Prevalence of human papillomaviruses in patients with head and neck squamous cell carcinoma in Lithuania and Belarus
Abstract
Overall, head and neck sqamous cell carcinoma accounts for more than 550,000 cases annually worldwide. It is well known that human papillomavirus (HPV) is the main risk factor for cervical cancer development. As the incidence and the mortality of cervical cancer are closely related to the HPV prevalence, we hypothesized that there is the same association between HPV prevalence and head and neck squamous cell carcinoma. Therefore we performed the study aiming to compare the level of HPV infection and HPV type distribution between two groups of Lithuanian and Belarusian patients with head and neck sqamous cell carcinoma. One hundred ninety head and neck sqamous cell carcinoma patients were included in the study, 75 from Lithuania and 115 from Belarus. PCR was used for HPV detection and typing. The distribution of HPV infection among head and neck sqamous cell carcinoma patients was similar in the Lithuanian (20.0%) and Belarusian (18.3%) patient groups, however differences were found in the distribution of HPV types. J. Med. Virol. 86:531–535, 2014. © 2013 Wiley Periodicals, Inc.
INTRODUCTION
Overall, head and neck cancer accounts for more than 550,000 cases annually worldwide [Parkin et al., 2005]. Males are affected significantly more than females with a ratio ranging from 2:1 to 4:1. In males the incidence rate exceeds 20 per 100,000 in regions of France, Hong Kong, the Indian subcontinent, Central and Eastern Europe, Spain, Italy, Brazil, and among African Americans in the Unites States. Mouth and tongue cancers are more common in the Indian subcontinent, nasopharyngeal cancer is more common in Hong Kong, and pharyngeal and/or laryngeal cancers are more common in other populations [Sankaranarayanan et al., 1998].
It is well known that human papillomavirus (HPV) is the main risk factor for cervical cancer development [Munoz et al., 1993; IARC Monographs 1995]. HPV belongs to the Papova family and Papovaviridae genera. The majority of HPV belongs to the genus Alpha papillomavirus. HPV 16 and 18 are the general oncogenic types in cervical carcinogenesis. However, some data show HPV association with other epithelial cancers—laryngeal, head and neck, anal, skin, etc. Despite the fact that the main risk factors for head and neck squamous cell carcinoma are alcohol and tobacco consumption (IARC Monographs 1988 and 2004), some tumors occur in persons who were not subject to these known risk factors. Evidence from clinical, population-based, and molecular studies has shown that HPV could also be an important risk factor for a subset of head and neck sqamous cell carcinoma [Braakhuis et al., 2009; O'Rorke et al., 2012]. However, the prevalence of HPV differs in various countries and regions. These variations in type and distribution of HPV could influence the different incidences and mortality rates of HPV related cancers. The aim of this study was to compare HPV infection level and HPV type distribution among two groups of Lithuanian and Belarusian patients with head and neck sqamous cell carcinoma.
MATERIALS AND METHODS
Study Patients
The study was performed during the period from March 2011 to October 2012. A total of 190 head and neck sqamous cell carcinoma patients were included: 75 patients from the Institute of Oncology, Vilnius University, Lithuania and 115 from N.N. Aleksandrov National Cancer Center, Belarus.
The study was supported by Lithuanian and Belarusian Scientific Councils according to the Bilateral Cooperation in Science and Technology Program for 2009–2013 (in Lithuania no. TAP-19/2011 and TAP LB 04/2012 and in Belarus no. Б11ЛИT-015 01/.2011). The study protocols, the Patient Information and Consent forms were approved by the Vilnius Regional Committee of Biomedical Research in Lithuania (permission no. 158200-6-062-16) and by the Belarusian Regional Committee of Biomedical Research (permission no. 34510-2-035). All patients signed the Informed Consent forms.
Data Collection
Fresh tumor samples were used for HPV detection. After surgical operation and pathological diagnosis, remaining tumor material was used for DNA extraction. Samples were collected in 0.5 ml PBS solution for transport and taken directly to the laboratory for DNA extraction.
HPV Testing and Typing
In the Institute of Oncology, Vilnius University (Lithuania) the DNA was extracted from all samples using GeneJet™ Genomic DNA Purification Kit (Thermo Scientific Fermentas Ltd., Vilnius, Lithuania). After DNA extraction all samples were tested for the beta-globin gene (internal control for the presence of DNA). HPV DNA detection was performed using two sets of general HPV primers: GP5+/GP6+ and PGMY09/11. As a positive control, the DNA extracted from HeLa and SiHa cells was used. For the negative control PCR with deionized water was used. The PCR was performed in 50 µl of PCR mix. DreamTaq™ Green PCR Master Mix containing DreamTaq™ DNA polymerase, optimized DreamTaq™ Green buffer, MgCl2 and dNTPs was used (Thermo Scientific Fermentas Ltd.).
For HPV typing the in-house developed and optimized multiplex PCR-based systems were used with four sets of primers specific for low and high risk HPV types: 6, 11, 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 73, and 82. The primers for HPV PCR system were designed according to the bioinformatics analysis of HPV L1 gene sequences. The quality of isolated DNA was tested using specific primers of two house-keeping genes: the β-globin gene and prostate specific antigen (PSA) gene. As a positive control, the DNA extracted from HeLa cells (ATCC NO. CCL-2) was used. The primers of HPV type-specific multiplex PCR system are complementary to HPV L1 and E gene sequences. The amplification products differ in 50–100 bp thus allowing their analysis in agarose gels. To develop a positive control for HPV genotyping, the plasmids based on λ DNA containing HPV DNA of the respective HPV type were constructed [Popendikyte et al., 2000]. The amplification products were analyzed by electrophoresis in 2% agarose gels and visualized using ethidium bromide under UV transilluminator (Herolab, GmbH Laborgerate, Germany). All results were documented by photoimaging and stored on the computer.
In N.N. Alexandrov National Cancer Center (Belarus) DNA-Sorb-V kit (Russia) for DNA extraction was used. Hot start TagF real-time PCR with TagF DNA polymerase was used to detect HPV (AmpliSens HPV genotype Kit, Moscow, Russia). PCR was performed using 13 µl PCR mix and 5 µl DNA. Twelve oncogenic HPV types were investigated during RT-PCR (HPV 16, 18, 31, 33, 35, 39, 45, 52, 53, 56, 58, 59) followed by fluorescence hybridization using specific probes.
Statistical Analysis
Results were calculated and presented as mean ± standard deviation (SD) or percentage (%) with 95% confidence intervals (CI).
RESULTS
Patients' Characteristics
Seventy-five patients with primary head and neck sqamous cell carcinoma diagnosed at the Institute of Oncology, Vilnius University (Lithuania) were included in the first study group. The average age of patients in this group was 61.8 ± 17.8 years.
One hundred fifteen patients with primary head and neck sqamous cell carcinoma diagnosed at the N.N. Aleksandrov National Cancer Center (Belarus) were included in the second study group. The average age of these patients was 54.3 ± 10.1 years.
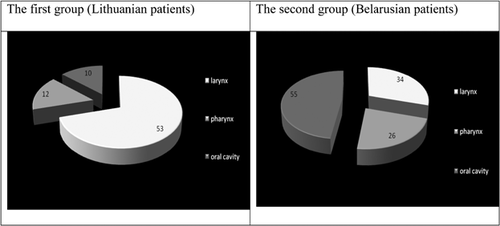
The distribution of both patient groups by tumor localization is presented in Figure 1.
Prevalence of HPV and Its Types
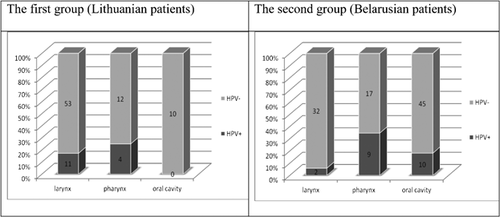
Among the 75 Lithuanian patients, 15 (20.0%, 95% CI 11.65–30.83) of them were HPV positive. HPV infection within the Belarusian patients was similar with 21 patients out of 115 (18.3%, 95% CI 11.67–26.55) testing positive.
Fifty-three patients from the Lithuanian group had larynx cancer, 12 had pharynx, and 10 had oral cavity cancer. HPV was detected in 20.8% (95% CI 10.84–34.11) of laryngeal and 33.3% (95% CI 9.92–65.11) of pharyngeal cancers, respectively. However, HPV was not detected in the 10 cases of oral cavity cancer.
Thirty-four patients from the Belarusian group had larynx cancer, 26 had pharynx cancer, and 55 had oral cavity cancer. HPV was detected in 5.9% (95% CI 0.72–19.68) of laryngeal, 34.6% (95% CI 17.21–55.67) of pharyngeal and 18.2% (95% CI 9.08–30.90) of oral cavity cancers, respectively.
Analyzing the distribution of HPV infection according to the cancer site in both studied groups, the highest incidence of HPV was in those patients with pharyngeal cancer (Fig. 2).
HPV types 6, 11, 16, 31, and 58 were found in both patient groups, but the distribution of HPV types depending on the location of head and neck squamous cell carcinoma varied significantly between two groups (Table I).
| Cancer location | HPV type | Lithuanian patients | Belarusian patients | ||
|---|---|---|---|---|---|
| % (n) | 95% CI | % (n) | 95% CI | ||
| Oral cavity | 6 | 0 | — | 3.64 (2) | 0.44–12.53 |
| 11 | 0 | — | 9.09 (5) | 3.02–19.95 | |
| 16 | 0 | — | 5.45 (3) | 1.15–15.12 | |
| Pharynx | 6 | 13.33 (2) | 1.66–40.46 | 0 | — |
| 11 | 0 | — | 7.69 (2) | 0.95–25.13 | |
| 16 | 0 | — | 19.23 (5) | 6.55–39.35 | |
| 31 | 0 | — | 3.85 (1) | 0.10–19.64 | |
| 58 | 0 | — | 3.85 (1) | 0.10–19.64 | |
| X | 13.33 (2) | 1.66–40.46 | 0 | — | |
| Larynx | 6 | 6.67 (1) | 0.17–31.95 | 2.94 (1) | 0.07–15.33 |
| 16 | 6.67 (1) | 0.17–31.95 | 2.94 (1) | 0.07–15.33 | |
| 31 | 6.67 (1) | 0.17–31.95 | 0 | — | |
| 31,39 | 6.67 (1) | 0.17–31.95 | 0 | — | |
| 58 | 6.67 (1) | 0.17–31.95 | 0 | — | |
| X | 40.00 (6) | 16.34–67.71 | 0 | — | |
DISCUSSION
The incidence of head and neck cancers is similar in Lithuania and Belarus. It was estimated that in 2008 head and neck cancer age-standardized incidence rates among males were 24.4 per 100,000 and 26.4 per 100,000 in Lithuania and Belarus, respectively [Ferlay et al., 2010]. The incidence of this cancer among females was much lower in both countries (2.5 and 2.2 per 100,000, respectively).
Despite the fact that head and neck sqamous cell carcinoma is a disease attributed to exposure to environmental risk factors, recent epidemiologic and molecular data suggest that HPV infection of upper airways may promote head and neck carcinogenesis [Braakhuis et al., 2009; O'Rorke et al., 2012]. Numerous studies have reported the presence of various HPV strains in head and neck sqamous cell carcinoma. In previous study from Lithuanian patients HPV infection was detected in 27.1% of patients with head and neck squamous cell carcinoma; 30.1% of HPV infected patients harbored HPV16 [Gudleviciene et al., 2009]. Present study provides the new data on HPV infection and distribution of HPV types among two studied groups of Lithuanian and Belarusian patients with head and neck squamous cell carcinoma.
In the case of oral cancer the presence of HPV infection ranged from 0% to 100% [Franceschi et al., 1996; Gillison et al., 2000; Gillison and Shah, 2001; Kreimer et al., 2005). This variation could be related to differences in populations, detection methods used, and other factors [Braakhuis et al., 2009]. In general, a lower HPV occurrence was detected in the western economically developed countries like Sweden, the Netherlands or the USA (2.4%, 4.4% and 4.9%, respectively); the highest occurrence was confirmed in India, China, and Japan (73.6%, 31.1% and 31.2%, respectively). The most common HPV type in the case of oral cancer was HPV16 [Herrero et al., 2003; Kreimer et al., 2005]. In this study 18.2% of Belarusian patients with oral cavity cancer had HPV infection. The most common HPV types detected in these patients were HPV6 (3.6%), 16 (5.5%), and 11 (9.1%). However, in Lithuanian patients with oral cancer (n = 10) no HPV was detected. This result could be related to the small number of cases included in the study group. On the other hand, these results are similar to the findings from other studies on European populations [IARC Monographs 1995 and 2007].
In the case of pharyngeal cancer, studies from Switzerland [Lindel et al., 2001] and the USA [Smith et al., 2004] reported HPV infection in 14% and 58.1% of patients, respectively. The predominant HPV infection was HPV16 which was confirmed in 35.6% of cases (95% CI 32.6–38.7%) [Kreimer et al., 2005]. Only sporadic cases were found to be infected with HPV types 6, 11, 31, and 33, while HPV type 18 was not detected at all. HPV infection rate was similar in both of present study groups—33.3% in Lithuanian and 34.6% in Belarusian patients. These reported results are comparable to the reported HPV infection rate for the rest of the world. In Belarusian patients HPV16 was found more frequently. On the other hand, low cancer risk type HPV6/11 was detected in 13.3% of Lithuanian and HPV11 in 7.7% of Belarusian patients.
HPV involvement in larynx cancer ranges from 5% in France [Fouret et al., 1995] to 60% in China [Ma et al., 1998] with a predominance of type HPV16 (in 74% of all HPV positive laryngeal carcinomas). HPV18 is the second most common type and in some cases low oncogenic risk HPV 6 and 11 were detected. This study showed results similar to those found for the rest of the world. It is well known that HPV6 and 11 are the most frequently detected types in the pathogenesis of recurrent respiratory papillomatosis which is the precursor of laryngeal cancer, so in addition to HPV16 and 18 these low risk types HPV 6 and 11 could play a significant role in the development of laryngeal cancer [Larson and Derkay, 2010; Sanchez et al., 2013].
In 2011, new data on HPV prevalence in cases from Central Europe and Latin America were presented by the International Agency for Research on Cancer [Ribeiro et al., 2011]. According to these data HPV16 E7 DNA prevalence among head and neck sqamous cell carcinoma cases was 3.1% (6/196), including 4.4% in the oropharynx (3/68), 3.8% in the hypopharynx/larynx (3/78), and 0% among 50 cases of oral cavity carcinomas. The authors concluded that the proportion of head and neck sqamous cell carcinoma caused by HPV varies substantially between different geographical regions. Some differences in HPV type distribution were stated in this study.
It is well known that vaccines against HPV are an effective prevention measure against cervical cancer. There is discussion in the literature about using these vaccines for head and neck sqamous cell carcinoma prevention [IARC Monographs, 2007]. However, according to the literature, HPV16 and 18 are not the most common types in these cancers. This was also confirmed in present study. On the other hand this study shows the intermediate prevalence of HPV6 and 11 in both countries, Lithuania and Belarus. These data could help to develop new vaccination strategies. Today, clinical trials of HPV vaccines focus only on cervical cancer and no vaccine efficacy studies focusing on head and neck sqamous cell carcinoma have been conducted. The new vaccination strategies against HPV6 and 11 infections using quadrivalent vaccines in children could play an important role in prevention of head and neck sqamous cell carcinoma development in both countries.
CONCLUSIONS
HPV prevalence in head and neck sqamous cell carcinoma patients in Lithuania (20.0%) and Belarus (18.3%) were similar. However, differences in the distribution of HPV types were found.




