Viral load of the highly pathogenic avian influenza H5N1 virus in infected human tissues
Abstract
The highly pathogenic avian influenza A (H5N1) virus is a virulent virus that causes an acute febrile respiratory disease with high mortality in humans. To gain a better insight of H5N1 viral distributions in infected human tissues, the levels of viral RNA were determined in the autopsy tissues from two patients who were infected with H5N1 virus by using real-time reverse transcription-polymerase chain reaction. In one patient who died on day 6 of the illness, the viral load in the lung was extremely high, whereas the levels of viral RNA in the other organs were more than 6 log lower. In the other patient who died on day 17 of the illness, the viral load was similar in the lung and other organs, and was comparable to the viral load in the extra-pulmonary tissues of the first patient. These results suggested that while the H5N1 virus can cause disseminated infection in humans, the lung is still the major site of viral replication, and viral replication in the lung in the later stages may decrease as a result of the depletion of the available target cells. In addition, the mRNA levels of the tumor necrosis factor-α (TNF-α) were found to be associated with the viral titers. J. Med. Virol. 83:1418–1423, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Most influenza viruses replicate only in the respiratory and gastrointestinal tracts. This is because of the requirement of trypsin-like enzymes for the cleavage of the precursor hemagglutinin (HA) 0 molecule to produce functional HA1 and HA2 [Klenk et al., 1975; Lazarowitz and Choppin, 1975; Palese and Shaw, 2007]. Without cleavage by these enzymes which are present only in the respiratory and gastrointestinal tract, newly produced virions are not infectious [Zhirnov et al., 1984, 2002; Barbey-Morel et al., 1987; Kido et al., 1992]. The highly pathogenic H5N1 avian influenza viruses posses a highly cleavable HA cleavage site, which makes the molecule cleavable by furin, a ubiquitous enzyme [Stieneke-Grober et al., 1992; Horimoto et al., 1994; Klenk and Garten, 1994; Steinhauer, 1999; Guo et al., 2008]. Because of this, the H5N1 virus can cause disseminated infection in various avian and mammalian species [Lu et al., 1999; Keawcharoen et al., 2004; Kuiken et al., 2004; Govorkova et al., 2005; Thanawongnuwech et al., 2005; Amonsin et al., 2006; Songserm et al., 2006a,b]. However, in primates and humans, the infection has been shown to be limited more to the respiratory tract [Rimmelzwaan et al., 2001, 2003; Kuiken et al., 2003; Zhou et al., 2009]. Although viral RNA was found in various organs such as the intestine, heart, brain, spleen, kidneys, liver, lymph node, and placenta by reverse transcription-polymerase chain reaction (RT-PCR) and by in situ hybridization, viral antigen could be demonstrated only in the alveoli and a few other tissues [Uiprasertkul et al., 2005; Gu et al., 2007; Korteweg and Gu, 2008; Piwpankaew et al., 2010]. On the basis of these data, it was assumed that H5N1 virus replicated poorly in the extra-pulmonary tissues of humans [Uiprasertkul et al., 2005, 2007; Zhang et al., 2009]. In addition, strand-specific RT-PCR analyses of viral RNA from various tissues suggested that the presence of viral RNA in some tissues might be due to the contamination of virions and viral genomic RNA (vRNA) from the blood [Uiprasertkul et al., 2005] because viremia was demonstrated in patients infected with the H5N1 virus [de Jong et al., 2005; Chutinimitkul et al., 2006]. In accordance with this assumption, no or little inflammation was usually observed in the extra-pulmonary tissues, which tested positive for viral RNA [Uiprasertkul et al., 2005, 2007]. However, these findings were still not conclusive. Detailed quantitative data on viral RNA distribution in human tissues were not available. Therefore, the levels of the H5N1 viral RNA were determined in human tissues obtained from autopsy cases by using a quantitative real-time RT-PCR method.
MATERIALS AND METHODS
Samples
Autopsy tissues were derived from two patients who were infected with HPAI H5N1 virus. Patient A was a 48-year-old man who had progressive viral pneumonia. The diagnosis of avian influenza virus was made on day 4 of the illness, after his history of direct contact with dying chickens was revealed. Respiratory secretion was confirmed positive for influenza A (H5N1) virus. He died on day 6 of the illness [Uiprasertkul et al., 2007]. Patient B was a 6-year-old boy who had pneumonia and respiratory failure; the virological diagnosis of an H5N1 infection was made on day 7 of the illness. He was treated with oseltamivir and methylprednisolone from day 15 of his illness until his death on day 17 [Chokephaibulkit et al., 2005; Uiprasertkul et al., 2005]. The autopsy tissues of patient A were stored as fresh tissues at −80°C, whereas the autopsy tissues of patient B were stored as formalin-fixed, paraffin-embedded tissues at room temperature. A list of autopsy tissues used in this study is shown in Table I.
| Lung | Trachea | Lymph node | Liver | Spleen | Intestine | Heart | Bone marrow | Remark | |
|---|---|---|---|---|---|---|---|---|---|
| Patient A | + | + | − | + | + | − | − | + | Fresh tissue |
| Patient B | + | − | + | + | − | + | + | − | Formalin-fixed, paraffin-embedded tissue |
- +, Tissue available; −, tissue not available.
Tissue RNA Extraction
Formalin-fixed, paraffin-embedded tissue sections from patient B were deparaffinized with xylene, rehydrated with a graded ethanol series, and then washed in distilled water. The deparaffinized sections were treated with proteinase K. Total RNA from the proteinase K-treated sections or fresh tissues was extracted by using the Trizol reagent (Invitrogen, Carlsbad, CA) and then purified by using the QIAGEN RNeasy kit (Qiagen, Hilden, Germany), in accordance with the manufacturer's instructions. The extracted RNA was stored at −80°C until assayed.
Quantitative Real-Time RT-PCR
A real-time RT-PCR protocol for H5N1 established by the World Health Organization (WHO) [World Health Organization, 2007] and a real time RT-PCR for RNaseP established by the Centers for Disease Control and Prevention (CDC) [World Health Organization, 2009] were used. For standard HA RNA, a 1,732 bp PCR product of the full-length HA gene of A/Thailand/5(KK-494)/2004 (H5N1) was purified and cloned into the pGEM®-T easy plasmid (Promega, Madison, WI). The pGEM-HA plasmid was linearized for in vitro transcription. Thereafter, the HA RNA was generated from 1 µg of the linearized plasmid DNA by in vitro transcription by using MAXIscript® T7 (Ambion, Austin, TX). The HA RNA transcript was checked for quality by using 1% agarose gel electrophoresis and treated with TURBO DNase (Ambion) to remove the DNA template. Finally, the in vitro transcribed HA RNA was purified by using TRIzol® LS reagent (Invitrogen, Carlsbad, CA). The HA RNA transcript was stored at −80°C until assayed. Concentration of the HA RNA transcripts was measured by using the Nanodrop® spectrophotometer ND-1000, and the copies number was calculated. This standard RNA was serially diluted and used to set a standard curve for HA RT-PCR. The sequences of the primers and probes used in this study are shown in Table II. The amplification was carried out in the Light Cycler 2.0 (Roche Diagnostics, Berlin, Germany). The results were analyzed by using the Light Cycler Software. One microgram of RNA was used for real-time RT-PCR. For total viral RNA, random hexamers were used as the primers for reverse transcription. For plus and minus strands, reverse and forward PCR primers, respectively, were used for the reverse transcription.
| Primer and probe | Sequence (5′ → 3′) | Refs. |
|---|---|---|
| H5HA-205-227v2-Forward | CGA TCT AGA YGG GGT GAA RCC TC |
World Health Organization [2007 ] |
| H5HA-326-302v2-Reverse | CCT TCT CCA CTA TGT ANG ACC ATT C |
World Health Organization [2007 ] |
| H5-Probe-239-RVa1 | AGC CAY CCA GCT ACR CTA CA |
World Health Organization [2007 ] |
| RNaseP Forward | AGA TTT GGA CCT GCG AGC G |
World Health Organization [2009 ] |
| RNaseP Reverse | GAG CGG CTG TCT CCA CAA GT |
World Health Organization [2009 ] |
| RNaseP Probe2 | TTC TGA CCT GAA GGC TCT GCG CG |
World Health Organization [2009 ] |
| TNF-α Forward | GGC TCC AGG CGG TGC TTG TTC |
Blaschke et al. [2000 ] |
| TNF-α Reverse | AGA CGG CGA TGC GGC TGA TG |
Blaschke et al. [2000 ] |
| IP-10 Forward | GAG CCT ACA GCA GAG GAA CC |
Okamoto et al. [2008 ] |
| IP-10 Reverse | GAG TCA GAA AGA TAA GGC AGC |
Okamoto et al. [2008 ] |
- 1 TaqMan® probe is labeled at the 5′-end with the reporter molecule 6-carboxyfluorescein (FAM) and at the 3′-end with the quencher carboxytetramethylrhodamine (TAMRA).
- 2 TaqMan® probe is labelled at the 5′-end with the reporter molecule FAM and at the 3′-end with the quencher Blackhole Quencher 1 (BHQ1).
Tumor necrosis factor-α (TNF-α) and interferon-induced protein-10 (IP-10) mRNA were analyzed by real-time RT-PCR using the Light Cycler® FastStart DNA MasterPLUS SYBR Green I (Roche Diagnostics) on a Light Cycler 2.0 (Roche Diagnostics). IP-10 and TNF-α gene expression levels were normalized with RNaseP expression. The sequences of the primers used in this study are shown in Table II.
RESULTS
The amount of HA copy numbers in the autopsy tissues was obtained by the extrapolation of the cycle number of each RNA sample against the standard curve. The HA RNA was detected in the bone marrow, lung, and trachea of patient A, but there was no detectable viral RNA in the liver and spleen. The viral load in patient A tissues was the highest in the lung at 109 copies/g of tissue, whereas the viral load in the bone marrow and trachea was around 102 copies/g of tissue (Fig. 1A). The difference between the viral loads in the lung and the trachea of this patient confirms the tropism of the highly pathogenic H5N1 avian influenza virus for the alveolar epithelial cells of the lungs. In patient B, viral RNA was detected in all the autopsy tissues (heart, intestine, lymph node, lung, and liver), and the range of viral load in those tissues was 102–104 copies/cm3 of tissue (Fig. 1B). All the RNA extracts were positive for RNaseP as the control for RNA integrity (data not shown). To understand the nature of the viral RNA, strand-specific RT-PCR was performed by using specific reverse or forward primers in the RT step for plus or minus strand amplification, respectively. Relatively constant plus/minus strand ratios of 2/1 to 4/1 were observed in all the tissues, except for the bone marrow of patient A, where the plus strand was not detectable, and the lung of patient A, where the ratio was 0.3 (Fig. 2).
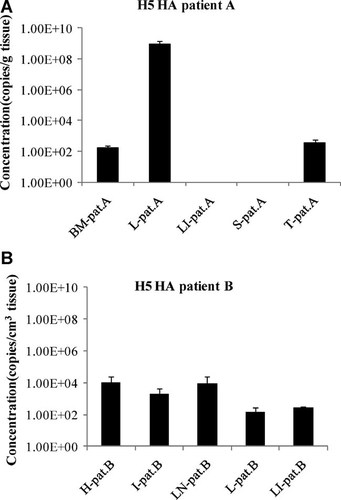
Real-time reverse transcription-polymerase chain reaction (RT-PCR) analysis of the HA copy number in two patients; patient A (fresh tissue) (A) and patient B (formalin-fixed, paraffin-embedded tissue) (B). BM-pat.A = bone marrow, patient A; L-pat.A = lung, patient A; LI-pat.A = liver, patient A; S-pat.A = spleen, patient A; T-pat.A = trachea, patient A; H-pat.B = heart, patient B; I-pat.B = intestine, patient B; LN-pat.B = lymph node, patient B; L-pat.B = lung, patient B; LI-pat.B = liver, patient B.
Ratio of plus (+) RNA strand to minus (−) RNA strand of patients A and B; sample abbreviations are as shown in Figure 1.
In both cases, severe inflammation and tissue damage were observed in the lungs, whereas only minimal non-specific changes were observed in the other organs [Uiprasertkul et al., 2005, 2007]. This suggested that low levels of viral infection in the extra-pulmonary tissues did not induce inflammation. Because hyperinduction of proinflammatory cytokines is believed to play an important role in the pathogenesis of the H5N1 influenza infection, it was investigated whether cytokine expression in those infected tissues correlated with the viral load and inflammation. TNF-α mRNA was detectable in high levels in the lung and spleen of patient A; whereas, it was undetectable in the bone marrow, liver, and trachea samples of the same patient. In patient B, TNF-α mRNA was detected in comparable levels in the heart, lung, liver, lymph node, and intestine (Fig. 3A). A different pattern was observed for IP-10 mRNA, which was detected in comparable levels in all the tested tissues from the same patient, although the overall levels in patient A appeared to be higher than those in patient B (Fig. 3B).
Real-time reverse transcription-polymerase chain reaction (RT-PCR) analysis of tumor necrosis factor-α (TNF-α) mRNA (A) and interferon-induced protein-10 (IP-10) mRNA (B) in two patients, patient A and patient B; sample abbreviations are as shown in Figure 1.
DISCUSSION
Although the H5N1 HPAI infection is a disseminated and systemic infection involving multiple organs in many avian and mammalian species [Lu et al., 1999; Keawcharoen et al., 2004; Kuiken et al., 2004; Govorkova et al., 2005; Thanawongnuwech et al., 2005; Amonsin et al., 2006; Songserm et al., 2006a,b], infections caused by this virus in primates and humans have been shown to be limited more to the respiratory tract [Rimmelzwaan et al., 2001, 2003; Kuiken et al., 2003; Uiprasertkul et al., 2005; Zhou et al., 2009]. The reason for this difference is unclear. Although previous reports have shown viral RNA in multiple organs in humans, their relative amounts were not known [Uiprasertkul et al., 2005; Gu et al., 2007; Korteweg and Gu, 2008; Piwpankaew et al., 2010]. In this study, the data clearly showed that in the acute phase, viral load in the lung was much higher than that in the other organs, and some organs were negative for viral RNA. Lower viral RNA levels in those extra-pulmonary tissues suggest that viral replication in those tissues was inefficient. Positive viral RNA in more organs in patient B suggests that viral dissemination was more extensive in the later stages. However, the comparable levels of viral RNA in the lung and other organs of patient B suggest that the viral load in the lung decreased in the later stages. This reduction was unlikely to be caused by the host immune response because the viral load in the other organs did not seem to be reduced. Pulmonary infection by the H5N1 virus can cause the massive apoptotic death of alveolar epithelial cells, which are the major target cells of the virus [Uiprasertkul et al., 2007]. Heavy infection in the early phase may burn out the target cells and cause a reduction in viral replication and viral load in the later phases.
The absence of positive-stranded viral RNA in the bone marrow of patient A suggests that the detected viral RNA in this tissue was because of the virions in circulation. The lower plus/minus strand ratio in the lung tissue of patient A suggests that the lung tissue, when actively infected, produced negative-stranded vRNA, and presumably virions, more efficiently than the other tissues, and the higher plus/minus strand ratios in the other tissues may indicate a less effective viral genome replication. The relatively higher ratio of positive-stranded viral RNA in those tissues suggests that the early phase of viral replication up to the synthesis of complementary RNA or mRNA was intact, while the later phase of vRNA synthesis may have been less effective.
Hyperinduction of proinflammatory cytokines such as TNF-α and IP-10 by the H5N1 avian influenza virus in the cell culture has been shown and considered a virulence factor of the virus [Cheung et al., 2002; Chan et al., 2005; Zhou et al., 2006]. Elevated levels of these cytokines in the sera of the H5N1 avian influenza patients were also observed [To et al., 2001; Peiris et al., 2004; de Jong et al., 2006]. The restriction of TNF-α expression to the lung and spleen of patient A suggests that this cytokine response was limited to the lung as the main target organ and the circulating immune cells in an acute phase, whereas the pattern of TNF-α expression in patient B suggests a widespread response in the later stages. It is surprising to see similar levels of IP-10 expression in all the tissues, regardless of the presence of viral RNA and inflammation. This suggests that IP-10 may not play a major role in the local inflammatory process.
Although the findings of the two cases were clearly different, and it is suggestive that the differences may be due to the phases of the infection, it is not yet conclusive as the differences could be due to other factors such as host variation, autopsy timing, and sample processing. Comparison of the absolute levels of RNA between the two cases should be therefore interpreted with care. However, the analyses and interpretations in this study relied mainly on the comparison of the viral RNA levels from the organs of the same patients, which underwent similar tissue handling processes. The data therefore demonstrate clearly that the lung is the main site for viral replication and TNF-α production in the acute phase of the H5N1 avian influenza infection in humans. Whether this can be generalized to most H5N1 avian influenza patients needs to be studied.




