Lack of association of RAD51 genetic variations with hepatitis B virus clearance and occurrence of hepatocellular carcinoma in a Korean population†
Conflict of Interest: The authors declare no conflicts of interest.
Abstract
The RecA homolog, E. coli (S. cerevisiae) (RAD51) may modulate hepatitis B virus (HBV) infection by maintaining genome integrity and mediating homologous DNA repairs. In this study, 16 sequence variations were detected by resequencing all exons, the exon–intron boundary, and promoter regions of the human RAD51 gene in DNA samples of 24 unrelated individuals. To investigate the association of common variations in the RAD51 locus with HBV infection and hepatocellular carcinoma (HCC) occurrence, six common polymorphisms were genotyped in a total of 1,103 Korean HBV cohort, composed of 433 spontaneously recovered patients as controls and 670 chronic carriers of HBV, who were stratified further into 327 cirrhosis/chronic hepatitis patients and 343 patients with HCC infected with HBV. Logistic analyses revealed no significant association of RAD51 polymorphisms and haplotypes with HBV clearance and HCC occurrence (P > 0.05). Furthermore, with age of infection as an important factor in disease progression to HCC, results from the Cox proportional hazards analysis showed no significant associations between any of the tested RAD51 variants and the age of onset of HCC (P > 0.05), suggesting that genetic polymorphisms of RAD51 may not play an important role in clearance of HBV and disease progression to HCC. Although studies in other populations are needed to confirm these findings, this preliminary data may contribute to the current knowledge on the pathogenesis of hepatitis. J. Med. Virol. 83:1892–1899, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Hepatitis B virus (HBV) infection is a potentially life-threatening condition. Persistent HBV infection is often associated with chronic liver diseases such as chronic hepatitis and cirrhosis that leave the patient at risk for hepatocellular carcinoma (HCC) [Beasley et al., 1981], conditions that are estimated to result in 500,000–700,000 deaths each year. According to the World Health Organization (WHO), estimates of the prevalence of HBV infection are around 2 billion people worldwide with more than 360 million cases of persistent infection. Humans and the higher apes are the only species susceptible to HBV [Tong et al., 1999], and there is a continuing need to develop efficient drugs and other intervention strategies for viral clearance.
Restoration of damaged DNA requires action of certain DNA-repairing enzymes that are necessary for maintaining genomic integrity and preventing neoplastic cellular transformation [Charames and Bapat, 2003]. Homologous recombinational repair is crucial for protecting chromosomes against endogenous damage affecting both DNA strands, such as double-strand breaks. The human RecA homolog, E. coli (S. cerevisiae) (RAD51) gene encodes a protein that promotes the key reaction in homologous recombinational repair by mediating ATP-dependent homologous pairing [Benson et al., 1994; Vispe and Defais, 1997], forming nucleoprotein filaments on tailed duplex DNA substrates that mimic the 3′-overhanging single-stranded DNA (ssDNA) tails to initiate strand exchange reaction between single- and double-stranded DNA during repair [Golub et al., 1998], and binding strongly to the Holliday junction for homologous recombination and replication structures [Ouyang et al., 2009; Compton et al., 2010]. In vitro studies have demonstrated that RAD51 binds DNAs at double-strand break sites and catalyzes homologous pairing and strand transfer [Benson et al., 1994; Baumann et al., 1996]. Furthermore, depletion of the human RAD51C, a RAD51 prolog, has been implicated in impaired homologous recombinational repair of chromosomal double-strand breaks and destabilized XRCC3, a DNA repair gene [Lio et al., 2004]. These results provide further evidence of the significant influence of RAD51 in promoting genomic stability.
As double-strand break sites are the specific targets for HBV X protein integration in inducing chronic infection and progression to cirrhosis and HCC [Capovilla et al., 1997; Bill and Summers, 2004], it was hypothesized that RAD51 polymorphisms are mechanisms for HBV clearance through homologous recombination-related repair of double-strand breaks.
MATERIALS AND METHODS
Study Patients
To serve as primary patients for the case–control study, a total of 1,103 individuals having either past or present evidence of HBV infection were recruited from patients enrolled in the outpatient clinic of the liver unit and the Center for Health Promotion at Seoul National University Hospital. Diagnoses of chronic carriers and spontaneously recovered patients were established by repeated seropositivity for the hepatitis B surface antigen (HBsAg; Enzygnost1 HBsAg 5.0; Dade Behring, Marburg, Germany) over a 6-month period, and for both anti-HBs (antibody to hepatitis B surface antigen; Enzygnost Anti-HBs II; Dade Behring) and anti-HBc (antibody to hepatitis B core antigen; AB-Corek; DiaSorin s.r.l., Saluggia, Italy) of the IgG type without HBsAg, respectively. The chronic carrier group consisted of patients who were HBsAg-positive for over 6 months and were assessed further for disease progression to cirrhosis or HCC. All of the patients in the chronic carrier group had undergone regular medical follow-ups and had been evaluated with serum alpha-fetoprotein level, abdominal ultrasonography, and/or a 2-phase spiral liver CT scan more than twice a year to detect early stages of HCC. Abdominal MRI, bone scan, chest CT, brain MRI, brain CT, hepatic angiography, or PET scan was also carried out in some patients based on the clinical results. HCC was diagnosed by pre-existing cirrhosis with a liver mass that is greater than 2 cm in diameter and further pathological assessment as described previously [Ryder, 2003]. Cirrhosis of the liver, on the other hand, was diagnosed pathologically or based on the clinical evidence of portal hypertension such as visible collateral vessels on the abdominal wall, esophageal varices on esophagogastroscopy, palpable splenomegaly, and sonographically definite findings of cirrhotic liver or ascites. The age of onset of HCC was determined according to the date of initial diagnosis.
Exclusion criteria for the study patients included the following: (i) tested positive for anti-HBs but not for anti-HBc; (ii) tested positive for anti-HCV or anti-HIV (GENEDIA®; Greencross Life Science Corp., Yongin-shi, Korea, HCV®3.2; Dong-A Pharmaceutical Co., Seoul, Korea); (iii) average alcohol consumption of ≥10 g/day or an average number of ≥1 cigarette pack/s smoked daily assessed through individual interviews; and (iv) incurrence of other types of liver diseases such as autoimmune hepatitis, toxic hepatitis, primary biliary cirrhosis, or Budd-Chiari syndrome. None of the patients had a previous history of immunosuppressant or anti-viral treatment. Finally, written consent was secured from the patients prior to conducting the study, and ethical approval was obtained from the Institutional Review Board for Human Research at Seoul National University Hospital.
Resequencing, Genotyping of RAD51 Polymorphisms, and Haplotype Construction
Using the Wizard genomic DNA purification kit (Promega, Madison, WI), genomic DNA was extracted from peripheral blood samples. Fifteen primer sets for PCR amplification (Supplementary Table I) were designed based on GenBank accession for the RAD51 gene (NM_002875.4) derived from reference sequence AC012476.8. All exons, the exon–intron boundary, and promoter regions (∼2 kb) of the RAD51 gene were analyzed by resequencing DNA samples of 24 unrelated healthy Korean individuals using the ABI 3730×l automated sequencer (Applied Biosystems, Foster City, CA). The sequence variants were then verified by chromatograms. SNP genotyping was performed using the TaqMan assay in the ABI prism 7900HT sequence detection system (Applied Biosystems). With an average call rate of 99.9%, a total of six common SNPs in the RAD51 gene were successfully genotyped in 1,103 Korean HBV patients. Using the PHASE algorithm, version 2.0 [Stephens et al., 2001], haplotypes were then inferred from the genotyped SNPs.
Statistical Analyses
To determine the association of RAD51 variations with HBV clearance and HCC occurrence, the odds ratio (95% confidence interval) was calculated using logistic analysis adjusted for age (continuous value) and sex (male = 0, female = 1) as covariates to eliminate or reduce any confounds that might influence the findings. Data were managed and analyzed using the Statistical Analysis System (SAS) version 9.1 (SAS Inc., Cary, NC). To demonstrate the relationship between RAD51 variants and the age at which HCC was diagnosed, Cox proportional hazards were used for relative risk analysis, controlling for sex (male = 0; female = 1), age (0 for age < 40; 1 for 40 ≤ age < 60; 2 for age ≥ 60), cirrhosis of the liver (with cirrhosis = 0, no cirrhosis = 1), and HBsAg (negative = 0; positive = 1) as covariates. Lewontin's D′ (|D′|) and the linkage disequilibrium (LD) coefficient r2 were then examined using the Haploview algorithm [Barrett et al., 2005] to measure the LD between all pairs of biallelic loci.
To achieve optimal correction for multiple testing of SNPs that are in LD, the effective number of independent marker loci in RAD51 (4.0639) was calculated using SNPSpD (http://genepi.qimr.edu.au/general/daleN/SNPSpD/), a program that is based on the spectral decomposition (SpD) of matrices of pair-wise LD between SNPs [Nyholt, 2004].
RESULTS
Characteristics of the Study Patients
Out of a total of 1,103 patients of Korean ethnicity infected with HBV, who were recruited for the case–control study, 670 were identified as chronic carrier cases, and 433 were categorized as spontaneously recovered controls according to serological markers. The case group was further evaluated for disease progression and was classified into 343 HCC patients and 327 cirrhosis/chronic hepatitis-infected patients according to results of the medical examination. The clinical profiles of the study patients included a mean age of 54.9 for the spontaneously recovered group, 49.8 for HCC patients, and 58.3 for the cirrhosis/chronic hepatitis group. Results of the repeated seropositivity test for HBsAg and for both anti-HBs and anti-HBc of the IgG type without HBsAg, as well as the clinical characteristics of the study patients, are summarized in Table I.
| Clinical profile | Spontaneously recovered | Chronic carrier | |
|---|---|---|---|
| Hepatocellular carcinoma | Cirrhosis of the liver/chronic hepatitis | ||
| No. of patients | 433 | 343 | 327 |
| Age [mean (range)] | 54.9 (28–79) | 49.8 (22–85) | 58.3 (25–79) |
| Sex (male/female) | 240/193 | 278/65 | 279/48 |
| HBeAg (positive rate, %) | 0 | 19.6 | 33.2 |
| HBeAb (positive rate, %) | 38.1 | 64.2 | 47.1 |
| HBsAg (positive rate, %) | 0 | 100 | 100 |
| HBsAb (positive rate, %) | 100 | 0 | 0 |
- Each clinical profile from the chronic carrier group was compared to the respective spontaneously recovered controls. Age was measured from the first medical examination.
Resequencing, Genotyping of RAD51 Polymorphisms, and Haplotype Analysis
By resequencing DNA samples of 24 patients, 16 sequence variants were identified in the RAD51 gene; three were localized in the promoter region, two in the 5′-UTR, three in the intron, six in the 3′-UTR, and two in the 3′ near region (Fig. 1A and Supplementary Table II). From the identified variants, six common SNPs were selected for larger-scale genotyping based on location, minor allele frequency (MAF > 0.05; Table II), and LD. Among these polymorphisms, five SNPs (−4720T/A, −3429G/C, +32922G/T, +33347C/T, and +33349A/G) were found in the regulatory regions and one SNP (+7386G/T) with the highest frequency was positioned in the intronic region of the gene. Two common SNPs, +33533C/G and +33552C/G in the 3′ near region, were excluded from genotyping due to their location and complete LD (r2 = 1) with +33349A/G and +32922G/T, respectively (Fig. 1A). Results from genotyping showed that not all samples were amplified for all SNPs. Using the genotyped SNPs, six major haplotypes with frequencies over 0.05 (Fig. 1B) were obtained and analyzed for possible association with HBV clearance and HCC occurrence. The haplotypes were contained in two LD blocks (Fig. 1C) that were established from pair-wise comparisons of the six genotyped SNPs.
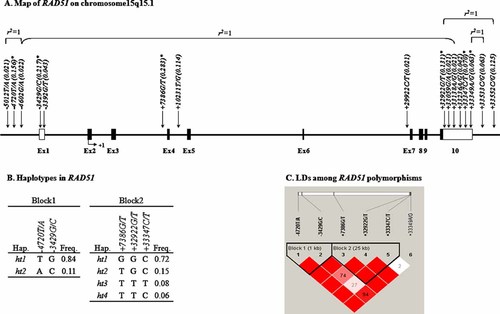
Physical map, haplotypes, and LD of the RAD51 gene. A: Schematic gene map and SNPs in the RAD51 gene on chromosome 15q15.1 (39 kb). Black blocks represent coding exons and white blocks represent 5′- and 3′-UTR. The first base of translation site is denoted as nucleotide +1. Absolute linkage between −5012T/A, −4602G/A, and +33138A/G is indicated by a bracket (r2 = 1). Asterisks (*) indicate SNPs that have been genotyped and included in the association analyses. B: Haplotypes of RAD51. C: LD coefficient (|D′|) among RAD51 SNPs. Numbers in blocks represent values of multiallelic D′. Shades of red/pink indicate LOD ≥ 2, whereas white blocks indicate LOD < 2 (D′ < 1).
| Loci | rs# | Position | Genotype | HWE* | Heterozygosity | MAF | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Korean | Caucasian | Chinese | Japanese | African | |||||||||
| −4720T/A | rs2619679 | Promoter | TT | TA | AA | N | 0.117 | 0.263 | 0.156 | 0.531 | 0.186 | 0.186 | 0.623 |
| 779 | 271 | 33 | 1083 | ||||||||||
| −3429G/C | rs1801320 | 5′-UTR | GG | GC | CC | N | 0.366 | 0.195 | 0.109 | 0.062 | 0.133 | 0.146 | 0.238 |
| 812 | 193 | 15 | 1020 | ||||||||||
| +7386G/T | rs45457496 | Intron3 | GG | GT | TT | N | 0.080 | 0.406 | 0.283 | — | — | — | — |
| 570 | 418 | 99 | 1087 | ||||||||||
| +32922G/T | rs12593359 | 3′-UTR | GG | GT | TT | N | 0.732 | 0.228 | 0.131 | 0.535 | 0.161 | 0.150 | 0.650 |
| 820 | 245 | 20 | 1085 | ||||||||||
| +33347C/T | rs11855560 | 3′-UTR | CC | CT | TT | N | 2.18E−08 | 0.132 | 0.070 | 0.540 | 0.168 | 0.170 | 0.654 |
| 929 | 114 | 17 | 1060 | ||||||||||
| +33349A/G | rs45507396 | 3′-UTR | AA | AG | GG | N | 0.715 | 0.123 | 0.066 | — | — | — | — |
| 953 | 136 | 4 | 1093 | ||||||||||
- The MAFs of Caucasian, Chinese, Japanese, and African patients were obtained from the International HapMap and dbSNP databases.
- MAF, minor allele frequency.
- * P-values for Hardy–Weinberg equilibrium (HWE).
Associations of SNPs in RAD51 With HBV Clearance and HCC Occurrence
Results from logistic analysis of the differences in frequency distribution of RAD51 variants between chronic carriers and spontaneously recovered patients adjusted for age and sex as covariates showed that RAD51 polymorphisms are not significantly associated with HBV clearance via co-dominant, dominant, and recessive genetic models after multiple testing corrections (Pcorr > 0.05, Table III). Similarly, no association between RAD51 haplotypes and clearance of HBV was detected (Pcorr > 0.05, Table III).
| Locus | Position | Genotype | Chronic carrier (%) | Spontaneously recovered (%) | Co-dominant | Dominant | Recessive | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P* | Pcorr** | OR (95%CI) | P* | Pcorr** | OR (95%CI) | P* | Pcorr** | |||||
| −4720T/A | Promoter | TT | 476 (73.34) | 301 (69.68) | 0.90 (0.71–1.14) | 0.39 | — | 0.77 (0.58–1.02) | 0.07 | — | 2.48 (1.03–5.97) | 0.04 | NS |
| TA | 147 (22.65) | 124 (28.70) | |||||||||||
| AA | 26 (4.01) | 7 (1.62) | |||||||||||
| −3429G/C | 5′-UTR | GG | 473 (80.44) | 337 (78.37) | 0.97 (0.72–1.29) | 0.81 | — | 0.83 (0.60–1.14) | 0.25 | — | 12.53 (1.58–99.47) | 0.02 | NS |
| GC | 101 (17.18) | 92 (21.40) | |||||||||||
| GG | 14 (2.38) | 1 (0.23) | |||||||||||
| +7386G/T | Intron3 | GG | 344 (52.60) | 224 (51.97) | 1.06 (0.87–1.29) | 0.56 | — | 1.02 (0.79–1.31) | 0.90 | — | 1.29 (0.82–2.04) | 0.27 | — |
| GT | 245 (37.46) | 173 (40.14) | |||||||||||
| TT | 65 (9.94) | 34 (7.89) | |||||||||||
| +32922G/T | 3′-UTR | GG | 495 (76.04) | 323 (74.77) | 0.95 (0.73–1.23) | 0.68 | — | 0.87 (0.65–1.17) | 0.35 | — | 2.38 (0.77–7.43) | 0.13 | — |
| GT | 140 (21.51) | 105 (24.30) | |||||||||||
| TT | 16 (2.46) | 4 (0.93) | |||||||||||
| +33347C/T | 3′-UTR | CC | 549 (87.84) | 378 (87.30) | 1.01 (0.73–1.41) | 0.94 | — | 0.91 (0.62–1.35) | 0.65 | — | 2.31 (0.71–7.47) | 0.16 | — |
| CT | 63 (10.08) | 51 (11.78) | |||||||||||
| TT | 13 (2.08) | 4 (0.92) | |||||||||||
| +33349A/G | 3′-UTR | AA | 558 (87.19) | 375 (86.61) | 0.93 (0.65–1.33) | 0.68 | — | 0.88 (0.60–1.28) | 0.50 | — | — | — | — |
| AG | 78 (12.18) | 58 (13.39) | |||||||||||
| GG | 4 (0.63) | 0 (0.00) | |||||||||||
| BL2_ht2 | −/− | 467 (72.85) | 312 (72.22) | 1.11 (0.86–1.43) | 0.44 | — | 1.07 (0.80–1.43) | 0.64 | — | 1.78 (0.71–4.46) | 0.22 | — | |
| ht2/− | 159 (24.81) | 112 (25.93) | |||||||||||
| ht2/ht2 | 15 (2.34) | 8 (1.85) | |||||||||||
| BL2_ht4 | −/− | 573 (89.39) | 378 (87.50) | 0.75 (0.51–1.12) | 0.16 | — | 0.75 (0.51–1.12) | 0.16 | — | — | — | — | |
| ht4/− | 68 (10.61) | 54 (12.50) | |||||||||||
| ht4/ht4 | 0 (0.00) | 0 (0.00) | |||||||||||
- Values significant at P < 0.05 are shown in bold.
- BL1-ht1, BL1-ht2, BL2-ht1, and BL2-ht3 were equivalent with −4720T/A, −3429G/C, +7386G/T, and +33347C/T, respectively.
- Logistic regression models were used to calculate odds ratios (95% CI).
- OR, odds ratio; CI, confidence interval; NS, not significant.
- * P-values for logistic analyses controlling for age (continuous value) and sex (male = 0, female = 1) as covariates in HBsAg-positive patients, assuming co-dominant, dominant, and recessive models.
- ** P-values after multiple testing corrections (4.0639).
Because persistent HBV infection is often associated with progression to HCC, the association between RAD51 and HCC occurrence was investigated further. Results from logistic analysis, however, also revealed a lack of association between RAD51 variants and HCC occurrence (P > 0.05, Table IV). Considering the fact that age of infection strongly affects disease progression to HCC, statistical analysis of association between this variable and RAD51 variants was performed using Cox proportional hazards. Similarly, none of the variants was significantly associated with age at onset of HBV infection (P > 0.05, Table V).
| Locus | Position | Genotype | Hepatocellular carcinoma (%) | Cirrhosis of the liver/chronic hepatitis (%) | Co-dominant | Dominant | Recessive | |||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P* | OR (95%CI) | P* | OR (95%CI) | P* | |||||
| −4720T/A | Promoter | TT | 228 (72.84) | 245 (74.24) | 0.98 (0.69–1.38) | 0.89 | 1.01 (0.66–1.54) | 0.97 | 0.80 (0.31–2.05) | 0.64 |
| TA | 74 (23.64) | 71 (21.52) | ||||||||
| AA | 11 (3.52) | 14 (4.24) | ||||||||
| −3429G/C | 5′-UTR | GG | 237 (83.15) | 232 (78.11) | 0.79 (0.52–1.21) | 0.28 | 0.71 (0.42–1.17) | 0.18 | 1.06 (0.31–3.63) | 0.93 |
| GC | 42 (14.74) | 58 (19.53) | ||||||||
| GG | 6 (2.11) | 7 (2.36) | ||||||||
| +7386G/T | Intron3 | GG | 162 (51.10) | 182 (54.98) | 1.07 (0.81–1.41) | 0.65 | 1.22 (0.84–1.78) | 0.30 | 0.80 (0.43–1.49) | 0.49 |
| GT | 123 (38.80) | 117 (35.35) | ||||||||
| TT | 32 (10.10) | 32 (9.67) | ||||||||
| +32922G/T | 3′-UTR | GG | 232 (74.60) | 260 (77.84) | 0.94 (0.65–1.37) | 0.75 | 1.03 (0.67–1.60) | 0.88 | 0.41 (0.12–1.38) | 0.15 |
| GT | 74 (23.79) | 64 (19.16) | ||||||||
| TT | 5 (1.61) | 10 (2.99) | ||||||||
| +33347C/T | 3′-UTR | CC | 263 (88.26) | 282 (87.85) | 0.85 (0.54–1.36) | 0.50 | 0.96 (0.54–1.70) | 0.88 | 0.36 (0.10–1.33) | 0.12 |
| CT | 31 (10.40) | 31 (9.66) | ||||||||
| TT | 4 (1.34) | 8 (2.49) | ||||||||
| +33349A/G | 3′-UTR | AA | 264 (86.56) | 290 (88.15) | 0.89 (0.54–1.50) | 0.67 | 0.91 (0.52–1.58) | 0.73 | 0.57 (0.05–6.15) | 0.64 |
| AG | 40 (13.11) | 36 (10.94) | ||||||||
| GG | 1 (0.33) | 3 (0.91) | ||||||||
| BL2_ht2 | −/− | 229 (72.24%) | 241 (73.70) | 1.16 (0.79–1.69) | 0.45 | 1.21 (0.79–1.87) | 0.38 | 0.99 (0.29–3.35) | 0.99 | |
| ht2/− | 81 (25.55) | 78 (23.85) | ||||||||
| ht2/ht2 | 7 (2.21) | 8 (2.45) | ||||||||
| BL2_ht4 | −/− | 280 (88.33) | 297 (90.83) | 1.18 (0.64–2.18) | 0.60 | 1.18 (0.64–2.18) | 0.60 | — | — | |
| ht4/− | 37 (11.67) | 30 (9.17) | ||||||||
| ht4/ht4 | 0 (0.00) | 0 (0.00) | ||||||||
- BL1-ht1, BL1-ht2, BL2-ht1, and BL2-ht3 were equivalent with −4720T/A, −3429G/C, +7386G/T, and +33347C/T, respectively.
- Logistic regression models were used to calculate odds ratios (95% confidential interval).
- * P-values for logistic analyses controlling for age (continuous value) and sex (male = 0, female = 1) as covariates in HBsAg-positive patients, assuming co-dominant, dominant, and recessive models.
| Locus | Position | Co-dominant | Dominant | Recessive | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n/event | x2 | RH | P* | n/event | x2 | RH | P* | n/event | x2 | RH | P* | ||
| −4720T/A | Promoter | 643/306 | 0.152 | 1.042 | 0.70 | 643/306 | 0.321 | 1.076 | 0.57 | 643/306 | 0.042 | 0.938 | 0.84 |
| −3429G/C | 5′-UTR | 582/278 | 0.917 | 0.875 | 0.34 | 582/278 | 1.112 | 0.842 | 0.29 | 582/278 | 0.035 | 0.924 | 0.85 |
| +7386G/T | Intron3 | 648/310 | 0.019 | 1.012 | 0.89 | 648/310 | 0.007 | 1.009 | 0.93 | 648/310 | 0.029 | 1.033 | 0.86 |
| +32922G/T | 3′-UTR | 645/304 | 0.039 | 1.023 | 0.84 | 645/304 | 0.269 | 1.072 | 0.60 | 645/304 | 0.671 | 0.690 | 0.41 |
| +33347C/T | 3′-UTR | 619/291 | 0.003 | 1.008 | 0.96 | 619/291 | 0.189 | 1.083 | 0.66 | 619/291 | 0.645 | 0.667 | 0.42 |
| +33349A/G | 3′-UTR | 634/298 | 0.013 | 0.982 | 0.91 | 634/298 | 0.0001 | 1.002 | 0.99 | 634/298 | 0.333 | 0.559 | 0.56 |
| BL2_ht2 | 635/301 | 0.162 | 0.954 | 0.69 | 635/301 | 0.146 | 0.951 | 0.70 | 635/301 | 0.046 | 0.920 | 0.83 | |
| BL2_ht4 | 635/301 | 0.243 | 1.093 | 0.62 | 635/301 | 0.243 | 1.093 | 0.62 | 635/301 | — | — | — | |
- BL1-ht1, BL1-ht2, BL2-ht1, and BL2-ht3 were equivalent with −4720T/A, −3429G/C, +7386G/T, and +33347C/T, respectively.
- The co-dominant model of the Cox relative hazards analyses was used to calculate relative hazards (RH) and P-values for SNPs and haplotypes controlling for sex, adjusted age (age < 40, adj. age = 0; 40 ≤ age < 60, adj. age = 1; age > 60, adj. age = 2), cirrhosis of the liver (cirrhosis = 0, no cirrhosis = 1), and HBeAg (negative = 0, positive = 1) in HBsAg-positive patients.
- * P-values for Cox relative hazards analyses.
DISCUSSION
Although HBV infection generally remains a major health problem worldwide, the prevalence in each region differs according to varying levels of endemicity as determined by HBsAg positivity, rate of infection, and age at which infection was acquired [Zanetti et al., 1993]. The modes of viral infection in highly endemic areas, such as Korea, include perinatal viral acquisition and early childhood infection transmission [Ahn, 1996]. In previous epidemiological studies, it was found that 6.5% of pregnant Korean women were HBsAg positive and as a result of mother–infant transmission, 16.4% of infants born to these women had perinatal HBV infection with an increasing trend as their age increases [Ahn, 1996]. This rate is especially alarming because HBV is a predisposing factor in some of the most prevalent chronic liver diseases, including HCC, cirrhosis, and chronic hepatitis. Findings from this study, therefore, may contribute to understanding of HBV pathophysiology, especially in the Korean population.
DNA repair pathways are thought to play a role in the early phase of viral clearance during HBV infection [Valerie and Povirk, 2003]. As the primary initiator of infection, the HBV X protein carries out its function by integrating with damaged DNA such as double-strand breaks [Capovilla et al., 1997; Bill and Summers, 2004]. Repair of double-strand breaks is mediated by both nonhomologous end-joining repair and homologous recombinational repair pathways through interplay of numerous genetic mechanisms [Valerie and Povirk, 2003]. The human RAD51 gene promotes pairing and exchange of strands between homologous DNA molecules during homologous recombinational repair [Benson et al., 1994; Vispe and Defais, 1997; Golub et al., 1998] and has been reported to work in conjunction with genes that are implicated in repair of DNA breaks, such as BRCA1 and BRCA2 [Ferguson et al., 1997; Wiese et al., 2002; Cousineau et al., 2005], leading to maintenance of genome integrity and prevention of double-strand break-specific HBV integration. These insights underpin the possible clinical relevance of RAD51 in HBV clearance and HCC progression.
In this case–control study, 16 SNPs were identified initially by resequencing (Build 126 of the public NCBI database). The identified sequence variants, however, have been reported previously in the current version of Build 131 (Supplementary Table II). From the identified resequenced variants, differences in the distributions of six genotyped SNPs and six major haplotypes were used to detect a relationship between RAD51 and HBV clearance as well as HCC occurrence in a Korean HBV cohort. Among the loci in this study, +33747C/T showed serious deviation from Hardy–Weinberg equilibrium (HWE) (P = 2.18E−08). To decipher the reason for the departure from HWE, we checked possible factors that could influence HWE in a population, including genotyping error and selection bias, but no obvious causes were observed. Further genotyping in different populations might be needed. Results from co-dominant, dominant, and recessive genetic modes of analyses revealed that RAD51 genetic variations are not associated with the risk of HBV clearance in a Korean population. Similarly, results failed to detect a statistically significant association signal between RAD51 variants and HCC occurrence as well as age at which HCC was diagnosed. Taken together, these data provide evidence that genetic variants of RAD51 do not affect clearance of HBV and progression to HCC.
Previous case–control studies have revealed a significant relationship between RAD51 and several types of cancer [Connell et al., 2006; Ko et al., 2008; Mitra et al., 2009; Willers et al., 2009] but no association with HBV infection. In fact, the G/C allele of the −3429G/C SNP (which corresponds to the 135G/C variant in previous reports) identified in this study has already been implicated in breast and ovarian cancer [Levy-Lahad et al., 2001; Wang et al., 2001; Jakubowska et al., 2003, 2007] with minor discrepancies among the findings. After comparing data from various ethnic groups using information from the International HapMap and dbSNP databases, a similar trend in the allelic frequencies of the analyzed variants was observed in Asian populations including Korean, but this trend differed from that in Caucasian and African cohorts. The MAF of +33347C/T is particularly low in the Korean population compared to other ethnic groups, including Chinese and Japanese populations (Table II). These findings suggest that further replications in other populations might provide evidence of more convincing relationships between the analyzed variants and HBV-related diseases. Differences in the frequencies of the RAD51 variants tested across various populations might explain the inconsistencies among previous reports. However, the absence of prior association does not exclude the possibility that RAD51 may induce HBV clearance either directly or via other functional elements. The findings of this study are important in that it is the first known test of the association between RAD51 variants and HBV clearance.
Refuting the hypothesis that RAD51 may modulate HBV infection by maintaining genome integrity and mediating homologous DNA repairs, the findings of this study provide evidence that RAD51 genetic variations are not implicated clinically in HBV clearance and progression to HCC, at least not in a Korean population. Although studies in other populations are warranted, this information may contribute to the current knowledge about hepatitis pathogenesis in relation to HBV infection and development of HCC.




