Prevalence of congenital cytomegalovirus infection in Slovenia: A study on 2,841 newborns
Abstract
Human cytomegalovirus (CMV) is the most frequent cause of congenital infection in humans. In the first prevalence study of congenital CMV infection in Eastern and Central Europe, all neonates born in a 22-month period in two Slovenian maternity units (total of 2,841 newborns) were screened prospectively for congenital CMV infection by a real-time polymerase chain reaction (PCR) in urine. In all newborns with positive screening results, plasma and dried blood spots (DBS) collected at birth were tested additionally for CMV DNA. Congenital CMV infection was confirmed by virus isolation from a urine sample collected within the first 2 weeks of life. Congenital CMV infection was identified in four out of 2,841 newborns tested (incidence 0.14%; 95% CI, 0.05–0.39%). In four newborns with confirmed congenital infection, the concentration of CMV DNA in urine ranged from 4.68 to 8.18 log10 copies/ml, all four newborns had detectable CMV DNA in plasma taken at birth (1.26–3.34 log10 copies/ml) and two out of four had detectable CMV DNA in DBS collected during newborn metabolic screening. None of the four newborns with confirmed congenital CMV infection was symptomatic. The study showed that the prevalence of congenital CMV infection at birth in Slovenia is among the lowest in the world and that CMV DNA PCR testing of urine is a suitable and affordable real-time screening strategy for congenital CMV infection. If it is performed in 24 mini-pools, the cost of screening is 1.4 €/newborn and the cost of detecting a single newborn with congenital CMV infection 1,000 €. J. Med. Virol. 84:109–115, 2011. © 2011 Wiley Periodicals, Inc.
INTRODUCTION
Human cytomegalovirus (CMV) is the most frequent cause of congenital infections, which can cause permanent disabilities such as hearing loss, vision loss, and mental retardation [Kenneson and Cannon, 2007]. Although congenital CMV infection is usually asymptomatic and undiagnosed at birth, its cumulative effect on morbidity and mortality is of considerable public health significance; for example, a recent study in the United States identified a total of 777 deaths associated with congenital CMV over the 17-year study period, resulting in 56,355 years of age-adjusted years of potential life lost [Bristow et al., 2011].
Congenital CMV infection is a leading non-genetic cause of sensorineural hearing loss in infants and children [American Academy of Pediatrics, 2007; Engman et al., 2008; Grosse et al., 2008]. Although sensorineural hearing loss can be detected by hearing screening of newborn, a substantial proportion of affected children are missed using this screening approach [Fowler et al., 1997; Foulon et al., 2008]. In contrast, virological screening of newborns for congenital CMV infection permits timely identification of children at increased risk of CMV-associated sensorineural hearing loss, allowing targeted monitoring of affected children in order to intervene at critical stages of acquiring speech and language skills [American Academy of Pediatrics, 2007; Boppana et al., 2010, 2011]. Conventional virus isolation from urine or saliva is still considered the “gold standard” for identification of newborns with congenital CMV infection, but the method is laborious, not amenable to automation and thus unsuitable for large-scale screening [Schleiss, 2008; Syggelou et al., 2010]. Molecular methods, such as the polymerase chain reaction (PCR), are considered currently to be the most appropriate methods for CMV screening of the newborn [Schlesinger et al., 2003; Paixão et al., 2005; Barbi et al., 2006; Boppana et al., 2010]. However, due to the diverse results obtained in recent major screening studies, the question of which clinical sample saliva, urine or dried blood spots (DBS), is best suited for large-scale neonatal CMV screening is still matter of intense debate [Dollard and Schleiss, 2010; Kharrazi et al., 2010; de Vries et al., 2010; Boppana et al., 2011; Cannon et al., 2011; Leruez-Ville et al., 2011].
The prevalence of congenital CMV infection at birth, defined as the number of CMV infected newborns divided by the total number of live-born infants [Kenneson and Cannon, 2007], varies in the published literature between 0.2% and 2.2% [Halwaschs-Baumann et al., 2000; Schlesinger et al., 2003; Barbi et al., 2006; Yamagashi et al., 2006; Engman et al., 2008; Inoue and Koyano, 2008; Soetens et al., 2008; Endo et al., 2009; Mussi-Pinhata et al., 2009; Boppana et al., 2010, 2011]. In contrast to high-income Western countries, reliable data on the prevalence of congenital CMV infection at birth in other parts of the world are quite scarce. In order to provide the first data on the prevalence of congenital CMV infection at birth in Slovenia, all neonates born in a 22-month period in two Slovenian maternity units were screened prospectively for the presence of CMV DNA in urine, using real-time PCR. This study is believed to be the first prevalence study of congenital CMV infection at birth performed on a representative newborn population in Eastern and Central Europe.
MATERIALS AND METHODS
Study Design
From March 13, 2007 to December 31, 2008, all neonates born in the maternity units of two Slovenian hospitals, General Hospital Slovenj Gradec, and General Hospital Murska Sobota, were included in the study. Three kinds of clinical specimens were collected from each newborn: urine, plasma, and DBS. Urine samples were collected during the first 3 days of life using the Pediatric Urine Collector (Dahlhausen, Köln, Germany) and stored in two 2 ml aliquots at 4°C until they were transported to the laboratory. Umbilical cord blood was collected in an EDTA-precoated sample tube and plasma was obtained and stored at 4°C until transportation to the laboratory. DBS were collected at the time of metabolic screening of newborns on the second or third day of life. Additional blood spots for CMV testing were collected on a separate filter paper, placed in individual envelopes and stored at room temperature in plastic resealable bags containing desiccant. All specimens (urine, plasma, and DBS) were shipped at least once weekly to the Institute of Microbiology and Immunology, Faculty of Medicine, University of Ljubljana, where all virological testing was performed.
All newborns included in the study were first screened for the presence of CMV DNA in urine samples. For neonates in whom CMV DNA was detected in the urine sample, PCR testing was also performed on the plasma specimen collected at birth and DBS sample collected at the time metabolic screening of newborns. Additionally, two of MRC-5 fibroblast shell vials were inoculated with 0.2 ml each of an independent urine sample. The shell vial culture was performed as described previously [Gleaves et al., 1984]. A confirmed congenital CMV infection was defined as identification of CMV by shell vial culture in urine sample(s) obtained during the first 2 weeks of life.
All neonates included in the study were examined clinically by a certified pediatrician following the standard procedure, which included Amiel-Tison neurological assessment, as described previously [Paro-Panjan et al., 2005] and transient-evoked otoacustic emissions assessment [Owens et al., 1993; American Speech Language Hearing Association, 2004] during the first 3 days of life. The study was approved by the Medical Ethics Committee of the Slovenian Ministry of Health (consent reference 105/01/07), and written informed consent was provided by the parents of all newborns included in the study.
DNA Extraction and CMV DNA Testing
DNA was extracted from 0.2 ml of urine using a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany), following the manufacturer's protocol for DNA isolation from blood or body fluids; DNA was eluted from the column with 50 µl of AE buffer. DNA was isolated from 0.4 ml of plasma using an EZ1 DSP Virus Kit (Qiagen), following the manufacturer's instructions; DNA was eluted with 60 µl of AVE buffer. For DBS samples, eight 2 mm discs were punched from half of a single DBS (diameter 10 mm) and DNA was extracted using a QIAamp DNA Mini Kit (Qiagen) following the manufacturer's protocol for DNA purification from DBS; DNA was eluted with 100 µl of AE buffer.
Quantitative detection of CMV DNA in urine, plasma, and DBS was performed using the commercial real-time PCR kit Artus CMV LC PCR Kit (Qiagen) on a Light Cycler 2.0 Instrument (Roche Applied Science, Mannheim, Germany). To control the efficiency of the DNA isolation procedure and to check for possible PCR inhibition, an internal control (CMV IC) supplied by the manufacturer was added to the lysis buffer, following the manufacturer's instructions. Analytical sensitivities of the assay at a 95% detection level for the detection of CMV DNA in urine, plasma, and DBS were 171.3 copies/ml, 78.9 copies/ml, and between 378 and 1,296 copies/ml, respectively.
RESULTS
According to data retrieved from the Perinatal Information System of the Republic of Slovenia, there were 37,906 live births in Slovenia between March 13, 2007 and December 31, 2008. Of them 2,893 children (1,472 boys and 1,421 girls) were born in the two maternity units that participated in the study. In Slovenj Gradec (SG) maternity unit, parents gave written informed consent for all of the 1,716 newborns to participate in the study, while in Murska Sobota (MS) maternity unit, parents of 35 out of 1,177 newborns declined to participate. In 17 newborns, the collection of urine samples failed, mainly due to logistic problems. In the end, 2,841 newborns were included in the study (Fig. 1), representing 7.6% of the total Slovenian neonate population born in the study period.
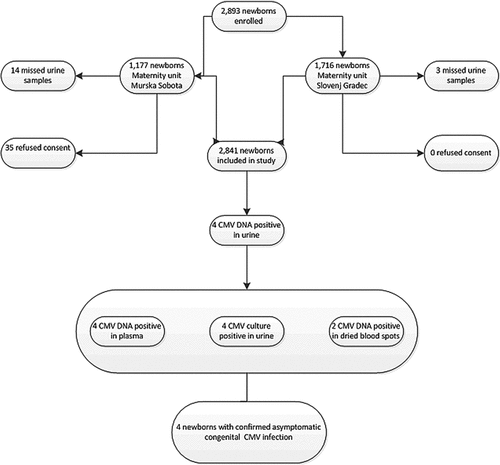
Flowchart of screening procedure for congenital CMV infection in two Slovenian maternity units.
Of the 2,841 neonates included in the study, 2,694 were born at term and 147 were premature. The mean birth weight in SG and MS maternity units was 3,350 ± 501 and 3,327 ± 450 g, respectively, and the mean gestational age was 38.9 ± 1.6 and 39.2 ± 1.5 weeks, respectively. There were no statistically significant differences in sex distribution, mean gestational age, or mean birth weight between the population of neonates included in the study and the total Slovenian population of neonates born in the study period.
The presence of CMV DNA in urine samples was detected in 4 out of 2,841 neonates tested. The concentration of CMV DNA in urine ranged from 4.68 to 8.18 log10 copies/ml. The characteristics of these four newborns and the results of their virological assessment are summarized in Table I, which also shows that CMV presence was confirmed by urine culture in all four newborns with a CMV DNA positive result in urine. The plasma samples of all four cases also tested positive for CMV DNA (concentration of CMV DNA 1.26–3.34 log10 copies/ml), while CMV DNA was detected in the DBS obtained from two out of four cases. The prevalence of congenital CMV infection in this study was thus 0.14% (95% confidence interval (CI), 0.05–0.39%).
| Patient no. | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Sex | Male | Male | Male | Female |
| Gestation age (weeks) | 39 | 40 | 40 | 39 |
| Birth weight (g) | 3,550 | 3,230 | 3,680 | 3,260 |
| Head circumference (cm) | 34 | 36 | 35 | 34 |
| Urine CMV DNA (log10 copies/ml) | 4.68 | 4.80 | 8.18 | 6.40 |
| Urine culture | Positive | Positive | Positive | Positive |
| Plasma CMV DNA (log10 copies/ml) | 2.22 | 1.26 | 3.34 | 2.26 |
| Dried blood spot CMV DNA | Negative | Negative | Positive | Positive |
| Complete blood count, liver enzymes, renal function | Normal | Normal | Normal | Normal |
| Neonatal cranial ultrasound | Normal | Normal | Hyperechogenic lesions in basal ganglia and thalamus | ND |
| Transient-evoked otoacustic emissions assessment | Normal | Normal | Normal | Normal |
| Brainstem evoked response audiometry | Right: 30 dB | Right: 40 dB | Right: 30 dB | ND |
| Left: 20 dB | Left: 20 dB | Left: 40 dB | ||
| Amiel-Tison neurological assessment | Normal | Palpable temporoparietal suture | Normal | ND |
- ND, not done, the infant was not available for follow-up assessment.
No symptomatic neonates with congenital CMV infection were born in either maternity unit during the study period. As summarized in Table I, all four neonates with confirmed congenital CMV infection were born at term, were appropriate for gestational age and had no evident clinical symptoms of congenital CMV infection, such as petechiae, hepatomegaly, or splenomegaly. No apparent hearing deficit was detected by transient-evoked otoacustic emission assessment in all four newborns. The neonates with confirmed congenital CMV infection were referred for further diagnostic evaluation to the Neonatal Unit of the University Children's Hospital Ljubljana and enrolled in the national program for neurological follow-up monitoring of children with congenital CMV infection [Paro-Panjan et al., 2005]. The results of additional tests performed on three neonates with confirmed congenital CMV infection are summarized in Table I. One of the four infants with confirmed congenital CMV infection was lost to follow-up, since the parents did not respond to repeated invitations for further diagnostic evaluation.
DISCUSSION
The prevalence of congenital CMV infection among 2,841 neonates born in a 22-month period in two Slovenian maternity units was found to be 0.14%, with a relatively wide CI (95% CI, 0.05–0.39%). This result is comparable with the prevalence established in similar prospective studies in the neighboring countries of Italy (0.18%; 95% CI, 0.09–0.26%) [Barbi et al., 2006] and Austria (0.21%) [Halwaschs-Baumann et al., 2000], and some other industrialized countries such as Sweden (0.20%; 95% CI, 0.1–0.3%) [Engman et al., 2008], Japan (0.17–0.19%) [Yamagashi et al., 2006; Endo et al., 2009] and the Netherlands (0.09%; 95% CI, 0.04–0.19%) [Gaytant et al., 2005]. Some other studies from the above mentioned countries showed higher prevalence of congenital CMV infection (Italy 0.47%; 95% CI, 0.22–1.00% [Barbi et al., 1998], Sweden 0.46%; 95% CI, 0.37–0.58% [Ahlfors et al., 1999], Japan 0.66%; 95% CI, 0.27–1.70% [Kamada et al., 1983] and 0.31%; 95% CI, 0.22–0.43% [Numazaki and Fujikawa, 2004]), however, due to their design they may suffer from selection bias. In contrast, it is rather unlikely that the relatively low prevalence of congenital CMV infection at birth established in this study is the result of selection bias, since no statistically significant differences in sex distribution, mean gestational age, or mean birth weight between the population of neonates included in the study and the total Slovenian population of neonates born in the study period were established, confirming that the study population is representative of the whole Slovenian newborn population. Alternative, although rather unlikely explanation for relatively low prevalence of congenital CMV infection at birth established in this study is the inability to detect cases of congenital CMV infection with CMV viral load in urine below the detection limit of PCR assay.
Molecular methods, such as PCR, are considered currently to be the most appropriate methods for CMV screening of newborns but the question of which clinical sample is best suited for large-scale neonatal CMV screening is still unresolved [Boppana et al., 2010, 2011; Vaudry et al., 2010; Cannon et al., 2011]. Although three clinical samples, urine, plasma, and DBS, were collected from each neonate for the present study, only urine was screened for CMV DNA due to financial constraints. An additional argument for a urine-only PCR-screening approach was that this sample in addition to saliva contains the highest quantity of CMV DNA in neonates infected congenitally, enabling the most sensitive screening [Halwachs-Baumann et al., 2002; Yamamoto et al., 2006; Inoue and Koyano, 2008]. According to published data, the present study is the third in which CMV DNA PCR testing in urine has been used to determine the prevalence of congenital CMV infection at birth. In similar previous studies, the prevalence at birth in Israel and Brasil were 0.7% and 1.1% (95% CI, 0.86–1.33%), respectively [Schlesinger et al., 2003; Mussi-Pinhata et al., 2009]. In the great majority of prevalence studies published to date, DBS have been used for PCR screening, prospectively or retrospectively [Barbi et al., 2006; Yamagashi et al., 2006; Engman et al., 2008; Boppana et al., 2010; Kharrazi et al., 2010]. PCR protocols for the detection of CMV DNA in DBS were first developed for retrospective diagnosis of congenital CMV infection in infants and children with hearing loss or other congenital CMV-related symptoms, which had not been diagnosed at birth [Johansson et al., 1997; Barbi et al., 2000, 2006; Yamamoto et al., 2001]. Subsequently, DBS has been proposed recently as an appropriate sample type for neonatal screening [Kharrazi et al., 2010; Leruez-Ville et al., 2011]. However, the only study that has evaluated the usefulness of DBS PCR for large-scale screening to date showed an unexpectedly low sensitivity of such approach in unselected newborns (sensitivity 34.4%; 95% CI, 18.6–53.2% for the two-primer DBS PCR assay) and raised the issue of whether the sensitivity of DBS PCR testing is adequate for screening a low-risk newborn population [Boppana et al., 2010]. It seems that the relatively low viral load present in DBS, particularly in asymptomatic newborns, contributed to the observed low sensitivity in this large screening trial. This presumption is supported by the significantly higher sensitivity of the DBS PCR approach observed in studies targeting high-risk populations such as symptomatic newborns (with higher viral loads) or in studies of infants with previously confirmed congenital CMV infection, in which the selection bias presumably leads to high sensitivity [Johansson et al., 1997; Barbi et al., 2000; Soetens et al., 2008; Atkinson et al., 2009; Leruez-Ville et al., 2011]. The suboptimal sensitivity of the DBS PCR approach was also observed in the present study, in which only half of the newborns with confirmed congenital CMV infection had positive PCR in their DBS samples (Table I). The main factors influencing the low sensitivity of the DBS PCR approach are a lower viral load present in the blood (also observed in this study), the size of DBS used for PCR testing, the choice of method used for DNA extraction and the PCR assay's analytical characteristics [Vaudry et al., 2010; Leruez-Ville et al., 2011]. Body fluids with a higher viral load, such as urine are presumably less affected when using one of the listed less efficient variables.
In addition to urine, saliva appears to be a very promising clinical specimen type for large-scale screening for congenital CMV infection. In a recent large-scale screening study performed on almost 35,000 newborns in the US the sensitivity and specificity of the liquid-saliva PCR assay for congenital CMV infection were 100% (95% CI, 95.8–100%) and 99.9% (95% CI, 99.9–100%), respectively, and the positive and negative predictive values were 91.4% (95% CI, 83.8–96.2%) and 100% (95% CI, 99.9–100%), respectively [Boppana et al., 2011]. Reasonable sensitivity and specificity were also obtained using a dried-saliva PCR approach. Non-invasive specimen collection, elimination of the DNA extraction step (providing considerable cost savings) and the fact that dried saliva specimens can be stored and transported at room temperature, are additional arguments for saliva-based screening [Boppana et al., 2011].
The price is one, if not the most important, of the parameters influencing the decision to carry out screening for serious medical conditions. Pooling samples is one of the approaches used most widely for reducing the cost of screening. A good example is pooling samples for the detection of blood-borne viruses, which has been used successfully for a decade in transfusion or non-transfusion settings [Allain, 2000; Roth et al., 2002; Mine et al., 2003; Stramer et al., 2004; Seme et al., 2011]. It has been demonstrated recently that urine pools can be used for PCR-based screening of congenital CMV infection without influencing the sensitivity [Paixão et al., 2005, 2011]. Previous studies and our limited experience indicate that median CMV viral loads detected in urine samples from newborns with congenital CMV infection were high enough to allow pooling of at least 24 urine samples without compromising sensitivity [Pass et al., 1983; Boppana et al., 2005; Yan et al., 2008; Cannon et al., 2011]. However, the positive experience with screening pooled samples for blood-borne viruses cannot be transferred simply to the CMV DNA urine screening of newborns, mainly due to lack of reliable data concerning the lower end of concentration of CMV DNA in urine at birth in newborns infected congenitally. Therefore, extensive well-designed studies are needed to verify the sensitivity, accuracy, and usefulness of screening pooled urine samples by PCR to detect congenital CMV infection. In order to gain an insight into the cost effectiveness of, for example, a 24 mini-pool CMV DNA screening strategy, a rough calculation of the approximate costs of such a strategy showed that considering the price of DNA extraction, PCR reagents, consumables and labor in Slovenia, the total cost for single urine CMV DNA PCR is currently 33 €, or would be 1.4 €/newborn if 24 mini-pool CMV DNA screening strategy were used. At the prevalence rate of congenital CMV infection at birth of 0.14% as determined in this study, the estimated cost of detecting a single newborn with congenital CMV infection would be 1,000 € (or between 359 and 2,800 € for the prevalence at the upper (0.39%) and lower (0.05%) end of 95% CI, respectively). In practice, this means that a single PCR performed once weekly would be enough to screen for congenital CMV infection all neonates born in an average size maternity unit in Slovenia.
In conclusion, the first prevalence study of congenital CMV infection in Eastern and Central Europe has shown a relatively low prevalence of congenital CMV infection in Slovenia. CMV DNA PCR testing of urine is a suitable, non-invasive and affordable real-time screening strategy for congenital CMV infection with great potential for pool testing.
Acknowledgements
We thank clinical colleagues and the whole staff at the Departments of Obstetrics and Gynecology and the Departments of Pediatrics at Slovenj Gradec and Murska Sobota General Hospitals for newborn recruitment and management. We are also grateful to Tina Močilnik for study monitoring, Robert Krošelj and Blanka Kušar for excellent laboratory assistance and Anja Oštrbenk for statistical and technical assistance.




