discordant analysis was described in details elsewhere [Poljak et al., 2011]. Briefly, all specimens were tested first in parallel with Hybrid Capture 2 HPV DNA Test (hc2) (Qiagen, Hilden, Germany) and RealTime High Risk HPV Test (RealTime) (Abbott, Wiesbaden, Germany). All specimens with RealTime/hc2 concordant positive and discordant results were tested using the Linear Array HPV Genotyping Test (Roche Molecular Diagnostics, Branchburg, NJ), and if necessary, with an HPV-52 type-specific real-time PCR assay, INNO-LiPA HPV Genotyping Extra Test (Innogenetics, Gent, Belgium), and an in-house GP5+/GP6+ PCR assay targeting a 150-bp fragment in HPV L1 gene with additional HPV-68 specific primers.
Of the total of 4,602 women invited to participate in the Slovenian HPV prevalence survey, only 88 declined (response rate 98.1%). From 4,514 participants, we excluded 59 women (because they were not 20–64 years old or missing information on age, specimen spill out during transport or missing specimen, and because they had been vaccinated already against HPV). Thus, the sampling frame included a total of 4,455 participants. For the purpose of this analysis, a random sample of 1,000 participants was selected to be genotyped for 37 HPV types: 6, 11, 16, 18, 26, 31, 33, 34sub, 35, 39, 40, 42, 44sub, 45, 51, 52, 53, 54, 56, 58, 59, 61, 62, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 82sub, 83, 84, and 89 using Linear Array HPV Genotyping Test.
Statistical analyses were performed using STATA package version 10.0 (Stata Statistical Software: release 10.0, College Station, TX: Stata Corporation). According to recommendations of the IARC Monograph Working Group 12 HPV types with the strongest carcinogenic potential included in Group 1 were considered as HR-HPV and all other 25 HPV types tested for were referred here as non-HR-HPV types. Among them some were considered by IARC as probably (HPV-68) or possibly (HPV-53, -66, -67, -69, -70, -73, -82) carcinogenic for humans, for the rest no clear IARC recommendation exists at present [Bouvard et al., 2009]. We estimated the overall prevalence of infections with any of the 25 non-HR-HPV types regardless of and without the coexistence of HR-HPV types with 95% confidence intervals (CI), overall and according to age and cytology result. Test for linear trend was used to assess the association between the prevalence of infection with any non-HR-HPV type and age as well as severity of cervical disease.
The study has been conducted in accordance with the code of Ethics of the World Medical Association (Declaration of Helsinki) and has been approved by the Medical Ethics Committee of the Republic of Slovenia (Consent number: 83/11/09).
RESULTS
The mean age of the 1,000 women included into this analysis was 36.5 years (median: 35.0 years). 48.9% of women were 20–34 years old, 37.0% 35–49, and 14.1% 50–64 years old. Among 997 women with available cytology result, 94.7% were negative for intraepithelial lesion or malignancy, 2.3% had atypical squamous cells of undetermined significance, 1.5% low-grade squamous intraepithelial lesions, 1.3% high-grade squamous intraepithelial lesions, and 0.2% atypical squamous cells-cannot exclude high-grade squamous intraepithelial lesion.
The overall prevalence of cervical infection with any of the 25 non-HR-HPV types was 10.0% (CI: 8.1–11.9%) and with any of the 25 non-HR-HPV types without the coexistence of any of the 12 HR-HPV 4.5% (CI: 3.2–5.8%). Prevalence of infection with any of the 12 HR-HPV types was 12.5% (CI: 10.4–14.5%), any of the 12 HR-HPV types without the coexistence of non-HR-HPV types was 7.0% (CI: 5.4–8.6%) and any of the 37 HPV types examined 17.0% (CI: 14.7–19.3%).
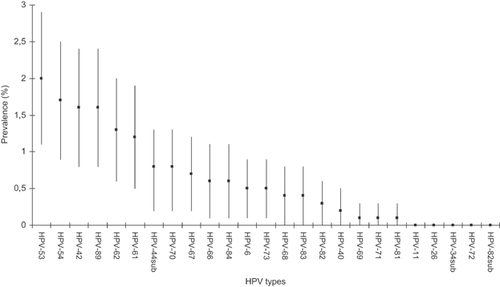
Among cervical infections with non-HR-HPV types, cervical infection with HPV-53 was most common (2.0%, CI: 1.1–2.9%), infections with HPV-69, HPV-71, and HPV-81 least common (0.1%, CI: 0.0–0.3%), while infections with HPV-11, HPV-26, HPV-34sub, HPV-72, and HPV-82sub were not detected (Fig. 1). The prevalence of infection with HPV-6 was 0.5% (CI: 0.1–0.9%). Prevalence of infections with any of the 25 non-HR-HPV types and individual non-HR-HPV types regardless of and without the coexistence of any HR-HPV types overall and according to age groups is shown in Table I. Infections with any of the 25 non-HR-HPV types were most common among women 20–34 years old (15.5%) and the prevalence decreased with increasing age to 2.8% among women 50–64 years old (P < 0.001).
| HPV types | All women (N = 1,000) | Age groups | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 20–34 years (N = 489) | 35–49 years (N = 370) | 50–64 years (N = 141) | |||||||
| N | P (%) | 95% CI | N | P (%) | N | P (%) | N | P (%) | |
| Non-HR-HPVa | 100 | 10.0 | 8.1–11.9 | 76 | 15.5 | 20 | 5.4 | 4 | 2.8 |
| Non-HR-HPVa + HR-HPVb | 55 | 5.5 | 4.1–6.9 | 43 | 8.8 | 8 | 2.2 | 4 | 2.8 |
| Only non-HR-HPVa | 45 | 4.5 | 3.2–5.8 | 33 | 6.7 | 12 | 3.2 | 0 | — |
| Single non-HR-HPVa | 35 | 3.5 | 2.3–4.6 | 25 | 5.1 | 10 | 2.7 | 0 | — |
| Multiple non-HR-HPVa | 10 | 1.0 | 0.4–1.6 | 8 | 1.6 | 2 | 0.5 | 0 | — |
| Specific non-HR-HPVa types | |||||||||
| HPV-6 | 5 | 0.5 | 0.1–0.9 | 4 | 0.8 | 0 | — | 1 | 0.7 |
| (without HR-HPVb) | 2 | 0.2 | 0.0–0.5 | 2 | 0.4 | 0 | — | 0 | — |
| HPV-11 | 0 | — | — | 0 | — | 0 | — | 0 | — |
| HPV-26 | 0 | — | — | 0 | — | 0 | — | 0 | — |
| HPV-34sub | 8 | 0.8 | 0.2–1.3 | 6 | 1.2 | 1 | 0.3 | 1 | 0.7 |
| (without HR-HPVb) | 3 | 0.3 | 0.0–0.6 | 2 | 0.4 | 1 | 0.3 | 0 | — |
| HPV-40 | 2 | 0.2 | 0.0–0.5 | 2 | 0.4 | 0 | — | 0 | — |
| (without HR-HPVb) | 0 | — | — | 0 | — | 0 | — | 0 | — |
| HPV-42 | 16 | 1.6 | 0.8–2.4 | 13 | 2.7 | 2 | 0.5 | 1 | 0.7 |
| (without HR-HPVb) | 4 | 0.4 | 0.0–0.8 | 3 | 0.6 | 1 | 0.3 | 0 | — |
| HPV-44sub | 0 | — | — | 0 | — | 0 | — | 0 | — |
| HPV-53 | 20 | 2.0 | 1.1–2.9 | 16 | 3.3 | 3 | 0.8 | 1 | 0.7 |
| (without HR-HPVb) | 8 | 0.8 | 0.2–1.3 | 6 | 1.2 | 2 | 0.5 | 0 | — |
| HPV-54 | 17 | 1.7 | 0.9–2.5 | 16 | 3.3 | 0 | — | 1 | 0.7 |
| (without HR-HPVb) | 5 | 0.5 | 0.1–0.9 | 5 | 1.0 | 0 | — | 0 | — |
| HPV-61 | 12 | 1.2 | 0.5–1.9 | 9 | 1.8 | 2 | 0.5 | 1 | 0.7 |
| (without HR-HPVb) | 3 | 0.3 | 0.0–0.6 | 2 | 0.4 | 1 | 0.3 | 0 | — |
| HPV-62 | 13 | 1.3 | 0.6–2.0 | 8 | 1.6 | 4 | 1.1 | 1 | 0.7 |
| (without HR-HPVb) | 6 | 0.6 | 0.1–1.1 | 3 | 0.6 | 3 | 0.8 | 0 | — |
| HPV-66 | 6 | 0.6 | 0.1–1.1 | 6 | 1.2 | 0 | — | 0 | — |
| (without HR-HPVb) | 3 | 0.3 | 0.0–0.6 | 3 | 0.6 | 0 | — | 0 | — |
| HPV67 | 7 | 0.7 | 0.2–1.2 | 7 | 1.4 | 0 | — | 0 | — |
| (without HR-HPVb) | 5 | 0.5 | 0.1–0.9 | 5 | 1.0 | 0 | — | 0 | — |
| HPV68 | 4 | 0.4 | 0.0–0.8 | 3 | 0.6 | 1 | 0.3 | 0 | — |
| (without HR-HPVb) | 2 | 0.2 | 0.0–0.5 | 2 | 0.4 | 0 | — | 0 | — |
| HPV-69 | 1 | 0.1 | 0.0–0.3 | 1 | 0.2 | 0 | — | 0 | — |
| (without HR-HPVb) | 1 | 0.1 | 0.0–0.3 | 1 | 0.2 | 0 | — | 0 | — |
| HPV-70 | 8 | 0.8 | 0.2–1.3 | 5 | 1.0 | 3 | 0.8 | 0 | — |
| (without HR-HPVb) | 4 | 0.4 | 0.0–0.8 | 2 | 0.4 | 2 | 0.5 | 0 | — |
| HPV-71 | 1 | 0.1 | 0.0–0.3 | 1 | 0.2 | 0 | — | 0 | — |
| (without HR-HPVb) | 1 | 0.1 | 0.0–0.3 | 1 | 0.2 | 0 | — | 0 | — |
| HPV-72 | 0 | — | — | 0 | — | 0 | — | 0 | — |
| HPV-73 | 5 | 0.5 | 0.1–0.9 | 4 | 0.8 | 1 | 0.3 | 0 | — |
| (without HR-HPVb) | 1 | 0.1 | 0.0–0.3 | 0 | — | 1 | 0.3 | 0 | — |
| HPV-81 | 1 | 0.1 | 0.0–0.3 | 0 | — | 1 | 0.3 | 0 | — |
| (without HR-HPVb) | 0 | — | — | 0 | — | 0 | — | 0 | — |
| HPV-82 | 3 | 0.3 | 0.0–0.6 | 3 | 0.6 | 0 | — | 0 | — |
| (without HR-HPVb) | 1 | 0.1 | 0.0–0.3 | 1 | 0.2 | 0 | — | 0 | — |
| HPV-82sub | 0 | — | — | 0 | — | 0 | — | 0 | — |
| HPV-83 | 4 | 0.4 | 0.0–0.8 | 2 | 0.4 | 2 | 0.5 | 0 | — |
| (without HR-HPVb) | 1 | 0.1 | 0.0–0.3 | 1 | 0.2 | 0 | — | 0 | — |
| HPV-84 | 6 | 0.6 | 0.1–1.1 | 5 | 1.0 | 1 | 0.3 | 0 | — |
| (without HR-HPVb) | 3 | 0.3 | 0.0–0.6 | 2 | 0.4 | 1 | 0.3 | 0 | — |
| HPV-89 | 16 | 1.6 | 0.8–2.4 | 13 | 2.7 | 3 | 0.8 | 0 | — |
| (without HR-HPVb) | 5 | 0.5 | 0.1–0.9 | 3 | 0.6 | 2 | 0.5 | 0 | — |
- Due to cervical infections with multiple non-HR-HPV types the sum of infections with individual non-HR HPV types does not add up to the number of women with any non-HR HPV type cervical infection.
- HPV, human papillomavirus; N, number of women; P, prevalence; CI, confidence interval; Non-HR-HPV, non-high-risk HPV types; HR-HPV, high-risk HPV types.
- a Non-HR-HPV types: 6, 11, 26, 34sub, 40, 42, 44sub, 53, 54, 61, 62, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 82sub, 83, 84, 89.
- b HR-HPV types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 (Group 1 by the IARC Monograph Working Group).
Prevalence of infections with any of the 25 non-HR-HPV types and individual non-HR-HPV types regardless of and without the coexistence of any HR-HPV types according to cytology result is shown in Table II. Prevalence of cervical infection with any of the 25 non-HR-HPV types among women who were negative for intraepithelial lesion or malignancy was 8.8%, those with atypical squamous cells of undetermined significance 30.4%, with low-grade squamous intraepithelial lesions 60.0%, and with high-grade squamous intraepithelial lesions 7.7%. Non-HR-HPV infection without coexisting HR-HPV types was found in 4.0% of women who were negative for intraepithelial lesion or malignancy, 26.1% with atypical squamous cells of undetermined significance, 6.7% with low-grade squamous intraepithelial lesions, and none of those with high-grade squamous intraepithelial lesions. HPV-6 infections without coexisting HR-HPV were found in 0.2% of women who were negative for intraepithelial lesion or malignancy and none with atypical squamous cells of undetermined significance or worse. Exclusively HPV-6 or HPV-11 cervical infections were not found.
| HPV types | All women (N = 997) | Cytology result | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NILM (N = 944) | ASC-US (N = 23) | LSIL (N = 15) | HSIL (N = 13) | ||||||||
| N | P (%) | 95% CI | N | P (%) | N | P (%) | N | P (%) | N | P (%) | |
| Non-HR-HPVa | 100 | 10.0 | 8.2–11.9 | 83 | 8.8 | 7 | 30.4 | 9 | 60.0 | 1 | 7.7 |
| Non-HR-HPVa + HR-HPVb | 55 | 5.5 | 4.1–6.9 | 45 | 4.8 | 1 | 4.3 | 8 | 53.3 | 1 | 7.7 |
| Only non-HR-HPVa | 45 | 4.5 | 3.2–5.8 | 38 | 4.0 | 6 | 26.1 | 1 | 6.7 | 0 | — |
| Single non-HR-HPVa | 35 | 3.5 | 2.3–4.6 | 28 | 3.0 | 6 | 26.1 | 1 | 6.7 | 0 | — |
| Multiple non-HR-HPVa | 10 | 1.0 | 0.4–1.6 | 10 | 1.1 | 0 | — | 0 | — | 0 | — |
| Specific non-HR-HPVa | |||||||||||
| HPV-6 | 5 | 0.5 | 0.1–0.9 | 5 | 0.5 | 0 | — | 0 | — | 0 | — |
| (without HR-HPVb) | 2 | 0.2 | 0.0–0.5 | 2 | 0.2 | 0 | — | 0 | — | 0 | — |
| HPV-11 | 0 | — | — | 0 | — | 0 | — | 0 | — | 0 | — |
| HPV-26 | 0 | — | — | 0 | — | 0 | — | 0 | — | 0 | — |
| HPV-34sub | 8 | 0.8 | 0.2–1.3 | 6 | 0.6 | 1 | 4.3 | 1 | 6.7 | 0 | — |
| (without HR-HPVb) | 3 | 0.3 | 0.0–0.6 | 3 | 0.3 | 0 | — | 0 | — | 0 | — |
| HPV-40 | 2 | 0.2 | 0.0–0.5 | 2 | 0.2 | 0 | — | 0 | — | 0 | — |
| (without HR-HPVb) | 0 | — | — | 0 | — | 0 | — | 0 | — | 0 | — |
| HPV-42 | 16 | 1.6 | 0.8–2.4 | 10 | 1.1 | 1 | 4.3 | 4 | 26.7 | 1 | 7.7 |
| (without HR-HPVb) | 4 | 0.4 | 0.0–0.8 | 3 | 0.3 | 0 | — | 1 | 6.7 | 0 | — |
| HPV-44sub | 0 | — | — | 0 | — | 0 | — | 0 | — | 0 | — |
| HPV-53 | 20 | 2.0 | 1.1–2.9 | 16 | 1.7 | 2 | 8.7 | 2 | 13.3 | 0 | — |
| (without HR-HPVb) | 8 | 0.8 | 0.2–1.3 | 6 | 0.6 | 0 | — | 0 | — | 0 | — |
| HPV-54 | 17 | 1.7 | 0.9–2.5 | 15 | 1.6 | 0 | — | 2 | 13.3 | 0 | — |
| (without HR-HPVb) | 5 | 0.5 | 0.1–0.9 | 5 | 0.5 | 0 | — | 0 | — | 0 | — |
| HPV-61 | 12 | 1.2 | 0.5–1.9 | 11 | 1.2 | 1 | 4.3 | 0 | — | 0 | — |
| (without HR-HPVb) | 3 | 0.3 | 0.0–0.6 | 3 | 0.3 | 0 | — | 0 | — | 0 | — |
| HPV-62 | 13 | 1.3 | 0.6–2.0 | 10 | 1.1 | 1 | 4.3 | 2 | 13.3 | 0 | — |
| (without HR-HPVb) | 6 | 0.6 | 0.1–1.1 | 6 | 0.6 | 0 | — | 0 | — | 0 | — |
| HPV-64 | 0 | — | — | 0 | — | 0 | — | 0 | — | 0 | — |
| HPV-66 | 6 | 0.6 | 0.1–1.1 | 3 | 0.3 | 1 | 4.3 | 2 | 13.3 | 0 | — |
| (without HR-HPVb) | 3 | 0.3 | 0.0–0.6 | 2 | 0.2 | 1 | 4.3 | 0 | — | 0 | — |
| HPV-67 | 7 | 0.7 | 0.2–1.2 | 6 | 0.6 | 0 | — | 1 | 6.7 | 0 | — |
| (without HR-HPVb) | 5 | 0.5 | 0.1–0.9 | 5 | 0.5 | 0 | — | 0 | — | 0 | — |
| HPV-68 | 4 | 0.4 | 0.0–0.8 | 4 | 0.4 | 0 | — | 0 | — | 0 | — |
| (without HR-HPVb) | 2 | 0.2 | 0.0–0.5 | 2 | 0.2 | 0 | — | 0 | — | 0 | — |
| HPV-69 | 1 | 0.1 | 0.0–0.3 | 1 | 0.1 | 0 | — | 0 | — | 0 | — |
| (without HR-HPVb) | 1 | 0.1 | 0.0–0.3 | 1 | 0.1 | 0 | — | 0 | — | 0 | — |
| HPV-70 | 8 | 0.8 | 0.2–1.3 | 7 | 0.7 | 0 | — | 1 | 6.7 | 0 | — |
| (without HR-HPVb) | 4 | 0.4 | 0.0–0.8 | 4 | 0.4 | 0 | — | 0 | — | 0 | — |
| HPV-71 | 1 | 0.1 | 0.0–0.3 | 1 | 0.1 | 0 | — | 0 | — | 0 | — |
| (without HR-HPVb) | 1 | 0.1 | 0.0–0.3 | 1 | 0.1 | 0 | — | 0 | — | 0 | — |
| HPV-72 | 0 | — | — | 0 | — | 0 | — | 0 | — | 0 | — |
| HPV-73 | 5 | 0.5 | 0.1–0.9 | 2 | 0.2 | 1 | 4.3 | 2 | 13.3 | 0 | — |
| (without HR-HPVb) | 1 | 0.1 | 0.0–0.3 | 0 | — | 1 | 4.3 | 0 | — | 0 | — |
| HPV-81 | 1 | 0.1 | 0.0–0.3 | 1 | 0.1 | 0 | — | 0 | — | 0 | — |
| (without HR-HPVb) | 0 | — | — | 0 | — | 0 | — | 0 | — | 0 | — |
| HPV-82 | 3 | 0.3 | 0.0–0.6 | 3 | 0.3 | 0 | — | 0 | — | 0 | — |
| (without HR-HPVb) | 1 | 0.1 | 0.0–0.3 | 1 | 0.1 | 0 | — | 0 | — | 0 | — |
| HPV-82sub | 0 | — | — | 0 | — | 0 | — | 0 | — | 0 | — |
| HPV-83 | 4 | 0.4 | 0.0–0.8 | 3 | 0.3 | 1 | 4.3 | 0 | — | 0 | — |
| (without HR-HPVb) | 1 | 0.1 | 0.0–0.3 | 1 | 0.1 | 0 | — | 0 | — | 0 | — |
| HPV-84 | 6 | 0.6 | 0.1–1.1 | 4 | 0.4 | 1 | 4.3 | 1 | 6.7 | 0 | — |
| (without HR-HPVb) | 3 | 0.3 | 0.0–0.6 | 2 | 0.2 | 1 | 4.3 | 0 | — | 0 | — |
| HPV-89 | 16 | 1.6 | 0.8–2.4 | 14 | 1.5 | 1 | 4.3 | 1 | 6.7 | 0 | — |
| (without HR-HPVb) | 5 | 0.5 | 0.1–0.9 | 4 | 0.4 | 1 | 4.3 | 0 | — | 0 | — |
- Due to cervical infections with multiple non-HR HPV types the sum of infections with individual non-HR HPV types does not add up to the number of women with any non-HR HPV type cervical infection. Three women without cytology result were excluded from the analyses.
- HPV, human papillomavirus; N, number of women; NILM, negative for intraepithelial lesion and malignancy; ASC-US, atypical squamous cells of undetermined significance; LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions; P, prevalence; CI, confidence interval; non-HR-HPV, non-high-risk HPV types; HR-HPV, high-risk HPV types.
- None of the two women with atypical squamous cells—cannot exclude HSIL (ASC-H) was infected with any of the non-HR-HPV types.
- a Non-HR-HPV types: 6, 11, 26, 34sub, 40, 42, 44sub, 53, 54, 61, 62, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 82sub, 83, 84, 89.
- b HR-HPV types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 (Group 1 by the IARC Monograph Working Group).
Table III shows the mixed and single infections with any of the 12 HR-HPV and/or 25 non-HR-HPV in 32 cases of infections with any of the 37 HPV types examined among 53 women with a cytology result of either atypical squamous cells of undetermined significance or worse among 997 women in cervical cancer screening with available cytology results.
| Cytology result | ||||||||
|---|---|---|---|---|---|---|---|---|
| ASC-US or ASC-H | LSIL | HSIL | ||||||
| Case | HR-HPV | Non-HR-HPV | Case | HR-HPV | Non-HR-HPV | Case | HR-HPV | Non-HR-HPV |
| 1a | 16, 52 | 11 | 16, 51, 59 | 42, 53, 84, 89 | 21 | 16 | ||
| 2a | 31, 39, 56 | 12 | 16 | 34sub, 53 | 22 | 16 | ||
| 3a | 56, 59 | 34sub, 42, 61, 62, 83 | 13 | 18, 31, 39, 59 | 66, 73 | 23 | 16, 42 | |
| 4a | 53 | 14 | 31, 52 | 54 | 24 | 16, 45, 51 | ||
| 5a | 53 | 15 | 33, 45, 58 | 54, 67, 70, 73 | 25 | 18 | ||
| 6a | 66 | 16 | 39, 51 | 42, 62 | 26 | 31 | ||
| 7a | 73 | 17 | 42 | 27 | 33 | |||
| 8a | 84 | 18 | 51 | 42, 62 | 28 | 33 | ||
| 9a | 89 | 19 | 45 | 29 | 33 | |||
| 10b | 33 | 20 | 45, 59 | 66 | 30 | 45 | ||
| 31 | 56 | |||||||
| 32 | 58 | |||||||
- ASC-US, atypical squamous cells of undetermined significance; ASC-H, atypical squamous cells—cannot exclude HSIL; LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions; HR-HPV, high-risk HPV types; non-HR-HPV, non-high-risk HPV types.
- The significance of shade in Table III is to highlight the cases that were infected with exclusively non-HR-HPV types (cases 4–9, and case 17).
- a A case with ASC-US.
- b A case with ASC-H.
DISCUSSION
The results of this study complement published estimates of pre-vaccination prevalence of cervical infections with HR-HPV types among 20–64 years old Slovenian women in cervical cancer screening obtained in the Slovenian HPV prevalence survey [Učakar et al., 2012] with data on pre-vaccination prevalence of cervical infections with 25 non-HR-HPV types in a subsample of 1,000 women.
We should be cautious in comparing these results to other similar studies, as study periods, age range as well as median age of the tested population, study design (surveys of women participating in organized or opportunistic screening programs, randomized trials of HPV testing in primary screening setting, population based surveys), exclusion criteria, and also HPV testing methodology differ.
The prevalence estimate from this study of cervical infection with any of the 25 non-HR-HPV types among women who were negative for intraepithelial lesion or malignancy was higher (8.8%) in comparison to the prevalence estimate of 1.9% cervical infections with 21 non-HR-HPV types reported by IARC from a pooled analysis of 5,141 women 15–75 years old from the Netherlands, Italy, and Spain [Clifford et al., 2005b]. Among women who were negative for intraepithelial lesion or malignancy, the most common non-HR-HPV types in our study were HPV-53 (1.7%), HPV-54 (1.6%), and HPV-89 (1.5%). In comparison, the meta-analysis of 194 studies with HPV type-specific data that enrolled more than 200,000 women worldwide (including more than 100,000 from Europe), the most common non-HR-HPV types globally were HPV-53 (0.6%), HPV-6, and HPV-66 (both 0.5%), and in Europe HPV-66 (0.6%), HPV-6 (0.5%), and HPV-53 (0.3%) [Bruni et al., 2010]. Estimated global and European HPV-11 prevalence was 0.2% and 0.3%, respectively. In comparison, among women who were negative for intraepithelial lesion or malignancy, the estimated prevalence of HPV-6 in our study was 0.5%, while we found no cervical infections with HPV-11.
Among women with low-grade squamous intraepithelial lesions in our study, HPV-42 was the most common non-HR-HPV genotype (26.7%), followed by HPV-53, HPV-54, HPV-62, HPV-66, and HPV-73 (all 13.3%), while no infections with HPV-6 or HPV-11 were detected. In meta-analysis of 21 studies from several European countries among more than 4,000 women with low-grade squamous intraepithelial lesions, the prevalence of three non-HR-HPV types examined was 3.7% (HPV-53), 6.0% (HPV-66), and 6.8% (HPV-6) [Clifford et al., 2005a].
Among women with high-grade squamous intraepithelial lesions in our study, we found only one infection with non-HR-HPV type, HPV-42 (7.7%). In meta-analysis of 38 studies from several European countries among 3,494 women with high-grade squamous intraepithelial lesions, the two non-HR-HPV types (among seven examined) detected most commonly were HPV-73 (3.5%) and HPV-66 (1.5%), while the prevalence of HPV-6 was 1.1% and HPV-11 0.4% [Smith et al., 2007].
We reported before that the prevalence of cervical infection with HR-HPV types among Slovenian women in cervical cancer screening increased with the severity of the cervical disease [Učakar et al., 2012]. In contrast, the cervical infections with any of the 25 non-HR-HPV types were less prevalent in women with high-grade squamous intraepithelial lesions (7.7%) and more prevalent in those with low-grade squamous intraepithelial lesions (60.0%) and atypical squamous cells of undetermined significance (30.4%). These findings are consistent with the results from a similar population based study of 40,000 Danish women 23–65 years old, where cervical infections with any of the 12 non-HR-HPV types examined were less prevalent among women with high-grade squamous intraepithelial lesions (12.7%) and more prevalent with low-grade squamous intraepithelial lesions (19.6%) and atypical squamous cells of undetermined significance (33.1%) [Nielsen et al., 2012]. The Danish researchers reported that only cervical specimens positive for 13 HR-HPV types (including HPV-68 considered as non-HR-HPV type by us) and 5 LR-HPV types with hc2 were genotyped for 24 HPV types, including the following 12 non-HR-HPV types: HPV-6, HPV-11, HPV-40, HPV-42, HPV-43, HPV-44, HPV-53, HPV-54, HPV-66, HPV-68, HPV-70, and HPV-74 [Nielsen et al., 2012] and that they may have underestimated the prevalence of infections with 12 non-HR-HPV types examined. In contrast, we tested with Linear Array HPV Genotyping Test also 825 women selected into a random subsample of 1,000 women from the Slovenian HPV prevalence survey participants, who were RealTime/hc2 negative and have thus not underestimated the prevalence of infections with any of the 25 non-HR-HPV types examined.
In the current study, cervical infections with any of the 25 non-HR-HPV types without coexisting HR-HPV types were relatively common among women with atypical squamous cells of undetermined significance (26.1%), less common in those with low-grade squamous intraepithelial lesions (6.7%) and not detected at all in those with high-grade squamous intraepithelial lesions. This is also consistent with Danish study where the prevalence of cervical infections with any of the 12 non-HR-HPV types examined without coexisting HR-HPV types was 11.3% among women with atypical squamous cells of undetermined significance and 2.6% with low-grade squamous intraepithelial lesions, and almost no non-HR-HPV types were present without coexisting HR-HPV types with high-grade squamous intraepithelial lesions [Nielsen et al., 2012]. The results from this study are also in line with the population based study among 3,410 women 25–64 years old from Italy, where prevalence of cervical infections with any of the 5 non-HR-HPV types examined without coexisting HR-HPV types was 5.1% among women with atypical squamous cells of undetermined significance and 5.6% among those with low-grade squamous intraepithelial lesions, and null among those with high-grade squamous intraepithelial lesions [Giorgi Rossi et al., 2011].
Although probability sampling was not used in the Slovenian HPV prevalence survey, a relatively large number of women eligible for cervical cancer screening were enrolled consecutively into a convenience sample in 22 outpatient gynaecology services all over the country and the refusal rate was very low (1.9%). With respect to age and cervical pathology, the population from this study had fairly similar characteristics to the total population of women in the NCCSP in 2010 (results not shown). Thus, it is assumed that our estimates of cervical infection prevalence with any of the 25 non-HR-HPV types as well as individual non-HR-HPV types reflect fairly well the prevalence of cervical infection with 25 non-HR-HPV types among 20–64 years old Slovenian women in cervical cancer screening. With respect to probability of finding exclusive or coexisting infections with non-HR-HPV and HR-HPV, it should be noted, that since the average duration of cervical infections with non-HR-HPV is shorter than average duration of infections with HR-HPV, we would expect, with our cross-sectional study design, to detect a lower prevalence of infections with non-HR-HPV in comparison to HR-HPV, if their incidence was the same.
In conclusion, in the first study performed in Central Europe on 1,000 women attending national cervical cancer screening programme with fairly good coverage (in last 3 years more than 70% of targeted women had at least one smear) we established pre-vaccination prevalence and assessed importance of cervical infection with 25 non-HR-HPV types with limited evidence or no-evidence to be carcinogenic for humans. These infections without coexisting HR-HPV infections were common among Slovenian women in cervical cancer screening programme with atypical squamous cells of undetermined significance, while rare in those with low-grade squamous intraepithelial lesions or worse. These results provide baseline data for monitoring the impact of Slovenian HPV vaccination program, development of future cervical cancer screening strategies in this part of Europe and for further health economic and modeling studies.
ACKNOWLEDGMENTS
We thank all women who participated in the study; our colleagues gynaecologists: Petra Bavčar, Irena Begič, Lara Beseničar Pregelj, Martina Bučar, Simona Čopi, Petra Eržen Vrlič, Andreja Gornjec, Mojca Grebenc, Nina Jančar, Mojca Jemec, Jožefa Kežar, Tatjana Kodrič, Zdravka Koman, Jasna Kostanjšek, Jasna Kuhelj Recer, Zlatko Lazić, Sonja Lepoša, Mili Lomšek, Sladjana Malić, Petra Meglič, Maja Merkun, Aleksander Merlo, Anamarija Petek, Suzana Peternelj Marinšek, Igor Pirc, Uršula Reš Muravec, Filip Simoniti, Lucija Sorč, Tina Steinbacher Kokalj, Mateja Darija Strah, Vesna Šalamun, Ksenija Šelih Martinec, Eda Vrtačnik Bokal, and Andrej Zore for patient recruitment and management; Petra Markočič for administrative management of the study in the laboratory; Petra Čuk, Boštjan J. Kocjan, Robert Krošelj, Mateja Jelen, and Katja Seme for laboratory support; Jasna Šinkovec, Marja Lenart, and Boštjan Luzar for managing cytology and histology results, personnel of the National Cervical Cancer Screening Program from Institute of Oncology; Ljubljana for providing National Cervical Cancer Screnning Programme population data; and Miha Pirc for sample transportation. The study was funded with the resources of the National Institute of Public Health of Slovenia; the Institute of Microbiology and Immunology, Faculty of Medicine, University of Ljubljana; the Health Insurance Institute of Slovenia, and financial support from Abbott Molecular. Abbott Molecular was not involved in the study design, data collection, data analysis and interpretation, writing the manuscript, or decision to submit the article for publication.




