Symptomatic and asymptomatic infections of rotavirus, norovirus, and adenovirus among hospitalized children in Xi'an, China†
Shuwan Zhang and Tsun-Hsuan Chen contribute equally to this work.
Abstract
Rotavirus (RV), norovirus (NoV), and adenovirus (AdV) have been reported as the common viral pathogens of acute gastroenteritis in children. To determine the prevalence of RV, NoV, and AdV infections among hospitalized children with and without symptoms of acute gastroenteritis, fecal specimens, and data on clinical symptoms were collected from 201 children with diarrhea and 53 children without diarrhea admitted to the Xi'an Children's Hospital in Xi'an, China between March 2009 and May 2010. RV, NoV, and AdV were identified in 68.7% (138/201), 20.4% (41/201), and 5.0% (10/201), respectively, of children with diarrhea. These three viruses were also detected in 13.2% (7/53), 35.9% (19/53), and 9.4% (6/53), respectively, of children without diarrhea. Diarrheal children infected with RV alone showed the average severity score of 6.5, statistically significant higher than the average score of 5.3 in children with unidentifiable viruses. GII.3 and GII.4 were the only two NoV genotypes identified, and the GII.4 sequences were genetically close to GII.4 2006b cluster. These findings highlight the importance of NoV as a causative agent of pediatric diarrhea after RV based on the clinical and epidemiological characteristics of NoV infection, and particularly convey information of asymptomatic infections of enteric viruses in young children. J. Med. Virol. 83:1476–1484, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Acute gastroenteritis is a common disease characterized by diarrhea, vomiting, nausea, abdominal pain, and fever in young children, and it is an important reason of hospitalization among children in industrialized countries [Oh et al., 2003; Olesen et al., 2005; Zintz et al., 2005] and the leading cause of childhood mortality in developing countries [Bern et al., 1992; Parashar et al., 2003]. Acute gastroenteritis can be caused by a broad spectrum of viral, parasitic, and bacterial enteropathogens. However, a large proportion of acute gastroenteritis cases are caused by enteric viruses [Reither et al., 2007]. Rotavirus (RV), calicivirus, adenovirus (AdV), and astrovirus are recognized as important etiological pathogens for clinically identified acute non-bacterial diarrheal diseases in young children [Bon et al., 1999; Wilhelmi et al., 2003; Nguyen et al., 2007].
RV is a major etiological agent of pediatric acute gastroenteritis. This virus is a double stranded RNA virus and belongs to the family of Reoviridae that includes seven serogroups (A–G). Group A, B, and C are known to cause gastroenteritis infections in humans. Group A RVs predominantly result in severe acute diarrhea in infants and young children in most areas of the world, and groups B and C RV infections have been occasionally detected in young children with acute diarrhea [Wu et al., 1998; Barman et al., 2006]. The genome of RV consists of 11 segments of double stranded RNA enclosed in a triple layered capsid protein. The outer-layer protein is composed of two independent neutralization antigens VP4 and VP7, in which VP4 determines P serotypes and VP7 defines G serotypes. At least 15 G genotypes and 26 P genotypes have been reported [Rao et al., 2000; Ghosh et al., 2011], but G1–G4, G9, P[4], and P[8] are the most prevalent genotypes responsible for RV infections worldwide [Chen et al., 2005; Nguyen et al., 2007; Jin et al., 2009]. In 2006 and 2008, two RV vaccines have been recommended for routine immunization programs of infants in the US. Before introduction of the RV vaccine, during the height of each season, about 40–50% of stool specimens submitted for RV analyses among children with diarrhea were positive [Parashar et al., 2006; Payne et al., 2008]. After introduction of the RV vaccine, a reduction in RV prevalence from 51 to 6% was observed [CDC, 2008; Parashar and Glass, 2009], clearly demonstrating successful protection against RV infection. In China, currently there is only one local RV vaccine in the market with a limited efficacy data available [Fu et al., 2007] and RV vaccination has not been broadly promoted.
Human caliciviruses including norovirus (NoV) and sapovirus are recognized as the second most frequent etiological agent of acute gastroenteritis in young children, following RV [Qiao et al., 1999; Nguyen et al., 2007; Patel et al., 2008; Tran et al., 2010]. NoV is single stranded, plus-sense RNA, approximately 7.6 kb in length that contains three open-reading frames (ORFs). ORF1 encodes a non-structural polyprotein including the RNA-depended RNA polymerase region. ORF2 and ORF3 encode a single major (VP1) and a minor (VP2) capsid protein, respectively [Jiang et al., 1990]. NoVs are classified into five genogroups (GI–GV) and each genogroup is subdivided into genotypes based on the sequence homology of the RNA dependent RNA polymerase region and the ORF2 region of the viral genome, and only GI, GII, and GIV infect humans. Currently, 8 genotypes in GI, 17 genotypes in GII, and 1 in GIV have been identified [Zheng et al., 2006]. GII.4 is the most common genotype responsible for the majority of gastroenteritis outbreaks [Zheng et al., 2010] and a high proportion of sporadic diarrhea cases in children [Jin et al., 2008]. New GII.4 variants have emerged every 2–3 years and have a global distribution [Siebenga et al., 2009].
AdV is a group of DNA viruses that belong to the family Adenoviridae. There are at least 52 immunologically distinct types that can cause human infections. AdVs are the most common cause of respiratory illness. However, depending on the infecting serotype, they may also cause various other illnesses, such as gastroenteritis, conjunctivitis, cystitis, and rash illness. Serotypes 40 and 41 AdVs in the subgenus F are the AdVs associated with approximately 5.0% of gastroenteritis cases in young children [Bon et al., 1999; Nguyen et al., 2007; Jin et al., 2009].
Several hospital-based pediatric diarrhea studies have been performed in China [Qiao et al., 1999; Liu et al., 2006; Jin et al., 2009]. However, all of these studies have only focused on laboratory detection of viral pathogens and have not reported the clinical and epidemiological characteristics of viral infections in young children. In addition, several studies on NoV from other parts of the world have indicated that not all pediatric infections with NoVs result in clinical symptoms and asymptomatic NoV infection is common in young children [Garcia et al., 2006; Monica et al., 2007; Reither et al., 2007]. It is unknown whether pediatric asymptomatic infection of NoVs occurs in China and what is the rate of asymptomatic NoV excretion and the genetic diversity of NoVs. The objectives of this study were to: (1) determine the symptomatic and asymptomatic infection rates of RV, NoV, and AdV among hospitalized children with and without clinical symptoms of diarrhea; (2) examine the severity scores of diarrhea in children with different virus infections; and (3) analyze the NoV genotypes in children with symptomatic and asymptomatic infections.
MATERIALS AND METHODS
Study Population and Sample Collection
Between March 2009 and May 2010, fecal specimens and clinical symptoms from children aged <5 years, primarily diagnosed as acute gastroenteritis (defined as ≥3 loose or watery stools/day), and admitted to the Department of Digestive Diseases of Xi'an Children's Hospital, Xi'an, China were collected. The fecal samples were collected within 48 hr of admission after prescreening for blood cells in stool samples was performed. Only fecal specimens without blood or mucus were collected in this study. Simultaneously, fecal specimens and relevant clinical data were also collected from age- and gender-matched children admitted to other departments (control) and primarily diagnosed as respiratory diseases or because of surgery admission in a ratio of 1–4 with diarrhea cases (a case–control study was originally designed). The control children did not have any diarrheal symptoms in the last 3 weeks prior to admission. Parents/guardians were asked to sign an informed consent (approved by the IRB committee at Emory University) before their children's participation into this study.
Scoring System for Severity of Viral Diarrhea
Because the duration of diarrhea and vomiting and the diarrhea treatment information were not collected in this study, the severity of diarrhea was assessed using a 11-point numerical scoring system that was modified according to Ruuska and Vesikari's description [Ruuska and Vesikari, 1990]. The severity of diarrhea and vomiting was quantified according to the maximum episode frequency within 24 hr. Fever was measured using axillary temperature and was categorized as three levels (≤37.5, 37.6–38.5, and >38.5) by the clinical pediatric diagnostic criteria in China. Dehydration was grouped only as “yes” or “no” according to the local pediatrician's diagnostic criteria. Table I presents the clinical symptoms and criteria used for grading the severity of diarrhea.
| Clinical symptoms | Score |
|---|---|
| Max episodes of diarrheal/24 hr | |
| 3 | 1 |
| 4–6 | 2 |
| ≥7 | 3 |
| Max episodes of vomiting/24 hr | |
| 0 | 0 |
| 1 | 1 |
| 2–4 | 2 |
| ≥5 | 3 |
| Fever | |
| ≤37.5°C | 0 |
| 37.6–38.5°C | 2 |
| >38.5°C | 3 |
| Dehydration | |
| No | 0 |
| Yes | 2 |
Viral RNA and DNA Extraction
A 20% (w/v) stool suspension was prepared in RNAse- and DNAse-free water (Mediatech, Manassas, VA) and 500 µl of the suspension was mixed with an equal volume of Vertrel (Miller-Stephenson, Danbury, CT). After incubation at 4°C overnight, the samples were centrifuged at 12,000 × g for 15 min at 4°C. Subsequently, a 140-µl supernatant was removed and used for NoV RNA extraction using the QIAamp Viral RNA Mini Kit (Qiagen, Valencia, CA.), and an additional 140-µl supernatant was used for AdV DNA extraction per QIAamp DNA Blood Mini Kit (Qiagen) in accordance with the manufacturer's instructions. The extracted RNA or DNA samples were aliquoted and stored at −80°C until assayed by NoV and AdV PCR assays.
Rotavirus Enzyme Immunoassay (EIA)
To detect group A RVs in fecal samples, the ProSpecT™ Rotavirus Commercial Kit (Oxoid Ltd, Basingstoke, UK) was used. A 100-µl 10% (w/v) stool suspension was added to the microwells that were precoated with RV specific polyclonal antibodies and incubated simultaneously with horseradish peroxidase conjugated RV specific polyclonal antibodies. RV antigens present in RVs were captured by the antibodies on the solid phase and the enzyme-conjugated antibodies. After 60-min incubation at room temperature, the microwells were washed five times with wash buffer provided in the kit to remove excessive specimens and unbound enzyme-labeled antibodies. Subsequently, a 100-µl of substrate agent (3,3′-5,5′-tetramethylbenzidinein) was added to the microwells. After incubation for 10 min at room temperature, a 100-µl of sulfuric acid (0.46 M) was added to stop the substrate reaction and then the optical density (OD) values were measured using a spectrophotometer set at 450 nm. To meet the quality control criteria, the OD value of the negative control should be ≤0.15 and the positive control must be ≥0.50. To determine the presence or absence of RV in stool specimens, positive was defined as OD values greater than the cut-off value (add 0.1 to the OD value of the negative control), and negative was defined as OD values smaller than the cut-off value. Samples were retested when the OD value is between 0.01 and the cut-off value.
TaqMan Real-Time RT-PCR for Detection of Noroviruses
A NoV TaqMan real-time RT-PCR (RT-qPCR) was performed to detect GI NoVs using the Qiagen OneStep RT-PCR Kit (Qiagen) and NoV GI broadly reactive primers and probe (Sigma, St. Louis, MO) that were previously described [Kageyama et al., 2003; Liu et al., 2010]. Briefly, a 25-µl PCR reaction mixture was prepared with 10 µl of 10-fold diluted RNA, 4.75 µl RNAse-free water, 1.0 µl dNTP mixture (10 mM), 1.0 µl of each primer (l0 µM), 0.75 µl of the TaqMan probe 1a (l0 µM), and 0.25 µl of the TaqMan probe 1b (l0 µM), 0.25 µl (40 U/µl) RNAse inhibitor (Promega, Madison, WI), 5.0 µl 5× Qiagen oneStep buffer, 1 µl Qiagen RT-PCR enzyme mixture of HotStart Taq DNA polymerase and reverse transcriptase. The reverse transcription and amplification reactions were performed on an ABI7500 Fast RT-PCR system (Applied Biosystems, Foster, CA). For detection of NoV GII RNA, the aforementioned PCR system with a different set of primers and probe that targeted the ORF1 and ORF2 junction region of the NoV GII genome was prepared [Kageyama et al., 2003]. Reverse transcription was performed at 50°C for 32 min followed by HotStart Taq DNA polymerase activation and reverse transcriptase inactivation at 95°C for 15 min. A total of 45 amplification cycles were carried out, each consisting of 95°C for 15 sec and 56°C for 1 min.
PCR for Detection of Adenoviruses
A conventional PCR was performed using HotStarTaq Plus Master Mix Kit (Qiagen) and primers that correspond to the conserved region within the hexon gene of human AdV were previously described [Allard et al., 2001]. Briefly, 2 µl of the DNA extract was added to 18 µl of PCR mixture containing 10 µl of HotStarTaq plus master mixture including 2× PCR buffer, MgCl2 (3 mM), dNTP mixture (400 µM), and HotStarTaq Plus DNA polymerase, 1 µl of each primer (10 µM), 2 µl of Coralload (10×), and 4 µl of RNAse-free water. An initial denature and activation of 95°C for 5 min was followed by 35 cycles of 95°C for 25 sec, annealing at 58°C for 30 sec, and extension at 72°C for 35 sec. The final cycle had a prolonged extension time of 7 min at 72°C. After amplification was completed, 10 µl of the PCR products were separated on 1.5% agarose gels, and the expect band was visualized by staining with ethidium bromide under UV light.
Norovirus Conventional RT-PCR
After the initial detection of NoVs using the GI and GII broadly reactive TaqMan real-time RT-PCR assays, the positive RNA samples (only GII strains were detected) were amplified using a degenerated primer set that spans 3′-end of the region B and 5′-end of the region C (capsid protein) [Dey et al., 2007]. A 25 µl of RT-PCR reaction mixture consisted of 5 µl of RNA, 400 µM dNTP mixture, 0.4 µM of each primer, 1× Qiagen OneStep RT-PCR buffer (Qiagen), 10 U RNAse inhibitor (Promega), and 1 µl of Qiagen RT-PCR enzyme mixture. The PCR was performed at 50°C for 32 min, 95°C for 15 min followed by 35 cycles of 95°C for 20 sec, 50°C for 30 sec, 72°C for 40 sec, and a final extension at 72°C for 7 min. The PCR products were resolved on a 1.5% agarose gel, stained with ethidium bromide, and visualized under UV light.
Norovirus Phylogenetic Analysis
The PCR amplicons from the conventional RT-PCR assay were sent to the Beijing Genomics Institute (Beijing, China) for determining the NoV sequences using the conventional primer set. All NoV nucleotide sequences obtained from this study were processed with EditSeq program in the DNASTAR software package (Madison, WI). The sequences in this study, and the reference NoV sequences downloaded from the GenBank database were aligned using the Clustal W program implemented in the MEGA version 4 (Tamura, Dudley). The phylogenetic tree was constructed using neighbor-joining method in the MEGA 4 software package with 1,000 pseudoreplicate data sets.
RESULTS
During March 2009–May 2010, a total of 201 stool samples were eligible and examined for RV, NoV, and AdV. Although children under 5 years of age were designed as the target age population for this study, finally 98.5% of the enrolled diarrhea children were under 2 years old (mean age: 10 ± 4.9 months, ranging 1.5–37.5 months). Fifty-three children (control) without gastroenteritis-associated symptoms were selected from other departments at the same hospital but without diarrhea and diarrhea associated clinical symptoms. The mean age of the control group was 7 months (±9 months) ranging from 1.1 month to 50.1 months. The mean ages between the symptomatic and asymptomatic groups were not statistically different (data not shown).
A 11-point scoring system (Table I) for grading the severity of diarrhea in this study was utilized. The average scores of children infected with RV alone, NoV alone, AdV alone, mixed infections, and non-identifiable virus were 6.5, 6.0, 4.6, 6.5, and 5.3, respectively (Table II). Comparisons of these scores showed a statistically significant difference only between RV alone versus unidentifiable viruses (P = 0.018).
| Characteristic | Rotavirus (n = 117a) | Norovirus (n = 27a) | Adenovirus (n = 6a) | Mixed (n = 15) | Unidentifiable (n = 27) |
|---|---|---|---|---|---|
| Max diarrhea episodes (days) | |||||
| 3 | 22 (18.8) | 5 (18.5) | 2 (33.3) | 1 (6.6) | 2 (7.4) |
| 4–6 | 46 (39.3) | 11 (40.7) | 3 (50.0) | 7 (46.7) | 16 (59.3) |
| ≥7 | 49 (41.9) | 11 (40.7) | 1 (16.7) | 7 (46.7) | 9 (33.3) |
| Max vomiting episodes (days) | |||||
| ≤1 | 37 (31.6) | 7 (25.9) | 4 (66.7) | 4 (26.7) | 16 (59.3) |
| 2–4 | 52 (44.4) | 11 (40.7) | 0 (0.0) | 6 (40.0) | 8 (29.6) |
| ≥5 | 28 (24.0) | 9 (33.4) | 3 (33.3) | 5 (33.3) | 3 (11.1) |
| Levels of fever (°C) | |||||
| ≤37.5 | 26 (22.2) | 11 (40.7) | 3 (50.0) | 5 (33.3) | 10 (37.0) |
| 37.6–38.5 | 40 (34.2) | 5 (18.6) | 2 (33.3) | 2 (13.3) | 8 (29.6) |
| ≥38.5 | 51 (43.6) | 11 (40.7) | 1 (16.7) | 8 (53.4) | 9 (33.4) |
| Dehydration | |||||
| Yes | 34 (29.1) | 7 (25.9) | 2 (33.3) | 3 (20.0) | 6 (22.2) |
| No | 83 (70.9) | 20 (74.1) | 4 (66.7) | 12 (80.0) | 21 (77.8) |
| Mean severity score | 6.5 | 6.0 | 4.6 | 6.5 | 5.3 |
| ≤4 | 24 (20.5) | 7 (25.9) | 3 (50.0) | 1 (6.7) | 10 (37.0) |
| 5–7 | 50 (42.7) | 11 (40.7) | 3 (50.0) | 11 (73.3) | 13 (48.2) |
| ≥8 | 43 (36.8) | 9 (33.3) | 0 (0.0) | 3 (20.0) | 4 (14.8) |
- a Not including mix-infected patients.
A total of 201 stool samples collected from symptomatic children were examined for the presence of RV, NoV, and AdV, and these three viruses were identified in 68.7%, 20.4%, and 5.0%, respectively, of children with diarrhea. These three viruses were also detected in 13.2%, 35.9%, and 9.4%, respectively, of the hospitalized children without diarrhea (Table III). Mixed infections were observed in 16 (8.0%) of the children with symptomatic diarrhea and the majority (81.3%) were combination of RV with NoV (data not shown). Of the 53 fecal samples without diarrhea (asymptomatic infection), 11.8% (6/51) mixed infections were observed, most occurring between RV and NoV or RV and AdV. RV was present more frequently in diarrhea children than in control children (P < 0.0001) (adjusted OR = 14.2). In contrast, NoV infection was observed more in control children than in diarrheal children with statistically significant difference (P = 0.02; adjusted OR = 0.5). Human AdV infection showed no significant difference between the two groups (5.0% vs. 9.4%) (Table III).
| Symptomatic (n = 201) | Asymptomatic (n = 53) | Odds ratio | P-value | |
|---|---|---|---|---|
| No. (%) | No. (%) | |||
| RV | 138 (68.7) | 7 (13.2) | 14.2 | <0.0001 |
| NoV | 41 (20.4) | 19 (35.9) | 0.5 | 0.02 |
| AdV | 10 (5.0) | 5 (9.4) | 0.5 | 0.25 |
Of 201 fecal samples with symptomatic diarrhea, 41 were tested positive for NoV GII and no NoV GI strains were detected. All the 41 NoV GII positive samples were further confirmed as positive by a conventional RT-PCR with a different set of primers spanning the 3′-end of region B and 5′-end of region C, but the high-quality nucleotide sequences were only obtained in 36 samples. Analyses of these 36 NoV sequences clearly showed two distinct NoV genotypes (Fig. 1): GII.3 (52.8%) and GII.4 (47.2%). The same PCR and sequencing approaches were also applied to the 53 control samples. Similar to the symptomatic diarrheal samples, there were no GI NoV identified. GII.3 and GII.4 were detected in 52.6% and 47.4% of the control samples, respectively (Table IV). Phylogenetic analysis of the GII.4 sequences indicated that all the GII.4 strains in this study belonged to GII.4 2006b cluster (Fig. 2).
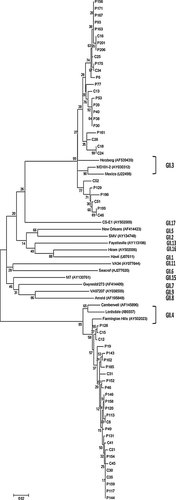
Phylogenetic tree of partial capsid nucleotide sequences (288 bp) from 55 fecal samples in children with symptomatic or asymptomatic infections of noroviruses, and NoV GII reference sequences. Dendrograms were generated using the neighbor-joining method, and the statistical analysis of the phylogenies was performed by bootstrap test with 1,000 replicates. The numbers in the branches indicate the bootstrap values. Sequence names beginning with p represent sequences from diarrheal patients, and c indicate sequences from control samples with asymptomatic infections.
| No. | Positive no (%) | Genogroup II | ||
|---|---|---|---|---|
| GII.3 (%) | GII.4 (%) | |||
| Symptomatic | 201 | 41 (20.4) | 19a (52.8) | 17a (47.2) |
| Asymptomatic | 53 | 19 (35.8) | 10 (52.6) | 9 (47.4) |
- a Sequences from 36 out of 41 NoV positive samples by RT-qPCR were obtained.
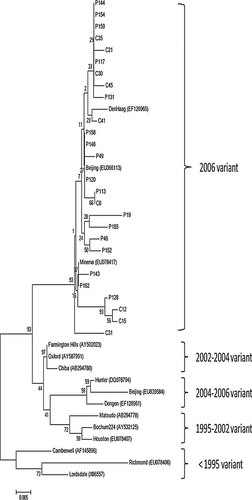
Phylogenetic tree of partial capsid sequences (288 bp) detected in children with symptomatic and asymptomatic infections of norovirus GII.4 variants. The tree was constructed using the neighbor-joining method. The GenBank accession numbers for the previously represent GII.4 variants include: Lordsdale (X86557); Richmond (EU078406); Camberwell (AF145896); Houston (EU078407); Bochum224 (AY532125); Matsudo (AB294778); Dongen (EF126961); Beijing (EU839584); Hunter (DQ078794); Chiba (AB294780); Farmington Hills (AY502023); DenHaag (EF126956); Oxford (AY587991); Minerva (EU078417); Beijing (EU366113).
DISCUSSION
In this study, RV infection was determined by a commercial immunological-based assay while NoV and AdV were detected using the nucleic acid amplification techniques. The reason for using immunoassay for RV was because of the high sensitivity/specificity of the RV enzyme-linked immunosorbent assay (ELISA) kit [Flewett et al., 1989] and the great consistency between Dako-ELISA and electron microscopy results [Herrmann et al., 1985]. On the other hand, RV genotyping information has been well documented in China [Duan et al., 2009; Li et al., 2009; Xu et al., 2009; Zeng et al., 2010]; therefore, application of rapid and accurate ELISA instead of complicated and time-consuming nucleic acid amplification assays for RV detection would be more feasible and efficient. In contrast, NoV, as the second most frequent viral agent of acute gastroenteritis, has no approved commercial ELISA kits in the market and little is known about NoV genotype diversity in developing countries.
NoVs were detected in 20.4% of the diarrheal children in this study, consistent with the findings from a previous study in this region [Jin et al., 2008]. Further investigation of NoV genotyping in this study showed that all the positive NoV samples were GII strains while NoV GI was not identified. Nucleotide acid sequencing analysis of the positive NoV specimens reveals that the identified NoV GII strains explicitly include two distinct genotypes, GII.3 and GII.4, which were two predominant GII NoV strains circulated in China [Cheng et al., 2010]. Further phylogenetic analysis of GII.4 strains using a 288-bp nucleotide spanning the 5′-end of the ORF2 showed that all the GII.4 sequences in this study belonged to GII.4 2006b cluster (Fig. 2) in comparison with GII.4 reference sequences from the GenBank database. This suggests that the new GII.4 strain in the study area has not emerged since the GII.4 2006a and 2006b were first detected in Europe in 2005–2006 [Kroneman et al., 2006]. In this study, 5.0% (10/201) of children with diarrhea have been detected as human AdV infection, similar to other reports [Olesen et al., 2005; Nguyen et al., 2007]. There were still 13.9% of the fecal specimens in the symptomatic children who were not identified with any of the three investigated viruses, suggesting that other enteric viruses such as astrovirus, sapovirus, and/or bacterial/parasitic pathogens may contribute to the remaining infections [Gallimore et al., 2004; Olesen et al., 2005; Nguyen et al., 2007].
Comparisons of the average severity scores categorized by different virus infections showed a statistical significant difference only between the group with RV infection alone versus the group with unidentifiable viruses, suggesting that children infected with RV alone likely experience more severe clinical symptoms than children infected with other unidentifiable enteric viruses and/or bacterial/parasitic pathogens. In addition, no statistical difference of the severity scores between RV and NoV infection alone indicates that NoV infection in young children causes severe clinical symptoms as RV infection.
Previous studies indicated that enteric virus infections were significantly associated with age, usually occurring in early lifetime [Colomba et al., 2006; O'Ryan et al., 2009; O'Ryan et al., 2010]. It is interesting that 99.5% of the pediatric diarrhea patients enrolled in this study were under 2 years of age although we aimed for <5 years, consistent with the findings from other studies in China [Qiao et al., 1999; Fang et al., 2005]. High NoV infections under 2 years suggest that children are more susceptible to NoV infection during their early lifetime and subsequently may result in protective immunity against symptomatic NoV infections after 2 years of age [Jing et al., 2000] while maternal antibodies only persist for several months [Parker et al., 1995].
Asymptomatic infections of enteric viruses in children were reported previously [Garcia et al., 2006; Monica et al., 2007; Reither et al., 2007]. In the present study, approximately half (47.5%) of the fecal specimens collected from the children without diarrhea were asymptomatic infections of NoV, RV, or AdV, which was surprising and unexpected. Interestingly, the infection rate of NoV in asymptomatic children was statistically higher than that in symptomatic children (P = 0.02), and these results were carefully confirmed by two different RNA extraction methods followed by the real-time RT-PCR assay. Among the children with asymptomatic NoV infection, some might recently asymptomatically infect with NoV but continued to excrete viruses. This phenomenon was observed in both human challenge and follow-up studies showing a significant proportion of infected people remained asymptomatic infection after NV oral inoculation [Hutson et al., 2002; Lindesmith et al., 2003] and virus shedding persisted for a prolonged period of time [Murata et al., 2007; Atmar et al., 2008; Siebenga et al., 2008]. Asymptomatic infections can also occur because of early life exposure to NoV infections and development of immunity that may protects late life NoV infections, in which NoV-specific mucosal IgA and/or NoV receptor likely contribute to the resistance of individuals to NoV infections [Lindesmith et al., 2003] although conflicting results [Rockx et al., 2005; Tan et al., 2008] were reported.
In summary, our findings align with previous etiological studies of pediatric acute gastroenteritis, and provide important insights on NoV study. First, RV was confirmed as the most common pathogen in childhood diarrhea in Xi'an, China. Due to the severity of RV infection, high prevalence, and potential economic burden, introduction of the RV vaccine into the existing national immunization program in China would be beneficial to children's health. Second, as the second most prevalent enteric virus after RV in pediatric diarrhea, more attention should be paid to NoV clinical diagnosis in China. Third, with high prevalence of asymptomatic NoV infections in young children, future studies are needed to understand the importance of asymptomatic infections acting as the potential reservoir of viral transmission in nurseries, schools, and childcare centers.




