Phenotypic assay of a hepatitis B virus strain carrying an rtS246T variant using a new strategy
Abstract
Phenotypic assays of hepatitis B virus (HBV) play an important role in research related to the problem of drug resistance that emerges during long-term nucleot(s)ide therapy in patients with chronic hepatitis B. Most of the phenotypic assay systems that are available currently rely on the transfection of recombinant replication-competent HBV DNA into hepatoma cell lines. Cloning clinical HBV isolates using conventional digestion-and-ligation techniques to generate replication-competent recombinants can be very difficult because of the sequence heterogeneity and unique structure of the HBV genome. In this study, a new strategy for constructing an HBV 1.1× recombinant was developed. The core of this strategy is the “fragment substitution reaction” (FSR). FSR allows PCR fragments to be cloned without digestion or ligation, providing a new tool for cloning fragments or genomes amplified from serum HBV DNA, and therefore making the assay of HBV phenotypes more convenient. Using this strategy, a phenotypic assay was performed on an HBV strain carrying an rtS246T variant isolated from a patient with chronic hepatitis B that was only responsive partially to entecavir therapy. The results indicated that this strain is sensitive to entecavir in vitro. J. Med. Virol. 84:34–43, 2011. © 2011 Wiley Periodicals, Inc.
INTRODUCTION
Nucleot(s)ide analogs with potent anti-viral efficacy represent common treatments for patients with chronic hepatitis B. Nucleot(s)ide analogs interfere with both the reverse transcription and the DNA elongation step of the hepatitis B virus (HBV) life cycle, but do not eliminate directly the covalently closed circular DNA that acts as the template for the transcription of HBV RNAs. Therefore, long-term treatment with these drugs is required to reduce HBV DNA to a low level.
A problem that occurs during the long-term management of HBV infection with nucleot(s)ide analogs is the emergence of drug resistance [Allen et al., 1998]. Specific amino acid changes in the viral reverse transcriptase that arise from mutations in the corresponding region of the HBV P gene are one major cause of this drug resistance [Allen et al., 1998; Angus et al., 2003; Villeneuve et al., 2003; Tenney et al., 2004, 2007]. The correlation between certain mutations and drug resistance has been established generally based on clinical data analyses and phenotypic assays. Phenotypic assays, by providing in vitro susceptibility data for HBV isolates of specific patients, play an important role in the verification of novel drug-resistant mutations and may also provide information useful for the selection of anti-viral drugs for therapy and the adjustment of treatment regimens when initial treatments fail.
In the absence of a robust cell culture system susceptible to HBV infection, most HBV susceptibility assays are based on the transfection of replication-competent HBV DNA into hepatoma cell lines that are able to replicate and secrete hepatitis B virions [Durantel et al., 2005]. To support HBV replication after it is transfected into cells, a properly constructed recombinant HBV DNA is crucial. Such a recombinant should be constructed to permit the transcription of functional pre-genomic RNA (pgRNA), through either a heterogeneous promoter, like the cytomegalovirus (CMV) promoter, or the intrinsic HBV core promoter. With its relatively low transcription activity, the core promoter produces a lower level of pgRNA and in turn a lower level of replication than the CMV promoter [Lucifora et al., 2008]. For susceptibility assays, a higher replication level is preferable for the detection of replicative intermediates by Southern blotting.
Two problems must be resolved in the construction of a replication-competent HBV 1.1× plasmid: One is cloning the 1.1× genome of HBV and the other is the accurate fusion of the HBV DNA downstream from a heterogeneous promoter. The longest fragment that can be amplified from serum HBV DNA in one independent PCR is one copy of the HBV genome. Therefore, at least two PCR fragments are required to construct HBV 1.1×. When ligating these two fragments to form an HBV 1.1, the problem arises that a single endogenous restriction site is needed to ensure that the organization of the HBV DNA is not disrupted. Although such restriction sites can be found in some genotypes of the HBV genome, as reported previously, these sites may not exist in other genotypes because of the heterogeneity of HBV, and the process of constructing an HBV 1.1× using these sites is usually laborious. For the precise fusion of HBV DNA to a heterogeneous promoter, an appropriate restriction site may need to be introduced at the correct position downstream from the promoter in a commercial vector, ensuring that the initiation site of pgRNA transcription is identical to that in HBV under natural conditions.
Durantel et al. [2004] and Yang et al. [2004] have both developed methods of constructing heterogeneous-promoter-driven HBV 1.1× genomic recombinants, which have been used productively in research related to HBV drug resistance. In the present study, a new strategy that circumvents the digestion and ligation processes in the construction of CMV-promoter-driven HBV 1.1× genomic recombinants was developed. Using this strategy, a susceptibility assay was performed for an HBV strain isolated from a patient who received entecavir (ETV) therapy but still had viral loads of >104 copies/ml serum at all the observed time points.
MATERIALS AND METHODS
Case Report
The patient selected was a 35-year-old Chinese man with a chronic HBeAg-positive HBV infection. He had never been treated with nucleot(s)ide analogs, and was enrolled in a registered clinical study [Guo et al., 2009]. The patient had received 0.5 mg ETV monotherapy daily for more than 3 years. The dynamics of his serum viral load and aminotransferase activities are shown in Figure 1. No HBeAg to anti-HBe conversion was observed during the whole period of therapy.
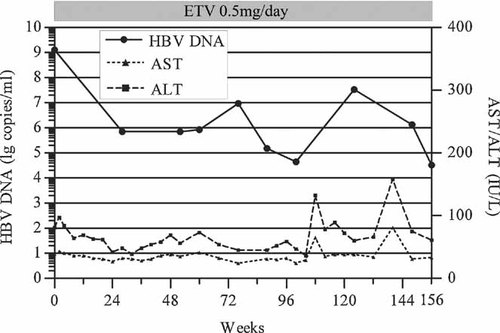
Dynamics of serum HBV DNA levels and serum aminotransferase activities of a patient during entecavir (ETV) therapy, at a dose of 0.5 mg/day for 156 weeks. Serum viral loads and aminotransferase activities at various time points were determined. ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Sequence Analysis of Serum HBV DNA
Viral DNA was extracted from 200 µl of serum using the High Pure Viral Nucleic Acid Kit (Roche Diagnostics, Mannheim, Germany), according to the manufacturer's instructions. The final volume of eluate was 50 µl and 1 µl was used for subsequent PCR amplifications. Primers F250 and R1,058 were used to amplify an 808-bp fragment encompassing domains A–E of the HBV reverse transcriptase. The amplicons were sequenced with an ABI PRISM® 3100 Genetic Analyzer (Applied Biosystems Inc., Foster City, CA). All PCR analyses in the present study were performed using PrimeStar HS DNA Polymerase (Takara Biotechnology, Dalian, China). Nine primers (all shown in the 5′ to 3′ direction) used are listed in Table I.
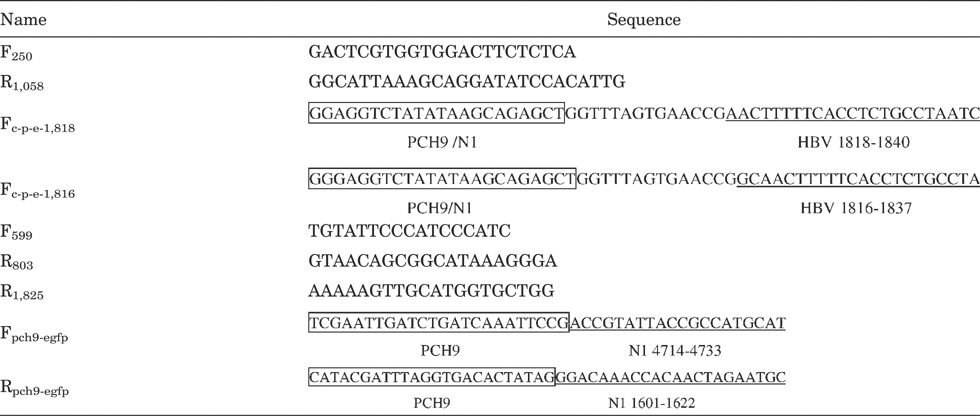
- Numbers indicate the positions on the HBV genome to which the primers would anneal (defining EcoRI as position 1).
- Underlined are sequences that anneal to the corresponding HBV sequence.
- Boxed Fc-p-e-1,818 and Fc-p-e-1,816 are sequences that can anneal to both PCH-9/3091 and pEGFP-N1.
- Boxed Fpch9-egfp and Rpch9-egfp are sequences that can anneal to PCH-9/3091.
HBV 1.1× Plasmid Construction
Fragment Substitution Reaction (FSR)
Plasmid pHBV1.3/1, which contains 1.3 copies of the HBV genome DNA (GenBank accession number: GU451682) from the serum of the patient described above, was constructed previously [Hu et al., 2009]. Plasmid PCH-9/3091, constructed by Nassal [1992] and containing a CMV-early-promoter-driven HBV 1.1× genome, was kindly provided by Lin Nan (Southwest Hospital, Chongqing, China). Figure 2 shows the procedure used to construct p1818-LL, a construct containing the HBV DNA from the patient. Fragment 1,818–803 (designated conventionally, with the ‘C’ corresponding to that in the EcoR I site of genotype A as position 1, although there is no EcoR I site in the genome of this strain), about 2.2 kb in length, was amplified from pHBV1.3/1 using primers Fc-p-e-1,818 and R803. After gel purification with the QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany), 800 ng of the fragment was used as the mega-primers in a 50 µl FSR system, together with 50 ng of PCH-9/3091, 4 µl of 2.5 mM dNTPs, 1.25 U of PrimeStar HS DNA Polymerase, and buffer. The cycling parameters used were: Initial denaturation at 94°C for 2 min, followed by 18 cycles of denaturation at 94°C for 15 sec, annealing at 55°C for 15 sec, and extension at 72°C for 4 min. The resultant FSR product was purified with a PCR Purification Kit (Roche Diagnostics), digested with Dpn I (Promega, Madison, WI), and then transformed into competent JM109 cells. That the clone contained the replaced fragment was confirmed by sequencing and it was designated p1818. Plasmid p1818-LL was generated by replacing nucleotides (nt) 599–1,825 of p1818 with a fragment (nt 599–1,825) amplified from pHBV1.3/1 with the same method. The fragment amplified from pHBV1.3/1 with Fc-p-e-1,816 and R803 was also used to replace the corresponding region in PCH-9/3091 by FSR to generate the plasmid p1816 (not shown in Fig. 2). The distance from the TATA box of the CMV promoter to nt 1,818 in p1818 is 2 nt shorter than in p1816 (Fig. 4A). This 2-nt deletion is expected to cause p1818 to transcribe pgRNA from nt 1,818. p1818 and p1816 were used to evaluate the influence of different pgRNA transcription start sites on the replication capacity of the construct.

Strategy for HBV 1.1× plasmid construction. A: Principle of the FSR. The principle of site-directed mutagenesis of Stratagene is illustrated graphically in the upper part. For FSR, mega-primers containing the target fragment (TF; indicated with red lines) were annealed to a plasmid via the homologous sequences at their ends. Each newly synthesized plasmid-like molecule had a nick on both strands, and the nicks are repaired after transformation into JM109. B: Procedure for HBV 1.1× plasmid construction. A fragment was amplified from the patient's HBV DNA (1). The fragment was substituted into PCH-9/3091 by FSR (2), yielding plasmid p1818 (3). Another FSR was performed with the PCR fragment 599–1,825 to produce plasmid p1818-LL (4.5), which contained a copy of the HBV genome derived from the patient's serum HBV. An EGFP-expressing fragment was inserted downstream from HBV 1.1 in p1818-LL to generate the plasmid pLL–EGFP. HBV could be replicated and EGFP expressed from this plasmid when it was transfected into HepG2 cells. FSR, fragment substitution reaction; PolyA, polyadenylation signal. PS, S, X, and C indicate pre-S, S, X, and core promoter, respectively.
Insertion of a CMV–EGFP Fragment Downstream From HBV DNA
A CMV–EGFP DNA fragment containing the CMV immediate early promoter, the gene encoding enhanced green fluorescent protein (EGFP), and the SV40 polyA signal was amplified from the plasmid pEGFP-N1 (Clonetech, Mountain View, CA) with primers Fpch9-egfp and Rpch9-egfp. The plasmid PCH9–EGFP, carrying CMV–EGFP downstream from HBV 1.1×, was generated with the CMV–EGFP DNA fragment using the same FSR method described in Fragment Substitution Reaction section, except that the FSR extension was performed at 72°C for 4 min 30 sec for 30 cycles. The CMV–EGFP DNA fragment was also inserted into p1818-LL to generate the plasmid pLL–EGFP. pLL–EGFP and PCH9–EGFP were the constructs used in susceptibility assays.
Cell Culture and Transfection
HepG2 cells were grown at 37°C under 5% CO2 in modified Eagle's medium supplemented with 10% (v/v) fetal bovine serum. For transfection, the cells were seeded into six-well plates or 3.5-cm-diameter plates and allowed to adhere overnight. On the following day, when the cells were 60–70% confluent, the culture medium was replaced with fresh medium and 4 µg of HBV construct was transfected into the cells in each well using 8 µl Lipofectamine 2000 (Invitrogen, Carlsbad, CA), according to the instructions provided by the supplier. The culture medium was changed every 2 days, and the cells were harvested on day 5 after transfection.
Viral DNA Analysis
Intracellular viral core DNA was extracted as described previously, with modifications [Hu et al., 2009]. Briefly, cells from one well or plate were lysed with 400 µl of lysis buffer (10 mM Tris–HCl [pH 8.0], 1 mM EDTA, 1% NP-40, and 2% sucrose) at 37°C for 10 min. After centrifugation at 15,000g for 3 min, the supernatant was transferred to a fresh microcentrifuge tube and 1 M MgCl2 was added to achieve a final Mg2+ concentration of 10 mM. DNase I (Promega; added to 40 U/ml) and RNase A (Calbiochem, Darmstadt, Germany; added to 1 mg/ml) were added to digest the contaminating plasmid and RNA, respectively. After 3 hr at 37°C, the reaction was terminated with 10 mM EDTA, and then 140 µl of 35% polyethylene glycol 8000 containing 1.5 M NaCl was added. After incubation for 1 hr on ice, the viral nucleocapsids were pelleted by centrifugation at 11,000g for 5 min at 4°C, and then digested (3 hr at 40°C) in 400 µl of buffer containing 1 mg/ml proteinase K (Promega). The digestion mixture was extracted twice with phenol. The DNA was precipitated with ethanol and dissolved in TE buffer (10 mM Tris–HCl [pH 8.0], 1 mM EDTA). The DNA was detected with an HBV DNA probe using the DIG DNA Labeling and Detection Kit (Roche Diagnostics), according to the manufacturer's instructions.
ETV Susceptibility Assay
For the drug susceptibility assays, six-well culture plates were seeded with 7.0 × 105 HepG2 cells/well. Twenty hours after seeding, the cells were transfected with 4 µg of plasmid DNA using Lipofectamine 2000 transfection reagent. One day after transfection, the cells were fed with fresh medium containing 0, 0.2, 0.5, 2, 5, or 10 nM ETV (Bristol-Myers Squibb, New York, NY). The medium containing ETV was changed on day 3, and the cells were harvested 5 days after their transfection. To assess the proportion of transduced cells (containing plasmid), cells expressing EGFP within 15 selected randomly fields of 200× magnification on an X71 microscope (Olympus, Tokyo, Japan) were counted before harvest. The counts in all the wells were divided by the counts in the well to which no ETV was added, to calculate the ratio of transduced cells in each well relative to the number of cells in the ETV-free well. The intracellular HBV replicative intermediates were analysed by Southern blotting and quantified with the Quantity One software (Bio-Rad). All experiments were performed in triplicate. GraphPad Prism software (GraphPad Software, Inc., San Diego, CA) was used to determine the best-fit values for individual dose–response equations.
RESULTS
Strategy for HBV 1.1× Plasmid Construction
A new strategy was developed to construct HBV 1.1. The strategy is characterized by a method called the fragment substitution reaction (FSR). The principle of this method resembles that of site-directed mutagenesis developed by Stratagene. In practice, a DNA fragment (1 bp to several kb in size) located between two primers (e.g., 25 bp in length) that can anneal to a plasmid template can be considered ‘one’ mutation. When the two primers of a DNA fragment both anneal to a plasmid, DNA polymerase starts to extend the two strands using the plasmid as the template. The extension products will form a plasmid-like structure with one nick in each strand, as is the case in a site-directed mutagenesis reaction. After the plasmid template is removed by Dpn I digestion, the transformation of the remaining DNA will generate clones containing the substituted fragment.
To construct HBV 1.1×, fragment 1,818–803 was first amplified from pHBV1.3/1 using primers Fc-p-e-1,818 and R803. Primer Fc-p-e-1,818 (Table I and Fig. 2B.2) contains the HBV sequence of nt 1,818–1,840 and the end part of the CMV promoter from the plasmid pEGFP-N1. In PCH-9/3091, part of the CMV promoter is identical to the 22 bp at the 5′ end of primer Fc-p-e-1,818, whereas the subsequent 16 bp, which comprises a Sal I site and the HBV sequence at nt 1,809–1,817, is different from the middle part of primer Fc-p-e-1,818 (Fig. 2B.2). The sequence of primer R803 is identical to the corresponding region in PCH-9/3091. With FSR, plasmid p1818 was obtained, which contains the substituted fragment nt 1,818–803 from pHBV1.3/1. Using p1818 as the template, another FSR was performed with fragment 599–1,825 amplified from pHBV1.3/1, producing plasmid p1818-LL, which contains one copy of the HBV genome from the patient serum plus less than 200 bp of the HBV sequence carried over from PCH-9/3091. A fragment containing the CMV promoter, the EGFP gene, and the SV40 polyA signal was then amplified from pEGFP-N1, and the fragment inserted downstream from HBV 1.1 in both p1818-LL and PCH-9/3091 by FSR. The resultant plasmids were designated pLL–EGFP and PCH9–EGFP, respectively.
Fragments can be Substituted Effectively Into a Plasmid With the Fragment Substitution Reaction
More than 20 experiments using FSR have been performed in our laboratory, including FSR with fragments amplified directly from serum containing HBV DNA, and most of them were successful (data not shown). When a fragment with high homology to the template is used as the mega-primers, such as the substitution of one genotype of HBV DNA for another, FSR is very efficient. The newly synthesized recombinants can be observed readily on a gel (Fig. 3A,B). When a fragment with relatively lower homology to the template was used, as in the FSR of CMV–EGFP, the FSR products could not be detected on a gel (Fig. 3C), despite an additional 12 cycles of reaction (30 cycles total; see Insertion of a CMV-EGFP Fragment Downstream From HBV DNA section) being added.

Electrophoresis of the FSR product. FSRs were performed with the method described in the Materials and Methods section. After the FSR products were treated with DpnI, they were separated by electrophoresis on agarose gels. As shown here, two bands were visible, for FSR products 1,816/1,818–803 (A) and 599–1,825 (B), representing the mega-primers that had not been used up and the newly synthesized molecules, respectively. The FSR products of CMV–EGFP were not visible compared with products A and B, indicating that the FSR of CMV–EGFP was less efficient than the FSR of 1816/1818–803 and 599–1,825.
After the transformation of the FSR products, three clones were usually picked for sequencing. In most cases, two of the three clones or three of the three clones contained a correctly substituted fragment. Figure 4 shows the sequence alignment of four FSR products with their templates. The sequence downstream from the TATA box in p1818 and p1816 (A) and the sequence downstream from nt 803 in p1818-LL differ from that in PCH-9/3091, confirming that these regions have been substituted.
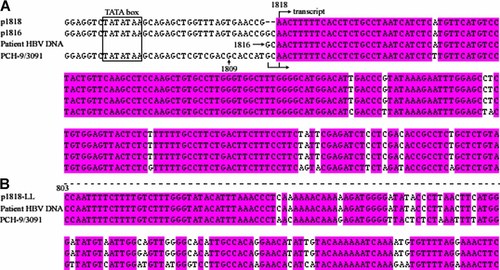
Sequence alignment of plasmids and the patient HBV DNA. The sequences around the end of the CMV promoter in plasmids p1818, p1816, and PCH-9/3091 were determined and aligned with the patient's HBV DNA sequence (A). Parts of the sequences downstream from nt 803 in p1818-LL, the patient's HBV DNA, and PCH-9/3091 were aligned (B). The HBV sequences analysed in p1818, p1816, and p1818-LL were identical with that in the HBV DNA of the patient and different from that of PCH-9/3091, suggesting that the fragments derived from the patient HBV DNA were successfully substituted into PCH-9/3091.
p1818 and p1816 Replicate HBV at Similar Levels in HepG2 Cells
PCH-9/3091 has been reported to contain an extra pgRNA transcription start site at nt 1,816 [Liu et al., 2004]. To analyse whether this extra transcription start site affects the level of replication, plasmids p1818 and p1816 were generated. P1818 and p1816 were each designed so that their transcription start sites coincide with nt 1,818 and nt 1,816, respectively (Fig. 4A); so, p1818 was expected to generate pgRNA transcripts starting from nt 1,818, whereas p1816 generated them from nt 1,816. p1816 or p1818 (4 µg) was transfected into HepG2 cells and the intracellular core replicative intermediates were analysed by Southern blotting. As shown in Figure 5, after the cell numbers were normalized to the cell viability, no significant difference was observed between the replication levels of p1816 and p1818. When the replication capacity of p1816 was set as 100%, the replication level of p1818 was 104% (mean value).
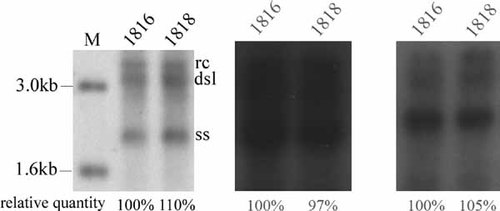
p1816 and p1818 replicated HBV at similar levels in HepG2 cells. p1816 and p1818 were transfected into HepG2 cells. The intracellular core DNA was extracted 5 days after transfection, and HBV replicative intermediates were detected by Southern blotting. The replication level of p1816 was set at 100%, and the replication capacity of p1818 was calculated as a relative value. All experiments were conducted in triplicate. M, DNA marker.
Sequence Analysis of Prevalent HBV Reverse Transcriptase at Different Time Points Following ETV Treatment
HBV reverse transcriptase fragments were amplified from the sera of the selected patient at 0, 28, 76, 124, and 144 weeks after ETV treatment and directly sequenced. All were shown to encode identical amino acid sequences (data not shown). No previously reported ETV-resistance-associated substitution, including M204V/I, L180M, I169T, S202G/I, M250V, or T184G, was found in the sequences. The nucleotide sequences of these fragments were classified as genotype B according to the classification system of Norder et al. [2004]. When compared with the alignment data of the 116 genotype B HBV strains described previously [Hu et al., 2009], these sequences from our patient encoded a threonine at reverse transcriptase amino acid 246 (numbering according to the nomenclature of Stuyver et al., 2001) instead of the serine present in the 116 genotype B strains. Amino acid 246 is located one codon 5′ to the boundary of the conserved subdomain E in HBV polymerase, as defined by Stuyver et al. [2001].
ETV Susceptibility of the Patient Isolate
As described above, an HBV 1.1× genomic recombinant plasmid (pLL–EGFP) was generated from the HBV genome DNA from the serum of a patient. To test the susceptibility of the isolate from the patient, HepG2 cells were transfected with pLL–EGFP and treated with various concentrations of ETV. The intracellular replicative intermediates were assayed by Southern blotting (Fig. 6A). The ETV IC50 of the isolate calculated from the dose–inhibition curve (Fig. 6C) was 0.99 ± 0.09 nM. As a wild-type control, PCH9–EGFP was transfected into HepG2 cells and their susceptibility to ETV was tested (Fig. 6B,C). This laboratory strain yielded an IC50 of 0.58 ± 0.10 nM. These results suggest that the HBV isolated from this patient was not resistant to ETV.
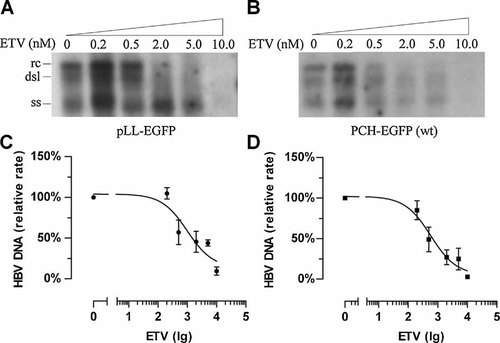
Susceptibility of HBV strains to ETV in vitro. HepG2 cells were transfected with plasmids pLL–EGFP and PCH–EGFP (wt) and treated with the indicated amounts of ETV. The intracellular replicative intermediates were analysed by Southern blotting. Representative results are shown in (A) and (B). The positions of the relaxed circular (rc), double-stranded linear (dsl), and single-stranded (ss) forms of HBV DNA are indicated. Dose–inhibition curves for the two HBV strains were used to estimate the ETV IC50 values for each HBV strain. The Y-axes show values relative to the no-ETV control for each strain. The X-axes show the log10 transformation of the ETV concentrations in pM. The experiments were performed in triplicate. Values are expressed as means ± SD.
DISCUSSION
FSR is an Effective Method for DNA Cloning
In this study, a new strategy for constructing HBV 1.1× recombinants with a CMV promoter to drive HBV pgRNA transcription was developed. The core of this strategy is the FSR method. FSR allows a fragment (the “substituted fragment”, SF) in a plasmid to be substituted with another fragment (the “target fragment”, TF). The homology between SF and TF can be 0–100%, which allows great flexibility. When the homology is 0, this method essentially becomes a cloning technique, with the same effect as the traditional digestion-and-ligation cloning technique. A non-homologous fragment, CMV–EGFP–PolyA, was successfully inserted into plasmids PCH-9/3091 and pLL1818, as in the construction of plasmids PCH–EGFP and pLL–EGFP, respectively. In this experiment, EGFP was expressed from the HBV 1.1× plasmid, although there was no appropriate site downstream from HBV 1.1×. Two 24-bp sequences (Table I) were added to the 5′ end of the primers for CMV–EGFP amplification respectively. These two sequences could anneal to the two sequences separated by 6-bp, downstream from HBV 1.1×. The CMV–EGFP PCR fragment was then inserted into the plasmids by FSR. Geiser et al. [2001] have used this method to insert fragments of 34–1,117 bp into plasmids. The present results demonstrate that longer fragments can also be inserted into a plasmid without restriction enzyme digestion or ligation. Recently, Stech et al. [2008] have used this method to clone complete sets of functional gene segments from influenza A strains, showing that fragments of up to 2,341 bp can be cloned effectively with this method.
When SF and TF have a relatively high degree of homology, as in the case of HBV fragment substitution, FSR can substitute the fragment with high efficiency. In the FSR for fragments 1,818–803 and 599–1,825, the differences between these two TFs and their SFs were 12.4% (including a 33-bp deletion of nt 2,897–2,929 from the genotype D HBV DNA in PCH-9/3091) and 10.0%, respectively. As shown in Figure 3A,B, 18 cycles of amplification resulted in electrophoresis-detectable FSR products. The transformation of FSR products digested with Dpn I led to clones with a low background of the template plasmid. There was always one or more correctly substituted clone in the three clones tested.
Advantages and a Disadvantage of FSR in HBV Phenotypic Assay
The major advantage of FSR over conventional cloning is that in FSR, the sequence specificity around the cloning site need not be considered, i.e., the presence of an appropriate restriction site is no longer a limitation. Most of the methods available currently to generate replication-competent HBV DNA constructs involve digestion and ligation process. Some restriction sites used in these methods include but are not limited to Sap I [Gunther et al., 1995; Yang et al., 2004], Nco I [Durantel et al., 2004], and Spe I [Hu et al., 2009]. Although these restriction sites are rather conserved in many HBV genomes, the high heterogeneity of HBV [Medley et al., 2001; De Maddalena et al., 2007] still makes it possible that these sites change in other HBV genomes. For example, the Sap I site is present in some HBV genomes [Gunther et al., 1995] and two Spe I sites have been found in some genotype G HBV genomes [Hu et al., 2009], thus making corresponding methods not feasible in some situations. The Nco I site seems to be conserved highly among all HBV genomes; however, sequences around the Nco I site are not conserved (data not shown). That could be a problem for designing desirable primers. In contrast with these methods, the problems arisen from restriction digestion are circumvented totally in FSR. This feature would provide FSR with good flexibility in the construction of recombinant HBV DNA.
Furthermore, with its high efficiency of homologous fragment substitution, FSR provides a simple way of cloning clinical HBV isolates. When a plasmid template already contains HBV 1.1×, two rounds of FSR can generate a plasmid containing an HBV 1.1× derived completely from serum HBV DNA with two appropriate PCR fragments; e.g., one fragment nt 1,818–3,215–1,654 (from the start of the minus strand to a certain position downstream from the start of the plus strand) for the first FSR and the other fragment nt 1,654–1,988 (amplified using the plus strand as the template) for the second FSR. If a region of HBV DNA, such as a reverse transcriptase region, is to be investigated, one round of FSR would be sufficient to produce a plasmid containing that region derived from a serum HBV DNA, and all that must be done is to select a pair of primers whose sequences are identical in SF and TF. Although there is no appropriate restriction site, the HBV DNA fragments from the serum HBV can be cloned in a simple way and do not interrupt any of the elements or open reading frames necessary for HBV replication in vitro. This method is especially useful in the analysis of HBV drug resistance, where an assay for the effect of reverse transcriptase mutations on the replication capacity and drug susceptibility of HBV are most often conducted. To the best of our knowledge, this is the first study to report methods resembling FSR to generate constructs that can be used in an HBV susceptibility assay.
A potential disadvantage of this method is that artificial mutations can occur during FSR, because Taq DNA polymerase is used in the reaction. However, this disadvantage is limited for two reasons. One is that the FSR amplifies DNA in a linear manner, not in an exponential manner like traditional PCR. Therefore, the entire effective FSR product is synthesized using the initial plasmid as the template, unlike a PCR product, which is mainly composed of DNA amplified from newly synthesized template molecules after several cycles. Therefore, even if a mutation occurs in a certain cycle of FSR, the mutation will not accumulate in subsequent cycles, and the end product of FSR may still be made up of the correct molecule. The other reason is that the TF itself, as the mega-primer, need not be synthesized in FSR. When the TF is long enough, the major part of the newly synthesized molecule is the backbone of the plasmid template. Lethal mutations in the backbone of the plasmid will be excluded through transformation, and the mutations retained in the backbone have only a minor probability of significantly affecting the subsequent experiment. For the reason of sensitivity, the initial DNA fragments for FSR have to be obtained by PCR. At present, potential artificial mutations during PCR cannot be avoided completely. An effective way to decrease artificial mutations is to use high-fidelity DNA polymerase in PCR.
rtS246T Does not Confer Significant ETV Resistance in vitro
Two characteristics of the dynamics of the serum HBV DNA load of our patient prompted the investigation of the viruses in the patient's serum. First, his serum HBV load was never reduced to a level <104 copies/ml during 156 weeks of therapy (Fig. 1), representing a partial virological response to ETV, which is uncommon in nucleot(s)ide-naïve patients. Second, his viral load increased by more than one log10 during therapy (Fig. 1), whereas no typical ETV-resistant mutant was detected. One hypothesis to explain these characteristics is that the HBV strains infecting this patient may be primarily resistant to ETV to some extent. Sequence analysis of the HBV reverse transcriptase at different time points identified a variant at amino acid 246, at which position most genotype B HBV strains analysed have a serine, rather than the threonine observed in the strain studied here. However, a susceptibility test of the patient's HBV DNA indicated that the strain containing the reverse transcriptase sequence was not resistant to ETV. The two strains yielded IC50 values of 0.99 ± 0.09 and 0.58 ± 0.10 nM. These values are close to the ETV IC50 (1 nM) of the wild-type HBV reported by Levine et al. [2002] with a pCMV–HBV plasmid, and nearly fall within the IC50 range (0.6–10.3 nM) calculated when several decades of wild-type strains of different genotypes were tested [Baldick et al., 2008]. The susceptibility data suggest that the S246T variant does not confer resistance to ETV, and the primary resistance of HBV strains to ETV does not explain the characteristics of the viral load dynamics observed in our patient very well. The rtS246C substitution has been observed in another nucleot(s)ide-naïve ETV-treated patient [Colonno et al., 2006]. However, a phenotype assay of HBV containing amino acid 246C found no significant resistance to ETV. These data, together with the fact that rtS246N does appear in some wild-type genotype F and H strains, although not in the genotype B strains assayed (data not shown), suggest that position rt246 could be a polymorphic site unrelated to anti-viral resistance.
Another possible explanation for the partial response observed in our patient is that the patient did not administer ETV as prescribed. The concentrations of ETV in the patient's serum at different time points, were not determined, so the possibility of a poor compliance with physician-recommended treatment cannot be ruled out.
Acknowledgements
We thank Dr. Rongtuan Lin (McGill University, Canada) and Dr. Russell. A. Nicholson (Simon Fraser University, Canada) for their critical review of the manuscript.




