Prevalence and circulation patterns of Variant, Atypical and Resistant HIV in Los Angeles County (2007–2009)
Abstract
The prevalence of transmitted HIV drug resistance (TDR) in Los Angeles County remains unknown, due in part to the absence of reliable genotypic data. The specific objectives of this study are to estimate the prevalence of TDR, to describe the demographic characteristics associated with TDR and to investigate the distribution of HIV-1 subtypes among persons newly diagnosed with HIV in Los Angeles County. From 2007 through 2009, 1,414 sequences were obtained from 7,100 persons newly diagnosed with HIV through HIV resistance surveillance. Overall, 257 (18%) sequences had some genetic evidence of drug resistance. Of these, 122 (9%) exhibited evidence of resistance to non-nucleoside reverse transcriptase inhibitors, 121 (9%) to nucleoside reverse transcriptase inhibitors and 76 (5%) to protease inhibitors. Subtype B was dominant (97%), followed by subtypes C (1.2%), CRF01_AE (0.8%), CRF02_AG (0.4%), A (0.3%), and F (0.1%). With a TDR prevalence of 18%, Los Angeles County ranks high compared with other jurisdictions across the nation. The prevalence of TDR in recent (19%) and long-standing (17%) HIV cases were similar, thus providing additional support for the notion that TDR-associated mutations may persist well beyond the period of recent infection. HIV-1 CRF01_AE, observed historically in central Africa and Asia, was observed to be circulating among men who have sex with men and heterosexuals in Los Angeles County. These findings underscore the need for continued and expanded HIV resistance surveillance to inform healthcare providers, policy makers and at-risk populations of emerging trends in HIV drug resistance. J. Med. Virol. 86: 1639–1647, 2014. © 2014 Wiley Periodicals, Inc.
INTRODUCTION
HIV-1 transmitted drug resistance (TDR) occurs when individuals are infected initially with drug resistant virus, whereas acquired drug resistance occurs when resistance mutations are selected for by drug selective pressure in individuals receiving antiretroviral therapy. Since the introduction of highly active antiretroviral therapy (ART) for routine clinical care, TDR has been an emerging public health challenge [Boden et al., 1999; Pomerantz, 1999]. Antiretroviral therapy has been effective in slowing the progression of immunodeficiency in persons infected with HIV-1 [Palella et al., 1998]. However, the benefits of ART have been mitigated somewhat by the emergence of drug resistant strains that both limit first-line treatment options and decrease the effectiveness of subsequent ART regimens.
Understanding current drug resistance patterns through HIV resistance surveillance enables us to anticipate trends that may affect the ability to treat HIV cases with current ART drugs. It can also inform antiretroviral regimen choices, improve guidelines for drug resistance testing, and identify potential gaps in treatment and/or prevention strategies. Molecular epidemiology may also be employed to track HIV transmission patterns and identify emerging transmission trends. However, to date, estimates of TDR have not been reported in Los Angeles County and molecular epidemiology research has yet to contribute to local HIV prevention policy.
As the largest local jurisdiction in the United States, Los Angeles County is highly diverse, both geographically and demographically. Preliminary findings from Los Angeles County Division of HIV and STD Programs/HIV Epidemiology (DHSP/HE) reported a total of 45,474 persons living with HIV/AIDS in Los Angeles County as of December 31, 2012 [http://publichealth.lacounty.gov/wwwfiles/ph/hae/hiv/2012AnnualHIVSurveillanceReport.pdf]. As a result of the US Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents, increasing numbers of newly diagnosed cases in Los Angeles County are receiving HIV genotype testing as part of the initial comprehensive patient assessment [http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf].
Since May 2006, the Los Angeles County DHSP has been working in collaboration with the Centers for Disease Control and Prevention (CDC) to incorporate Variant, Atypical and Resistant HIV Surveillance into routine HIV surveillance activities. Through HIV resistance surveillance, we are able to estimate the prevalence of transmitted HIV drug resistance and investigate the distribution of non-B subtypes and circulating recombinant forms (CRFs) among persons newly diagnosed with HIV in Los Angeles County.
MATERIALS AND METHODS
Variant Atypical and Resistant HIV Surveillance in Los Angeles County
Variant Atypical and Resistant HIV Surveillance collected genetic sequence data from the pol region (Protease and Reverse Transcriptase) of HIV-1 from newly diagnosed persons. During the course of this activity, the efforts changed from active to passive surveillance due to insufficient remnant serum and resources to support HIV genotyping. Through active surveillance, remnant diagnostic sera were obtained from 17 collaborating laboratories that account for approximately one-third of the cases reported to Los Angeles County's enhanced HIV/AIDS Reporting System (eHARS) annually. Collected sera were sequenced at the Stanford University Clinical Virology Laboratory in Palo Alto, California. Through passive surveillance, electronic HIV-1 genetic sequence data were obtained from four collaborating laboratories that perform HIV genotyping—namely, Advance Medical Analysis LLC, Laboratory Corporation of America, ARUP Laboratory and Los Angeles County Public Health Laboratory.
The study patients consisted of Los Angeles County HIV cases reported as new diagnoses during 2007–2009, who were determined to be antiretroviral naïve and for whom HIV genetic sequence data obtained from a blood specimen drawn within 90 days of HIV diagnosis was available.
Cases with an HIV-1 subtype B, E, and D Capture Enzyme Immunoassay (EIA) result, indicating recent infection, and no laboratory or medical records signifying an AIDS diagnoses within 6 months of their HIV diagnoses, were classified as “recent” infections. Cases with an EIA result indicating long-standing infection, or cases diagnosed with AIDS less than 6 months after their HIV diagnosis, were classified as long-standing.
HIV-1 Subtypes and Mutation List
CDC methods were used for subtyping HIV-1 sequences [Pieniazek, 2011]. First, sequences were analyzed using Sierra in Perl Language, a Stanford HIV Web Service developed by the Stanford Online HIV Drug Resistance Database [Liu and Shafer, 2006]. Sequences were classified as subtype B and potential non-B variants. Assignment to subtype B was based on ≥90% similarity to subtype B standard sequences in protease and reverse transcriptase (RT). Subsequently, we used phylogenetic methods to confirm subtype classification for non-B subtypes and recombinant assignments. For the phylogenetic reconstruction, sequences were aligned using the BioEdit program [Hall, 1999] with reference sequences of Group M (including CRFs) taken from the Los Alamos Laboratory HIV database [http://www.hiv.lanl.gov/]. A neighbor-joining tree using Kimura's-2 parameter method and 1,000 bootstrap replications was constructed using the Molecular Evolutionary Genetics Analysis program [Tamura et al., 2011]. The robustness of the nodes was estimated with a bootstrap value of 80% to indicate significance.
To identify drug resistance associated mutations in Los Angeles County, we adopted a mutation list developed by World Health Organization and modified by CDC [Bennett et al., 2009; Wheeler et al., 2010]. Since subtype B is predominant in the US, the CDC mutation list includes additional mutations that are nonpolymorphic in subtype B to maximize sensitivity. Some drug resistance associated mutations were excluded from consideration in the analysis of specific subtypes, as they are likely to represent naturally occurring polymorphisms in drug-naïve HIV-infected individuals [Wheeler et al., 2010].
Statistical Analysis
Chi-square and Fisher's exact tests were used to compare study patients with all reported newly diagnosed cases on demographics and other characteristics. We also compared TDR cases with non-TDR cases. Multivariate Log-binomial regression was used to estimate prevalence ratios (PR) and 95% confidence intervals (CI). The following variables were considered: age at diagnosis, sex, race/ethnicity, HIV transmission mode, and recency of HIV-1 infection. All P-values were two-sided and the significance level set at 0.05. All statistical calculations were performed using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
Study Patients
Of the 7,100 newly diagnosed HIV persons reported to eHARS in Los Angeles County from 2007 through 2009, 1,414 (20%) eligible sequences were obtained. Of these sequences, 493 (35%) were collected by genotyping remnant sera and 921 (65%) were obtained directly from collaborating laboratories.
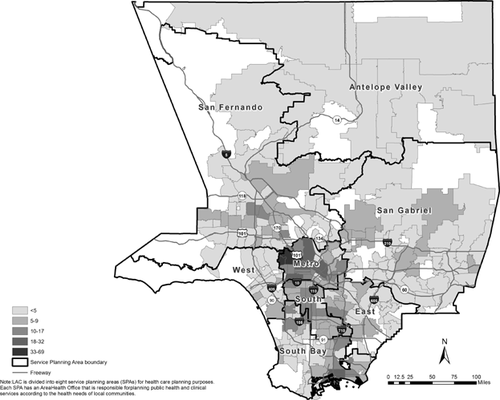
As shown in Table I, 91% of the study patients were male, 72% were below the age of 40, 48% were Hispanic/Latino and 70% were men who have sex with men. Thirty percent were classified as recent infections and approximately 44% were diagnosed at an HIV clinic. Figure 1 displays the study patients by ZIP code and delineated by Service Planning Areas (see definition in the footnotes). Over one-third of the study patients resided in the Metro Service Planning Area at the time of their HIV/AIDS diagnosis. Study patients differed from the overall population of newly diagnosed HIV cases in Los Angeles County by age at HIV diagnosis, race, gender, mode of transmission, HIV/AIDS diagnosis facility, and country of origin.
| Characteristics | Total HIV diagnoses | Study patients | P | TDR | PR (95% CI)a | Pa |
|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||||
| 7,100 | 1,414 | 257 | ||||
| Sex | ||||||
| Male | 6,238 (87.9) | 1,282 (90.7) | <0.01 | 239 (93.0) | Ref. | |
| Female | 862 (12.1) | 132 (9.3) | 18 (7.0) | 0.73 (0.47, 1.14) | 0.17 | |
| Age at diagnosis (years) | ||||||
| <20 | 208 (2.9) | 29 (2.1) | <0.01 | 5 (1.9) | 1.11 (0.58, 2.11) | 0.76 |
| 20–29 | 2,079 (29.3) | 513 (36.3) | 85 (33.1) | 0.83 (0.54, 1.27) | 0.38 | |
| 30–39 | 2,131 (30.0) | 475 (33.6) | 96 (37.4) | 1.04 (0.69, 1.57) | 0.86 | |
| 40–49 | 1,763 (24.8) | 285 (20.2) | 49 (19.1) | 0.88 (0.56, 1.39) | 0.59 | |
| ≥50 | 919 (12.9) | 112 (7.9) | 22 (8.6) | Ref. | ||
| Race/ethnicity | ||||||
| Black/African American | 1,725 (24.3) | 278 (19.7) | <0.01 | 52 (20.2) | 1.06 (0.76, 1.47) | 0.73 |
| Hispanic/Latino | 3,092 (43.5) | 684 (48.4) | 122 (47.5) | 1.01 (0.77, 1.33) | 0.93 | |
| White | 1,882 (26.5) | 369 (26.1) | 65 (25.3) | Ref. | ||
| Other | 401 (5.6) | 83 (5.9) | 18 (7.0) | 1.23 (0.77, 1.96) | 0.38 | |
| Transmission category: male | ||||||
| MSM | 4,430 (62.4) | 1,009 (71.4) | <0.01 | 189 (73.5) | — | |
| IDU | 99 (1.4) | 12 (0.8) | 2 (0.8) | |||
| MSM/IDU | 190 (2.7) | 34 (2.4) | 3 (1.2) | |||
| High-risk heterosexual contact | 65 (0.9) | 14 (1.0) | 1 (0.4) | |||
| No identified risk | 1,449 (20.4) | 213 (15.1) | 44 (17.1) | |||
| Transmission category: female | ||||||
| IDU | 61 (0.9) | 11 (0.8) | 0.93 | 1 (0.4) | — | |
| High-risk heterosexual contact | 214 (3.0) | 33 (2.3) | 2 (0.8) | |||
| No identified risk | 592 (8.3) | 88 (6.2) | 15 (5.8) | |||
| HIV/AIDS diagnosis facility | ||||||
| Outpatient/private physician's office | 2,180 (30.7) | 266 (18.8) | <0.01 | 52 (20.2) | 1.22 (0.82, 1.81) | 0.32 |
| SDR: HIV counseling & testing site/STD clinic | 775 (10.9) | 212 (15.0) | 34 (13.2) | Ref. | ||
| Inpatient/hospital | 972 (13.7) | 225 (15.9) | 36 (14.0) | 1.00 (0.65,1.53) | 0.99 | |
| Outpatient/adult HIV specialty clinicb | 2,334 (32.9) | 625 (44.2) | 119 (46.3) | 1.19 (0.84,1.68) | 0.34 | |
| Other/missing | 839 (11.8) | 86 (6.1) | 16 (6.2) | 1.16 (0.68,1.99) | 0.59 | |
| Country of birth | ||||||
| United States | 3,296 (46.4) | 706 (49.9) | <0.01 | 140 (54.5) | Ref. | |
| Foreign | 1,822 (25.7) | 458 (32.4) | 74 (28.8) | 1.05 (1.00, 1.10) | 0.10 | |
| Unknown | 1,982 (27.9) | 250 (17.7) | 43 (16.7) | 1.03 (0.97, 1.10) | 0.35 | |
| Service planning areas at diagnosisc | ||||||
| Antelope | 116 (1.6) | 13 (0.9) | 0.08 | 3 (1.2) | — | |
| San Fernando | 963 (13.6) | 172 (12.2) | 35 (13.6) | |||
| San Gabriel | 553 (7.8) | 122 (8.6) | 25 (9.7) | |||
| Metro | 2,562 (36.1) | 529 (37.4) | 86 (33.5) | |||
| West | 309 (4.4) | 62 (4.4) | 15 (5.8) | |||
| South | 918 (12.9) | 183 (12.9) | 31 (12.1) | |||
| East | 489 (6.9) | 91 (6.4) | 14 (5.4) | |||
| South Bay | 1,009 (14.2) | 219 (15.5) | 43 (16.7) | |||
| Recency of HIV infection (N = 1,171) | ||||||
| Recent infections, ≤6 months | 290 (24.8) | — | 55 (27.1) | 1.13 (0.85, 1.49) | 0.40 | |
| Long-standing | 881 (75.2) | 148 (72.9) | Ref. | |||
- Abbreviations: MSM, men who have sex with men; IDU, injection drug user; PR, prevalence ratio; CI, confidence interval; Ref., reference group; HIV, human immunodeficiency virus; STD, sexually transmitted disease; SDR, screening, diagnostic, referral agency.
- a PRs and P-values are not calculated for “transmission category: male,” “transmission category: female,” and “SPA of residence.”
- b This group also includes outpatient from other outpatient clinics.
- c LAC is divided into eight service planning areas (SPAs) for health care planning purposes. Each SPA has an Area Health Office that is responsible for planning public health and clinical services according to the health needs of local communities.
HIV-1 Drug Resistance
As shown in Table II, evidence of TDR was found in 257 (18%) patients. No association was observed between TDR and sex, race, age, mode of transmission or recency of infection. One hundred and twenty two patients (9%) exhibited evidence of resistance to non-nucleoside reverse transcriptase inhibitors (NNRTIs), 121 (9%) to nucleoside reverse transcriptase inhibitors (NRTIs), and 76 (5%) to protease inhibitors (PIs). Forty-seven percent of patients with evidence of resistance to PIs had only one mutation, while 50% of patients with evidence of resistance to NRTIs and 66% of patients with evidence of resistance to NNRTIs had only one mutation. The mean number of drug resistance associated mutations to any drug class was 1.5 (range, 1–10). Two hundred and seven cases (15%) had drug resistance associated mutations to one drug class, 38 (3%) to two drug classes, and 12(<1%) to all three drug classes. Among these with dual drug class resistance, 13 patients had evidence of resistance to both NNRTIs and NRTIs, 9 to both PIs and NNRTIs, and 16 to both PIs and NRTIs.
| Study patients | TDR | 1-class TDR | 2-class TDR | 3-class TDR | PI TDR | NRTI TDR | NNRTI TDR | |
|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |||
| 1,414 | 257 (18.2) | 207 (14.6) | 38 (2.7) | 12 (0.8) | 76 (5.4) | 121 (8.6) | 122 (8.6) | |
| Sex | ||||||||
| Male | 1,282 | 239 (18.6) | 191 (14.9) | 36 (2.8) | 12 (0.9) | 72 (5.6) | 112 (8.7) | 115 (9.0) |
| Female | 132 | 18 (13.6) | 16 (12.1) | 2 (1.5) | 0 | 4 (3.0) | 9 (6.8) | 7 (5.3) |
| Age at diagnosis (years) | ||||||||
| <20 | 29 | 5 (17.2) | 5 (17.2) | 0 | 0 | 1 (3.4) | 0 | 4 (13.8) |
| 20–29 | 513 | 85 (16.6) | 76 (14.8) | 9 (1.8) | 0 | 22 (4.3) | 33 (6.4) | 39 (7.6) |
| 30–39 | 475 | 96 (20.2) | 74 (15.6) | 18 (3.8) | 4 (0.8) | 28 (5.9) | 50 (10.5) | 44 (9.3) |
| 40–49 | 285 | 49 (17.2) | 38 (13.3) | 5 (1.8) | 6 (2.1) | 16 (5.6) | 26 (9.1) | 24 (8.4) |
| ≥50 | 112 | 22 (19.6) | 14 (12.5) | 6 (5.4) | 2 (1.8) | 9 (8.0) | 12 (10.7) | 11 (9.8) |
| Race/ethnicity | ||||||||
| Black/African American | 278 | 52 (18.7) | 46 (16.5) | 4 (1.4) | 2 (0.7) | 6 (2.2) | 26 (9.4) | 28 (10.1) |
| Hispanic/Latino | 684 | 122 (17.8) | 97 (14.2) | 21 (3.1) | 4 (0.6) | 49 (7.2) | 56 (8.2) | 46 (6.7) |
| White | 369 | 65 (17.6) | 49 (13.3) | 11 (3.0) | 5 (1.4) | 18 (4.9) | 31 (8.4) | 37 (10.0) |
| Other | 83 | 18 (21.7) | 15 (18.1) | 2 (2.4) | 1 (1.2) | 3 (3.6) | 8 (9.6) | 8 (9.6) |
| Transmission category: male | ||||||||
| MSM | 1,009 | 189 (18.7) | 154 (15.3) | 27 (2.7) | 8 (0.8) | 55 (5.5) | 89 (8.8) | 88 (8.7) |
| IDU | 12 | 2 (16.7) | 1 (8.3) | 1 (8.3) | 0 | 1 (8.3) | 1 (8.3) | 1 (8.3) |
| MSM/IDU | 34 | 3 (8.8) | 3 (8.8) | 0 | 0 | 1 (2.9) | 1 (2.9) | 1 (2.9) |
| High-risk heterosexual contact | 14 | 1 (7.1) | 0 | 1 (7.1) | 0 | 1 (7.1) | 1 (7.1) | 0 |
| No identified risk | 213 | 44 (20.7) | 33 (15.5) | 7 (3.3) | 4 (1.9) | 14 (6.6) | 20 (9.4) | 25 (11.7) |
| Transmission category: female | ||||||||
| IDU | 11 | 1 (9.1) | 0 | 1 (9.1) | 0 | 1 (9.1) | 1 (9.1) | 0 |
| High-risk heterosexual contact | 33 | 2 (6.1) | 2 (6.1) | 0 | 0 | 0 | 1 (3.0) | 1 (3.0) |
| No identified risk | 88 | 15 (17.0) | 14 (15.9) | 1 (1.1) | 0 | 3 (3.4) | 7 (8.0) | 6 (6.8) |
| HIV/AIDS diagnosis facility | ||||||||
| Outpatient/private physician's office | 266 | 52 (19.5) | 41 (15.4) | 10 (3.8) | 1 (0.4) | 17 (6.4) | 27 (10.2) | 20 (7.5) |
| SDR: HIV counseling & testing/STD clinic | 212 | 34 (16.0) | 29 (13.7) | 2 (0.9) | 3 (1.4) | 8 (3.8) | 14 (6.6) | 20 (9.4) |
| Inpatient/hospital | 225 | 36 (16.0) | 28 (12.4) | 5 (2.2) | 3 (1.3) | 12 (5.3) | 20 (8.9) | 15 (6.7) |
| Outpatient/adult HIV specialty clinica | 625 | 119 (19.0) | 97 (15.5) | 17 (2.7) | 5 (0.8) | 33 (5.3) | 55 (8.8) | 58 (9.3) |
| Other/missing | 86 | 16 (18.6) | 12 (14.0) | 4 (4.7) | 0 | 6 (7.0) | 5 (5.8) | 9 (10.5) |
| Country of birth | ||||||||
| United States | 706 | 140 (19.8) | 108 (15.3) | 23 (3.3) | 9 (1.3) | 42 (5.9) | 69 (9.8) | 70 (9.9) |
| Foreign | 458 | 74 (16.2) | 63 (13.8) | 9 (2.0) | 2 (0.4) | 21 (4.6) | 34 (7.4) | 32 (7.0) |
| Unknown | 250 | 43 (17.2) | 36 (14.4) | 6 (2.4) | 1 (0.4) | 13 (5.2) | 18 (7.2) | 20 (8.0) |
| Recency of HIV infection (N = 1,171) | ||||||||
| Recent infections, ≤6 months | 290 | 55 (19.0) | 43 (14.8) | 10 (3.4) | 2 (0.7) | 17 (5.9) | 27 (9.3) | 25 (8.6) |
| Long-standing | 881 | 148 (16.8) | 124 (14.1) | 17 (1.9) | 7 (0.8) | 37 (4.2) | 76 (8.6) | 66 (7.5) |
- Abbreviations: MSM, men who have sex with men; IDU, injection drug user; PR, prevalence ratio; CI, confidence interval; Ref., reference group; HIV, human immunodeficiency virus; STD, sexually transmitted disease; SDR, screening, diagnostic, referral agency; TDR, transmitted drug resistance; PI, protease inhibitors class; NRTI, nucleoside reverse transcriptase inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitors.
- a This group also includes outpatient from other outpatient clinics.
The most common mutation was K103N (84/122, 69%; in Table III), which is associated with high-level resistance to first-line NNRTIs-Efavirenz and Nevirapine. The second most common drug resistance associated mutation, was L90M (36/76, 47%; in Table III), which confers resistance to Nelfinavir and Saquinavir/Ritonavir. M41L (28/121, 23%; in Table III) was the most prevalent NRTI mutation. The M41L mutation is known to be selected by thymidine analogs and confers reduced susceptibility to all approved NRTIs.
| Protease inhibitor class (PI, N = 76) | Nucleoside reverse transcriptase inhibitor class (NRTI, N = 121) | Non-nucleoside reverse transcriptase inhibitor class (NNRTI, N = 122) | ||||||
|---|---|---|---|---|---|---|---|---|
| Amino acid | Mutationb | N (%) | Amino acid | Mutationb | N (%) | Amino acid | Mutationb | N (%) |
| V11 | IAE AG G | 2 (2.6) | M41 | L | 28 (23.1) | L100 | I | 1 (0.8) |
| L24 | I | 2 (2.6) | E44 | A | 1 (0.8) | K101 | E | 4 (3.3) |
| D30 | N | 6 (7.9) | DD F | 7 (5.8) | P | 1 (0.8) | ||
| M46 | I | 12 (15.8) | A62 | VA | 3 (2.5) | K103 | N | 84(68.9) |
| L | 12 (15.8) | K65 | R | 1 (0.8) | S | 8 (6.6) | ||
| I50 | L | 1 (1.3) | D67 | E | 1 (0.8) | V106 | A | 3 (2.5) |
| V | 1 (1.3) | N | 17 (14) | M | 1 (0.8) | |||
| F53 | L | 1 (1.3) | T69 | AD F | 3 (2.5) | Y181 | C | 10 (8.2) |
| I54 | L | 1 (1.3) | D | 4 (3.3) | Y188 | L | 2 (1.6) | |
| M | 3 (3.9) | N | 23 (19) | G190 | A | 17 (13.9) | ||
| V | 5 (6.6) | i | 1 (0.8) | E | 1 (0.8) | |||
| Q58 | ED | 7 (9.2) | K70 | E | 1 (0.8) | S | 1 (0.8) | |
| G73 | S | 3 (3.9) | R | 5 (4.1) | P225 | H | 8 (6.6) | |
| T | 1 (1.3) | L74 | I | 2 (1.7) | ||||
| T74 | P | 1 (1.3) | V75 | A | 3 (2.5) | |||
| SA AE AG C F G | 6 (7.9) | ID | 4 (3.3) | |||||
| L76 | V | 2 (2.6) | M | 3 (2.5) | ||||
| V82 | A | 5 (6.6) | Y115 | F | 1 (0.8) | |||
| T | 1 (1.3) | M184 | I | 1 (0.8) | ||||
| I84 | V | 2 (2.6) | V | 13 (10.7) | ||||
| I85 | V | 4 (5.3) | L210 | W | 9 (7.4) | |||
| N88 | D | 6 (7.9) | T215 | C | 6 (5) | |||
| L90 | M | 36 (47.4) | D | 7 (5.8) | ||||
| E | 6 (5) | |||||||
| F | 1 (0.8) | |||||||
| I | 2 (1.7) | |||||||
| S | 24 (19.8) | |||||||
| V | 4 (3.3) | |||||||
| Y | 3 (2.5) | |||||||
| K219 | E | 2 (1.7) | ||||||
| Q | 15 (12.4) | |||||||
| R | 2 (1.7) | |||||||
- a Limited to cases with sequences that can be assessed using CDC mutation list.
- b Some mutations excluded from CDC mutation list for specific subtypes (in superscript) due to possible naturally-occurring polymorphisms among HIV-infected persons.
Among cases classified as recent HIV infections, 19% (55/290) exhibited evidence of TDR. NRTI drug resistance was present in 9.3% of recent HIV infections, followed by NNRTI (8.6%), and PI (5.9%). Ten (3.4%) individuals had two drug class associated mutations, and two (0.7%) individuals had three drug class associated mutations. Among cases classified as long-standing HIV infections, 16.8% (148/881) exhibited evidence of TDR. NRTI drug resistance was present in 8.6% of long-standing HIV infections, followed by NNRTI (7.5%), and PI (4.2%). Seventeen (1.9%) individuals had two drug class associated mutations, and 7 (0.9%) individuals had three drug class associated mutations. There was no significant difference in the prevalence of TDR in recent and long-standing infection.
Non-B Subtype and CRFs
While most study patients (97.2%) had HIV-1 subtype B, other subtypes and circulating recombinant strains of HIV-1 were observed. In decreasing order of prevalence, these included: subtypes C (1.2%), CRF01_AE (0.8%), CRF02_AG (0.4%), A (0.3%), and F (0.1%). Thirty eight percent of patients with non-B subtype were Black/African American followed by Hispanic/Latino (24%), White (19%), Asian (17%), and Native Hawaiian/Pacific Islander (2%). Forty-six percent of these patients identified as men who have sex with men and 60% were foreign-born. Furthermore, among Black/African American, Hispanic/Latino, and White cases with non-B subtypes, 62%, 60%, and 50%, respectively, were foreign-born.
Phylogenetic analysis of study patients with non-B subtypes revealed CRF01_AE circulating among men who have sex with men and heterosexuals in Los Angeles County. As shown in Figure 2, four of the five patients were men who reported having sex with men and one was a heterosexual female. Of the four men, two were Hispanic/Latino, one was Asian and another was a Pacific Islander. Three of the five patients were foreign-born and two were US-born.

DISCUSSION
Estimated Prevalence of Transmitted HIV Drug Resistance in Los Angeles County
Compared with the national estimates of TDR prevalence, which range from 12% to 18% [Wheeler et al., 2010], the observed prevalence of TDR in Los Angeles County in the years 2007, 2008, and 2009 (17.1%, 18.4%, and 17.4%, respectively) ranks high. This is the first population-based study to estimate the prevalence of HIV-1 drug resistance associated mutations in newly diagnosed HIV cases in Los Angeles County. By focusing on newly diagnosed persons for whom HIV genotyping was performed on blood specimens obtained within 90 days of HIV diagnosis, this study attempts to obtain a prevalence estimate that approximates TDR.
It is noteworthy that this dataset of newly diagnosed cases was largely (70%) comprised of long-standing HIV cases. Compared with recent infections, newly diagnosed long-standing HIV cases may not provide as accurate a reflection of TDR because over time in the absence of ARVs, the tendency is for resistant HIV to revert to the wild-type [Pingen et al., 2011]. This tendency to revert to the wild-type is the reason some medical providers have argued that there is little benefit in conducting resistance testing in persons for whom there is no evidence of recent HIV infection. In this study sample, however, the prevalence of TDR in recent infections and long-standing infections was not significantly different, which may indicate that TDR-associated mutations persist well beyond the period of recent infection. Other studies have suggested that TDR-associated mutations may persist for a longer period than was previously believed [Barbour et al., 2004; Pao et al., 2004]. Furthermore, certain RT mutations such as M41L, K103N, T69N, and T215 and protease mutations, such as L90M have been observed to show little reversion to wild-type over time [Pao et al., 2004; Bezemer et al., 2006]. The persistence of these resistance associated mutations underscores the utility of performing baseline resistance testing on patients even when there is no evidence of recent infection, as such testing can provide resistance information that may be useful in guiding future ARV treatment choices.
Demographic Correlates of TDR
Similar to other studies, this study did not find any associations between drug resistance mutations and basic demographics [Booth and Geretti, 2007; Palma et al., 2007; Yerly et al., 2007; Wheeler et al., 2010]. By contrast, Youmans reported a negative association between age at HIV and total number of mutations among newly diagnosed treatment-naïve individuals [Youmans et al., 2011]. Readhead et al. found a significant association between TDR and risk group and year of diagnosis [Readhead et al., 2012].
Although HIV-1 subtype B was dominant, 3.1% of study patients were infected with non-B subtypes, indicating the need for continued monitoring of HIV genetic diversity throughout the US to track the transmission and prevalence of non-B subtypes. Compared with other racial/ethnic groups, African-Americans, primarily African immigrants, comprised the largest proportion of non-B subtype HIV-1 infections. The most prevalent non-B subtype was subtype C, which is the main genetic form in southern and eastern Africa. There are, in the literature, examples of subtypes initially occurring solely in immigrants that overtime have been found to represent a major proportion of cases in the non-immigrant population. In France Couturier et al. [2000] reported that, from 1996 to 1998, 15.5% of newly diagnosed HIV cases were non-B subtypes compared with 47.7% from 2003 to 2005. Semaille et al. [2007] also reported evidence of transmission of non-B subtypes within non-immigrants living in France. Furthermore, Hughes et al. [2009] reported that in the United Kingdom, increasing immigration from Southern and Eastern Africa heralded a shift from the observed majority subtype B during the mid-1990s to subtype C (35%) and subtype A (15%) in 2000.
Distribution of CRFs
This phylogenetic analysis indicates that the HIV-1 CRF01_AE virus is currently circulating among Los Angeles County men who have sex with men [Hemelaar et al., 2011]. CRF01_AE is the predominant CRF in Southeast Asian countries. It originated in central Africa in the 1970s and was first identified in Thailand in 1989. It later spread to neighboring countries [McCutchan et al., 1992]. To our knowledge, there has been no published report on the circulation of CRF01_AE strains among Los Angeles County men who have sex with men.
In a recent study in Maryland, the presence of non-B subtype infections in US-born individuals and subtype B infections in African immigrants was shown to be an early indicator of crossover between two initially distinct epidemics [Carr et al., 2010]. Similarly, the circulation of CRF01_AE virus in Los Angeles County may be evidence of the interconnected sexual networks of foreign-born and US-born patients as well as homosexual and heterosexual patients. This circulation pattern may be indicative of crossover of non-B HIV subtypes into US-born populations from immigrants. As has previously been argued in the literature, the impact of increased HIV non-B subtypes infections among US-born individuals could have important implications for antiretroviral treatment and vaccine development, since varying subtypes may influence pathogenicity, antiretroviral drugs susceptibility, and development of resistance [Carr et al., 2010].
Limitations
This study is not without limitations. First, study results may not be generalizable as the sample differs from the overall population of all newly diagnosed cases in Los Angeles County on several influential demographic characteristics. Second, use of antiretrovirals by study participants may have been misclassified due to incomplete or inaccurate reporting of previous antiretroviral drug use. Third, current HIV resistance testing methods may not be sensitive enough to detect low frequency mutations. Finally, the HIV-1 subtypes B, E, and D Capture EIA test is best utilized, as a tool for population-based HIV incidence estimation rather than to determine whether any given case is a recent infection [http://www.cdc.gov/hiv/topics/surveillance/resources/factsheets/pdf/bed.pdf].
CONCLUSION
This is the first population-based study that seeks to analyze HIV genetic sequence data in Los Angeles County. It is estimated that 18% of newly diagnosed HIV cases in Los Angeles County contracted drug resistant HIV at infection. This prevalence (18%) ranks high among TDR prevalence estimates (12–18%) reported across the nation and may be indicative of failures in HIV prevention among HIV-infected persons and less than optimal adherence to ARVs. These results provide additional support for the notion that TDR-associated mutations may persist well beyond the period of recent infection.
Furthermore, TDR is not associated with demographics—such as race/ethnicity, age—or behavioral risk group. While HIV subtype B remains dominant among newly diagnosed cases, HIV-1 CRF01_AE, historically observed in central Africa and Asia, is currently circulating among men who have sex with men in Los Angeles County. Together, these findings underscore the need for continued and expanded HIV resistance surveillance to inform healthcare providers, policy makers and at risk populations of emerging trends in HIV drug resistance. In particular, these findings highlight the importance of performing baseline HIV genotype testing as recommended in the US Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents.
ACKNOWLEDGMENTS
The authors would like to thank Centers for Disease Control and Prevention (CDC) for providing data analysis assistance and the mutation list.




