Antibody persistence and immune memory in adults, 15 years after a three-dose schedule of a combined hepatitis A and B vaccine†‡§
Potential conflicts of interest: PVD acts as chief and principal investigator for clinical trials conducted on behalf of the University of Antwerp, for which the University obtains research grants from vaccine manufacturers; speaker's fees for presentations on vaccines are paid directly to an educational fund held by the University of Antwerp. PVD receives no personal remuneration for this work; GLR acts as chief and principal investigator for clinical trials conducted on behalf of the Ghent University, for which the University obtains research grants from vaccine manufacturers. GLR received no personal remuneration for this work; KVH declares that his institution (University of Antwerp) has obtained grants to conduct the study and he was supported to travel to meetings in order to present the study results; outside the scope of this study as well, his institution has received research grants, fees for consultancy and educational presentations on vaccines provided by KVH from different vaccine manufacturers; PC is employed at GSK Biologicals; MM was employed by GSK Biologicals as a consultant (from the CRO CHILTERN) at the time of this trial; JJ is employed at GSK Biologicals and also has stock ownership at GSK Biologicals.
Registration: ClinicalTrials.gov number: NCT00289770 and NCT00289718.
Twinrix is a trademark of the GlaxoSmithKline group of companies. Enzymun is a registered trademark of Boehringer Mannheim, Enzygnost of DADE Behring, AUSAB RIA and AUSAB EIA of Abbott Laboratories.
Abstract
A combined hepatitis A and B vaccine is available since 1996. Two separate open-label primary studies evaluated the immunogenicity and safety of this hepatitis A and B vaccine (720 EI.U of HAV and 20 µg of HBsAg) in 306 healthy subjects aged 17–43 years who received three doses of the vaccine following a 0, 1, and 6 months schedule. These subjects were followed up annually for the next 15 years to evaluate long-term persistence of anti-HAV and anti-HBs antibodies. The subjects whose antibody concentrations fell below the cut-offs between Year 11 and Year 15 (anti-HAV: <15 mIU/ml; anti-HBs: <10 mIU/ml) were offered an additional dose of the appropriate monovalent hepatitis A and/or B vaccine. In subjects who received the additional vaccine dose, a blood sample was collected 1 month after vaccination. At the Year 15 time point, all subjects in Study A and Study B were seropositive for anti-HAV antibodies and 89.3% and 92.9% of subjects in the respective studies had anti-HBs antibody concentrations ≥10 mIU/ml. Four subjects (two in each study) received an additional dose of monovalent hepatitis B vaccine and mounted anamnestic responses to vaccination. No vaccine-related serious adverse events were reported. This study confirms the long-term immunogenicity of the three-dose regimen of the combined hepatitis A and B vaccine, as eliciting long-term persistence of antibodies and immune memory against hepatitis A and B for up to at least 15 years after a primary vaccination. J. Med. Virol. 84:11–17, 2011. © 2011 Wiley Periodicals, Inc.
INTRODUCTION
Active immunization is a well-established, safe and effective method of conferring long-term protection against hepatitis A and B infections in susceptible persons [Van Herck et al., 1999, 2007; Van Der Wielen et al., 2000; Burgess and Rodger, 2001; Van Damme et al., 2001]. Improvements in sanitation, better hygiene and availability of clean water—complemented with hepatitis A immunization programs over the last two decades, have lowered the hepatitis A disease burden [Feinstone and Gust, 1999; WHO, 2000, 2008]. Similarly, universal hepatitis B immunization programs beginning at birth and other vaccination strategies have reduced the incidence of hepatitis B infections [Mahoney and Kane, 1999; WHO, 2009]. However, the two viruses still cause considerable morbidity and mortality worldwide [WHO, 2000, 2009; Jacobsen and Wiersma, 2010].
Although there is distinction between the two viruses including the difference in their modes of transmission, considerable similarities in epidemiological aspects warrant combined vaccination in specific situations [Thoelen et al., 1999]. A combined vaccine against hepatitis A and B (Twinrix™, GlaxoSmithKline [GSK] Biologicals, Rixensart, Belgium) has been available since 1996, as Adult and Paediatric formulations to be administered following a 0, 1, and 6 months schedule. Both formulations have demonstrated a good immunogenicity and safety profile [Bruguera et al., 1996; Thoelen et al., 1999; Van Damme et al., 2001; Murdoch et al., 2003].
In two open-label primary studies [Leroux-Roels et al., 1996; Thoelen et al., 1999] initiated at two center in Belgium in 1993, 306 healthy adults (Study A: subjects aged 17–39 years [N = 150] and Study B: subjects aged 17–43 years [N = 156]), without history of hepatitis A and B infection or vaccination were enrolled to receive three doses of the Adult formulation of hepatitis A and B vaccine according to a 0, 1, and 6 months schedule. After completion of primary vaccination (Month 7), all subjects were seropositive for antibodies against hepatitis A (anti-HAV; cut-off for seropositivity: ≥33 mIU/ml) and all were seroprotected for antibodies against hepatitis B (anti-HBs; cut-off for seroprotection: ≥10 mIU/ml).
The protocols for these studies were subsequently amended to allow annual blood sampling. After 10 years of follow-up (per-protocol cohort for immunogenicity; Study A: N = 34; Study B: N = 29), 100% of subjects included in the per-protocol cohort for immunogenicity in both studies were seropositive for anti-HAV antibodies and 94.1% and 86.2% of subjects in Study A and Study B, respectively, had anti-HBs antibody concentrations ≥10 mIU/ml [Van Herck et al., 2007]. This article presents data on the persistence of humoral immune response against hepatitis A and B in subjects 15 years after primary vaccination with the hepatitis A and B vaccine and the anamnestic response induced by a challenge vaccine dose in subjects whose antibody levels dropped below pre-defined cut-offs.
MATERIALS AND METHODS
Study Design and Subjects
Subjects were invited for yearly follow-up visits up to Year 15. An additional dose of a monovalent hepatitis A or B vaccine was offered to subjects whose anti-HAV antibody concentrations dropped to <15 mIU/ml or anti-HBs antibody concentrations reduced to <10 mIU/ml at any time point in the Years 11–15 timeframe, either between 6 and 12 months after the Year 15 time point (Study A) or within 12 months after the decrease in antibody concentrations (Study B). The challenge doses were administered as deep intramuscular injections (needle length: 25 mm) in the deltoid of the non-dominant arm. Additional serum samples were collected 1 month after vaccination.
Written informed consent was obtained from subjects prior to conducting any study-related procedures. The study was conducted as per Good Clinical Practice guidelines, the Declaration of Helsinki and applicable local regulations, after receiving necessary approvals from the Ethic Review Committees.
Study Vaccines
In the primary studies, the subjects were administered Twinrix™ Adult (GSK Biologicals, Belgium: 720 EI.U of HAV and 20 µg of HBsAg) following a 0, 1 and 6 month schedule. The challenge dose of monovalent HBV vaccine was Engerix™-B (GSK Biologicals: 20 µg of HBsAg).
Laboratory Assays
Anti-HAV antibody concentrations in serum samples up to the Year 6 blood sampling time point were measured using a commercial enzyme-linked immunosorbent assay (Enzymun®, Boehringer Mannheim; cut-off: 33 mIU/ml), while for the subsequent yearly time points up to Year 15, a separate enzyme immunoassay (EIA) was used (Enzygnost®, DADE Behring; cut-off: 15 mIU/ml). Anti-HBs antibody concentrations in serum samples up to Year 6 were measured using a commercial radioimmunoassay (AUSAB RIA®, Abbott Laboratories, Chicago, IL; cut-off: 1.0 mIU/ml); for the subsequent yearly time points up to Year 12, an EIA was used (AUSAB EIA®, Abbott; cut-off: 3.3 mIU/ml), while for Years 13–15, the commercial enzyme-linked immunoassay was replaced by an equivalent validated in-house enzyme-linked immunosorbent assay (ELISA) (Centre for Vaccinology, Ghent University and Hospital [CEVAC Laboratories, Ghent, Belgium]; cut-off: 3.3 mIU/ml) [Cambron et al., 2009]. The serum samples at the Year 6 time point were re-tested for anti-HAV and anti-HBs antibodies with the new EIAs, in order to achieve comparability of data and evaluate antibody kinetics. Table I presents information on the assays used at different time points along with their cut-offs.
| Marker | Time point | Assay method | Test kit/manufacturer | Assay cut-off (unit; mIU/ml) | Laboratory |
|---|---|---|---|---|---|
| Anti-HAV | M7 to Y6 | ELISA | Enzymun (Boehringer Mannheim) | 33 | GSK Biologicals |
| Anti-HAV | Y6, Y7 to Y15 | EIA | Enzygnost DADE Behring | 15 | GSK Biologicals |
| Anti-HBs | M7 to Y6 | RIA | AUSAB RIA-Abbott | 1.0 | GSK Biologicals |
| Anti-HBs | Year 6 | EIA | AUSAB/Abbott | 3.3 | GSK Biologicals |
| Anti-HBs | Y7 to Y12 | EIA | AUSAB/Abbott | 3.3 | CEVAC |
| Anti-HBs | Y13 to Y15 | ELISA | In house | 3.3 | CEVAC |
- EIA, enzyme-linked immunoassay; ELISA, enzyme-linked immunosorbent assay; RIA, radioimmunoassay; mIU/ml, milli International Units per milliliter; CEVAC, Centre for Vaccinology, Ghent University and Hospital.
Assessment of Immunogenicity
The anti-HAV and anti-HBs seropositivity rates, percentage of subjects with anti-HBs antibody concentration ≥10 mIU/ml, anti-HAV and anti-HBs antibody geometric mean concentrations (GMCs) were calculated with 95% confidence interval (CI). GMCs were calculated as the antilog of the mean of log-transformed anti-HAV and anti-HBs concentrations.
Anamnestic response for anti-HBs antibodies was defined as post-challenge dose anti-HBs antibody concentrations ≥10 mIU/ml in subjects who were seronegative before the challenge dose or at least a 4-fold increase in pre- versus post-challenge dose anti-HBs antibodies in subjects were seropositive before the challenge dose.
Assessment of Safety
For the challenge dose, solicited local and general adverse events and unsolicited adverse events were recorded during the 4-day and 30-day post-vaccination follow-up periods.
Serious adverse events (SAEs) considered by the investigator to be causally related to vaccination were recorded throughout the study period.
Statistical Analyses
Descriptive immunogenicity analyses were performed on the long-term per-protocol (LT-per-protocol) cohort for immunogenicity at all yearly time points and the safety analyses were performed on the long-term total cohort.
The long-term total cohort included all subjects who belonged to the total cohort of the primary study and returned at any of the annual blood sampling time points between Years 11 and 15.
The LT-per-protocol cohort for immunogenicity at Year 15 time point included those subjects who completed the three-dose primary vaccination course, were not excluded from the per-protocol cohort in the primary study or any of the subsequent follow-ups, complied with the blood sampling interval at Year 15, had not received any additional hepatitis vaccine (study vaccine or other) since the previous follow-up time point or gamma globulin in the 6 months preceding the current time point, have had no concomitant hepatitis infection and did not demonstrate an abnormal increase in antibody concentrations suggestive of natural boosting since the previous follow-up time point (i.e., not showing at least a 2-fold increase in antibody concentration when the concentration was ≥100 mIU/ml or at least 4-fold increase when the concentration was <100 mIU/ml at the reference time point).
These statistical analyses were performed using Statistical Analysis Software (SAS) version 9.1.
RESULTS
Study Population
Fifteen years after primary vaccination, 50 and 51 subjects from Study A and Study B, respectively, returned for the long-term follow-up (total cohort in the primary studies: 150 and 156, respectively); of these, 28 subjects in each study were included in the LT-per-protocol cohort for immunogenicity analyses (per-protocol cohort for immunogenicity in the primary studies: 142 and 131, respectively). The reasons for elimination from analyses at Year 15 were: elimination at a previous time point: 19 and 22 subjects; abnormal increase in antibody concentration during long-term follow-up: 3 and 1 subjects, respectively.
The mean ages of subjects in the two studies at the Year 15 time point were: Study A—34.2 years (range: 33–38 years) and Study B—36.9 years (range: 33–57 years). Post hoc analyses showed that the demographic characteristics at the time of first vaccination for the LT-per-protocol cohorts at the Year 15 time point (age—Study A: 19.2 years [range: 18–23 years]; Study B: 21.9 years [range: 18–40 years]; male:female ratio—Study A: 25%:75%; Study B: 17.9%:82.1%) were similar to that for the per-protocol cohort in the primary studies (age—Study A: 19.5 years [range: 18–36 years]; Study B: 21.5 years [range: 18–43 years]; male:female ratio—Study A: 19%:81%; Study B: 20.6%:79.4%).
In addition, post hoc analyses of the immune response post-primary vaccination in all subjects at Month 7 (anti-HAV antibody GMCs: Study A: 6965.4 mIU/ml; Study B: 5201.3 mIU/ml; anti-HBs antibody GMCs: Study A: 5564.4 mIU/ml; Study B: 3846.1 mIU/ml) was of the same magnitude to the immune response at Month 7 for subjects included in the Year 15 analyses (anti-HAV antibody GMCs: Study A: 6324.3 mIU/ml; Study B: 4577.8 mIU/ml; anti-HBs antibody GMCs: Study A: 5751.8 mIU/ml; Study B: 2678.3 mIU/ml).
Immunogenicity
Long-term antibody persistence 15 years after primary vaccination
Fifteen years after primary vaccination, all subjects in both studies (100% [95% CI: 87.7–100]) were seropositive for anti-HAV antibodies while 89.3% (Study A: 95% CI: 71.8–97.7) and 92.9% (Study B: 95% CI: 76.5–99.1) of subjects continued to have anti-HBs antibody concentrations ≥10 mIU/ml. The anti-HAV seropositivity rate and percentage of subjects with anti-HBs antibody concentrations ≥10 mIU/ml across all long-term follow-up time points are presented in Tables II and III, respectively.
| Time points | N | Study A, % (95% CI) | N | Study B, % (95% CI) |
|---|---|---|---|---|
| Cut-off ≥33 mIU/ml (ELISA kits) | ||||
| Month 7 | 107 | 100 (96.6;100) | 116 | 100 (96.9;100) |
| Year 1.5 | 86 | 100 (95.8;100) | 93 | 100 (96.1;100) |
| Year 2 | 75 | 100 (95.2;100) | 86 | 100 (95.8;100) |
| Year 3 | 54 | 100 (93.4;100) | 80 | 100 (95.5;100) |
| Year 4 | 49 | 100 (92.7;100) | 71 | 100 (94.9;100) |
| Year 5 | 43 | 100 (91.8;100) | 65 | 100 (94.5;100) |
| Year 6 | 40 | 100 (91.2;100) | 47 | 100 (92.5;100) |
| Cut-off ≥15 mIU/ml (EIA kits) | ||||
| Year 6a | 40 | 100 (91.2;100) | 51 | 100 (93;100) |
| Year 7 | 35 | 100 (90.0;100) | 45 | 100 (92.1;100) |
| Year 8 | 35 | 100 (90.0;100) | 42 | 100 (91.6;100) |
| Year 9 | 28 | 100 (87.7;100) | 40 | 100 (91.2;100) |
| Year 10 | 29 | 100 (88.1;100) | 34 | 100 (89.7;100) |
| Year 11 | 25 | 100 (86.3;100) | 33 | 100 (89.4;100) |
| Year 12 | 28 | 100 (87.7;100) | 33 | 100 (89.4;100) |
| Year 13 | 23 | 100 (85.2;100) | 32 | 100 (89.1;100) |
| Year 14 | 24 | 100 (85.8;100) | 30 | 100 (88.4;100) |
| Year 15 | 28 | 100 (87.7;100) | 28 | 100 (87.7;100) |
- N, number of subjects with available results; CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; EIA, enzyme-linked immunoassay.
- a Serum samples at Year 6 time point were re-tested with the new assay kit and the results compared with the old assay kit.
| Time points | N | Study A, % (95% CI) | N | Study B, % (95% CI) |
|---|---|---|---|---|
| Cut-off ≥10 mIU/ml (RIA kits) | ||||
| Month 7 | 107 | 100 (96.6;100) | 116 | 100 (96.9;100) |
| Year 1.5 | 86 | 98.8 (93.7;100) | 92 | 95.7 (89.2;98.8) |
| Year 2 | 75 | 97.3 (90.7;99.7) | 86 | 97.7 (91.9;99.7) |
| Year 3 | 54 | 96.3 (87.3;99.5) | 80 | 98.8 (93.2;100) |
| Year 4 | 49 | 93.9 (83.1;98.7) | 71 | 95.8 (88.1;99.1) |
| Year 5 | 43 | 93.0 (80.9;98.5) | 65 | 96.9 (89.3;99.6) |
| Year 6 | 40 | 95.0 (83.1;99.4) | 47 | 89.4 (76.9;96.5) |
| Cut-off ≥10 mIU/ml (EIA kits) | ||||
| Year 6a | 40 | 95.0 (83.1;99.4) | 51 | 96.1 (86.5;99.5) |
| Year 7 | 35 | 91.4 (76.9;98.2) | 45 | 97.8 (88.2;99.9) |
| Year 8 | 35 | 91.4 (76.9;98.2) | 42 | 100 (91.6;100) |
| Year 9 | 28 | 89.3 (71.8;97.7) | 40 | 97.5 (86.8;99.9) |
| Year 10 | 29 | 86.2 (68.3;96.1) | 34 | 94.1 (80.3;99.3) |
| Year 11 | 25 | 92.0 (74.0;99.0) | 33 | 100 (89.4;100) |
| Year 12 | 28 | 89.3 (71.8;97.7) | 33 | 97.0 (84.2;99.9) |
| Cut-off ≥10 mIU/ml (ELISA kits) | ||||
| Year 13 | 23 | 87.0 (66.4;97.2) | 32 | 100 (89.1;100) |
| Year 14 | 24 | 87.5 (67.6;97.3) | 30 | 96.7 (82.8;99.9) |
| Year 15 | 28 | 89.3 (71.8;97.7) | 28 | 92.9 (76.5;99.1) |
- N, number of subjects with available results; CI, confidence interval; RIA, radioimmunoassay; EIA, enzyme-linked immunoassay; ELISA, enzyme-linked immunosorbent assay.
- a Serum samples at Year 6 time point were re-tested with the new assay kit and the results compared with the old assay kit.
The corresponding anti-HAV antibody GMCs in the two studies (Study A and Study B) at the Year 15 time point were 599.1 mIU/ml (95% CI: 422.7–849.1) and 277.4 mIU/ml (95% CI: 200.9–383.1), respectively and the anti-HBs antibody GMCs were 197.3 mIU/ml (95% CI: 113.9–341.7) and 80 mIU/ml (95% CI: 47.8–133.9), respectively. The anti-HAV and anti-HBs antibody evolution across all long-term follow-up time points is presented in Figures 1 and 2, respectively.
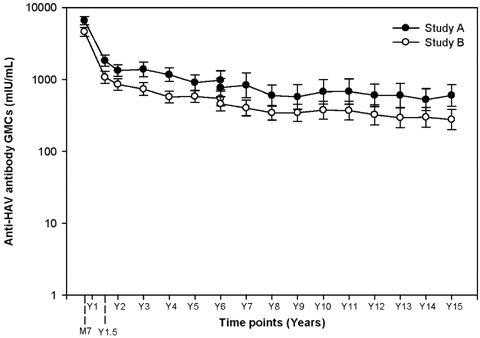
Evolution of anti-HAV antibody geometric mean concentration across the yearly follow-up time points (long-term total cohort). Serum samples up to Year 6 were tested with ELISA kits, while serum samples from Years 7 to 15 were tested with EIA kits.
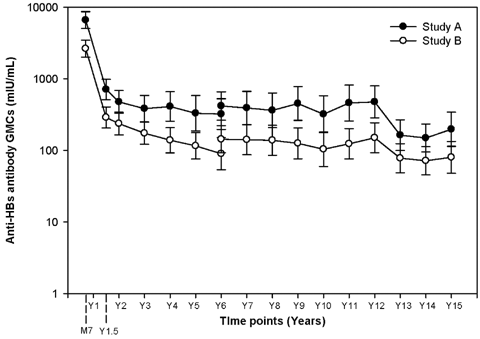
Evolution of anti-HBs antibody geometric mean concentration across the yearly follow-up time points (long-term total cohort). Serum samples up to Year 6 were tested with RIA kits, serum samples from Years 7 to 12 were tested with EIA kits, while serum samples from Years 13 to 15 were tested with in-house ELISA kits.
Anamnestic response to a challenge dose in subjects with anti-HAV antibody concentration <15 mIU/ml or anti-HBs antibody concentration <10 mIU/ml
At the Year 15 time point, all subjects had anti-HAV antibody concentration >15 mIU/ml and hence were not administered a challenge dose of monovalent hepatitis A vaccine; a total of five subjects were eligible to receive the challenge HBV vaccine dose (Study A: two subjects at Year 11 and one subject at Year 12 had anti-HBs antibody concentration <10 mIU/ml; Study B: one subject each at Years 11 and 12 had anti-HBs antibody concentration <10 mIU/ml). Of these, in Study A two subjects received a challenge dose of monovalent hepatitis B vaccine, 6–12 months after the Year 15 time point (one subject refused consent); for Study B, the two subjects received the challenge HBV vaccine dose within 12 months of eligibility time point. In addition, there were seven subjects (Study A: 4; Study B: 3) who were eligible for the challenge HBV vaccine dose at the Year 10 time point; of these, one subject did not give consent, while the remaining six subjects did not return for further follow-ups after Year 10 time point.
One month after the challenge dose, an anamnestic response to the challenge dose was observed in all four subjects; pre- versus post-challenge dose anti-HBs antibody concentrations described in Table IV.
| Study | n | Time-point determining eligibility | Anti-HBs (mIU/ml) at time-point determining eligibility | Anti-HBs (mIU/ml) pre-vaccination | Anti-HBs (mIU/ml) at Day 30 post-vaccination | Anamnestic responsea |
|---|---|---|---|---|---|---|
| Study A | 1 | Year 11 | <3.3 | <3.3 | 6548.1 | Yes |
| 2 | Year 11 | <3.3 | 14.5 | 554.0 | Yes | |
| Study B | 1 | Year 12 | 9.5 | 12 | 15022.3 | Yes |
| 2 | Year 11 | 9.7 | Blood sample not taken | 2138.0 | Yes |
- n, number of subjects who received the additional HBV dose.
- a Anamnestic response defined as: (i) post-challenge dose anti-HBs antibody concentrations ≥10 mIU/ml in subjects who were seronegative before the challenge dose or (ii) at least a 4-fold increase in pre- versus post-challenge dose anti-HBs antibodies in subjects were seropositive before the challenge dose.
Safety and Reactogenicity
No vaccine-related SAEs were reported at the Year 15 time point since the previous publication [Van Herck et al., 2007].
Overall, the hepatitis B challenge dose was well-tolerated; one subject reported an unsolicited adverse event which was assessed by the investigator to be vaccination-related; this subject reported heaviness sensation above eyes which was of Grade 1 intensity and persisted for 4 days following vaccination. No SAEs related to the hepatitis B challenge dose were recorded.
DISCUSSION
This article presents the results of the longest follow-up studies with the three-dose vaccination schedule of the combined hepatitis A and B vaccine in adults. After 15 years of follow-up, all subjects remained seropositive for anti-HAV antibodies (cut-off: ≥15 mIU/ml) and >89% of subjects continued to have anti-HBs antibody concentrations ≥10 mIU/ml. These results confirmed that the anti-HAV and anti-HBs antibodies persisted for at least 15 years after receiving three doses of the Adult formulation of the combined hepatitis A and B vaccine. This re-enforces observation from previous studies in children, adolescents, and adults that have demonstrated that the two- or three-dose primary vaccination schedules of this combined hepatitis A and B vaccine were highly immunogenic and induced long-term persistence of anti-HAV and anti-HBs antibodies [Lee et al., 1999; Van Herck et al., 2007; Beran et al., 2010; Burgess et al., 2010]. In the present study, the minor difference in the immunogenicity observed between the two study cohorts was apparent throughout the follow-up period of the study up to Year 15. Although the anti-HAV seropositivity rates and percentage of subjects with anti-HBs antibody concentrations ≥10 mIU/ml at Year 15 were comparable, the anti-HAV and anti-HBs antibody GMCs in Study A were comparatively higher than those in Study B (small overlap in 95% CIs), a trend already observed at a few follow-up time points. This observation probably measures subtle differences between the two study cohorts, along with natural variation of the GMC, an aggregate log value.
The pattern of decline in the anti-HAV and anti-HBs antibody GMCs over time in this study was comparable to that observed in previous studies with the hepatitis A and B vaccine—a rapid decline in the first year followed by a more gradual decrease over the subsequent years [Van Damme et al., 2001; Van Herck et al., 2007]; this has also been observed for monovalent hepatitis A and B vaccines [Duval et al., 2005; Rendi-Wagner et al., 2007]. The decrease in anti-HAV and anti-HBs antibody concentrations is not considered to be correlated to loss of long-term protection and immune memory against hepatitis A and B disease, as shown for monovalent vaccines [Banatvala et al., 2000; Van Damme et al., 2003; Gabbuti et al., 2007] and the same principle is expected to hold true for this combined hepatitis A and B vaccine. Persistence of immune memory (after 15 years) was demonstrated in the form of a robust anamnestic response to a challenge dose of monovalent hepatitis B vaccine in the four subjects whose antibody concentrations had declined below 10 mIU/ml. No subjects had anti-HAV antibody concentrations <15 mIU/ml in the 15 years following the primary vaccination, which illustrates the established long-term persistence of anti-HAV antibodies following vaccination with monovalent hepatitis A vaccines [Rendi-Wagner et al., 2007].
The evaluation of the long-term persistence of the humoral immune response following immunization with hepatitis vaccines is of critical importance, considering that lower seroprevalence of hepatitis A virus in most industrialized regions is correlated to an increased risk of hepatitis A infection in older age groups [Chatproedprai et al., 2007; Jacobsen, 2009]. In addition, in this age of international travel, an increasing number of adults travel to areas endemic for hepatitis A and B, thus increasing the risk of infection [Zuckerman and Hoet, 2008; Askling et al., 2009]. A recent report showed that in the United States, universal mass vaccination with hepatitis A vaccine has drastically reduced the incidence of hepatitis A; and the majority of recent hepatitis A cases and outbreaks have been attributed to international travelers to areas endemic for hepatitis A and immigrants [Andersson and Friedman, 2010]. Similar observations indicating a negative correlation between universal mass immunization against hepatitis A and HAV-associated morbidity have been reported in other countries [Andre, 2006; Torner et al., 2011]. In this context, a combined hepatitis A and B vaccine that can confer dual protection against hepatitis A and B in the long term may be beneficial in minimizing the risk of infection and disease in adults.
In conclusion, the data from these two longest follow-up studies of a combined hepatitis A and B vaccine in adults demonstrated that the three-dose regimen of the Adult formulation of this combined hepatitis A and B vaccine, in addition to being well-tolerated and highly immunogenic, elicited long-term persistence of antibodies and immune memory against hepatitis A and B for up to at least 15 years after a three-dose primary vaccination.
Acknowledgements
We thank the subjects for participating in this trial. We are indebted to the study nurses and other staff members for contributing in many ways to this study. We also thank Jyothi Kumari for providing statistical analysis support, Avishek Pal for medical writing assistance and Manjula K for publication coordination (all employed by GSK Biologicals). GlaxoSmithKline was the funding source and was involved in all stages of the study conduct and analyses. GlaxoSmithKline Biologicals also bore all costs associated with the development and the publishing of the present manuscript. The corresponding author had full access to the data and had final responsibility to submit for publication.




