A prospective longitudinal study of BK virus infection in 120 Czech renal transplant recipients†
Conflict of Interest Disclosure: None of the authors have a financial interest in a business or commercial entity or intellectual property interests relating to data or results presented in this manuscript.
Abstract
Polyomavirus BK (BKV) is a common human polyomavirus that rarely causes clinical symptoms in immunocompetent individuals. However, BK virus reactivation occurs in 20–40% of kidney transplant patients and 1–10% of cases present with BK virus-associated nephropathy (BKVN) and reduced kidney allograft survival. In this study, 120 consecutive renal allograft recipients were monitored for BK virus replication by real–time PCR (qPCR) in the blood and urine during the first year post-transplantation and risk factors for BK viremia, viruria, and polyoma BKV-associated nephropathy were evaluated. Receiver operating characteristic curve analysis was used to determine the cutoff points for assessing the risk of developing BKVN. In total, 1,243 samples were tested. BK-DNAuria >107 copies/ml and BK-DNAemia >104 copies/ml were found in 25.8% and 5% of the samples screened, respectively, during the 12 month follow-up period. BKVN was confirmed histologically in 3/120 patients and viremic patients were treated with dialysis for longer time periods and had higher levels of anti-BKV-reactive antibodies. Patients with viruria were also treated longer with dialysis and had impaired graft function 12 months post-transplantation. Patients with sustained viruria exhibited more acute rejection episodes than patients with transient viruria. Using receiver operating characteristic curve analysis, the cutoff point for viremia and viruria was redefined to 103 copies/ml serum for BK viremia and a cutoff point of 6.7 × 107 copies/ml in urine. In conclusion, polyoma BK viremia and viruria are frequent findings in kidney transplant recipients that warrant intensive monitoring as a means of preventing graft rejection. J. Med. Virol. 83:1395–1400, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Polyomavirus BK (BKV) is a common human polyomavirus that causes disease of little clinical significance in immunocompetent patients. Seroprevalence studies have demonstrated that most adults had been infected during childhood [Shah et al., 1973] with reported adult seropositivity rates of approximately 80% [Knowles et al., 2003].
However, BKV reactivates in 20–40% of kidney transplant patients and in 1–10% of cases develop BK virus-associated nephropathy (BKVN) [Hariharan, 2006; Almeras et al., 2008]. Although kidney biopsies remain the “gold standard” for diagnosing and managing BKVN, clinical interventions often are based on surrogate markers of the disease rather than on the tissue histology. This is supported conceptually by the fact that early BKVN is focal and liable to tissue sampling errors [Drachenberg et al., 2006; Wiseman, 2009]. Polymerase chain reaction-based screening for BKV replication is the basic strategy used to predict early BKVN onset [Hirsch et al., 2005] and has been recently implemented in transplant centers during routine screening procedures [Babel et al., 2009].
Previous studies reported graft loss in 47–60% of patients presenting with BKVN within 5 years [Vasudev et al., 2005; Babel et al., 2009] and current studies using early screening procedures have demonstrated a decreased graft survival rate of 15–30% in BKVN patients [Brennan et al., 2005; Babel et al., 2009]. The aim of this study was to monitor prospectively BK virus replication in blood and urine samples collected from a well-defined patient cohort. Additionally, risk factors for BK viremia, viruria, and polyoma BKVN were evaluated.
MATERIALS AND METHODS
Patient Cohort
Between March 2007 and March 2008, 120 patients who underwent renal allograft transplantation at the Institute for Clinical and Experimental Medicine, Prague, were enrolled into this study. Serum from peripheral blood and urine samples were collected from patients on day 1 and 1, 3, 6, 9, and 12 months post renal transplant surgery. The study protocol was approved by the Ethics Committee of the Institute for Clinical and Experimental Medicine in Prague and written informed consent was obtained from all patients. Physicians were not aware of PCR results but were aware of the patients' polyoma BKVN status during the course of the study. Renal function was assessed by determining serum creatinine and eGFR levels and by monitoring proteinuria. Patients' demographic and clinical data are summarized in Table I. For study purposes, the standard definition of positive serum viremia was >104 copies/ml and for viruria >107 copies/ml in urine as described [Hirsch et al., 2005].
| Age (yr) | 54 (18–77) |
| Gender (male, %) | 77 (65%) |
| BMI (kg/m2) | 25.02 (17.05–34.54) |
| Case of ESRD: (%) | |
| Polycystic kidney disease | 19 |
| GN | 47 |
| Hypertension | 18 |
| PN/IN | 24 |
| Diabetes | 8 |
| Other | 4 |
| HLA mismatches (avg ± SD) | 3.13 ± 1.55 |
| First graft (n, %) | 97 (81%) |
| PRA | |
| <20% | 77 (64%) |
| 20–80% | 35 (29%) |
| >80% | 8 (7%) |
| CMV seroconversion status | |
| D−/R− | 9% |
| D−/R+ | 20% |
| D+/R− | 13% |
| D+/R+ | 58% |
| Immunosuppression | |
| Induction with either antithymocyte globuline or basiliximab (n, %) | 54 (43%) |
| Tacrolimus (n, %) | 92 (77%) |
| Cyclosporin A (n, %) | 22 (18%) |
| Sirolimus or sotrastaurin (n, %) | 6 (5%) |
| Living donor (n, %) | 24 (20%) |
| CIT (hours) | 17.39 (0–25.9) |
- Data are shown as median (minimum–maximum) values unless indicated otherwise. ESRD, end-stage renal disease; PN/IN, pyelonephritis/interstitial nephritis; CIT, cold ischemia time; PRA, panel reactive antibody; BMI, body mass index; CMV, cytomegalovirus, induction: Thymoglobuline or basiliximab.
Immunosuppressive Regimens
Immunosuppressive therapy consisted of triple drug regimen based on either tacrolimus (Tac, n = 90) or cyclosporine A (CsA, n = 22). Six patients received sirolimus or sotrastaurin instead of calcineurin inhibitors according to the study protocol. All patients received mycophenolate mofetil (MMF) and steroids. Patients with panel reactive antibodies (PRA) >50% received induction immunosuppressive therapy with Thymoglobulin (n = 30) and all living donor recipients received basiliximab induction (n = 24) (Table I).
BKV-DNA Quantification
Extraction and purification of BKV-DNA from serum and urine samples was performed using the Chemagic viral DNA/RNA Kit (Chemagen, Baesweiler, Germany). Extracted DNA was tested for the presence of BK virus DNA by real-time PCR using the BKV Q-PCR Detection Alert Kit (Chemagen) for the detection of the viral target gene encoding for the BKV large T-antigen using the ABI Prism® 7900 H.T. Sequence Detection system (Applied Biosystems, CA, USA). According to the manufacture's instructions, PCR amplifications were set up in a 25 µl reaction volumes containing 5 µl of extracted sample DNA or negative control (reaction tubes without DNA). Each 96-well plate used for quantitation consisted of target samples measured in duplicate, standard curves and negative and positive controls used for comparisons between experiments. Standard curves for quantification were constructed by plotting the threshold cycle against the logarithm of serial 10-fold dilutions, ranging from 102 to 105. Measurements were repeated when differences >0.5 threshold cycle Ct value were observed between duplicates. Each sample was subjected to simultaneous TaqMan PCR for the human β-globin housekeeping gene. Results were acceptable only when they were also positive for β-globin. Probes were designed to detect BK virus specifically and did not cross-react with sequences present in the related JC or SV-40 polyomaviruses.
Clinical Data
The following clinical variables were recorded for the patients: gender, age at the time of transplantation, BMI (body mass index), the origin of the kidney graft (deceased or living donor), donor age, number of HLA mismatches, CMV (cytomegalovirus) seropositivity/negativity, maximal PRA, serum creatinine levels (Cr), the type of immunosuppressive therapies employed, and the presence and type of induction therapy used.
Statistical Analysis
Continuous variables were described as the median value and range. Statistical analysis of categorical characteristics was performed using either the χ2 or Fisher's tests. The Student's t-test and Mann-Whitney U-test were used, respectively, to identify differences between continuous parametric and non-parametric variables. To summarize the data from receiver operating characteristic (ROC) curves and to calculate the area under the curve (AUC), sensitivity and specificity, the SigmaPlot Version 11.0 was used. Differences in time of rejection were assessed by Kaplan–Meier survival analysis using Graph Pad Prism Version 4.0.
RESULTS
Prevalence of BK Viruria and Viremia
A total of 1,243 samples from a 120 patient cohort were analyzed (622 and 621 urine and serum samples, respectively). The 2005 recommended BKV quantitative load guidelines associated with risk of developing BKVN are >107 copies/ml in urine and >104 copies/ml in serum [Hirsch et al., 2005].
BK-DNAuria >107 and BK-DNAemia >104 in this study occurred in 25.8% (n = 31) and 5% (n = 6) of patients, respectively, during the 12-month follow-up period. The BKVN diagnosis was confirmed histologically in 3/120 patients; giving an incidence of 2.5%. The highest incidence of BK viruria and viremia occurred during the third and twelfth months post transplantation, respectively.
Patients with BK Virus Nephropathy
BKVN was histologically confirmed in 3/120 patients, incidence rate of 2.5% (Table II). None of these 3 patients lost their grafts during follow-up. In patients the 1 and 2, the original immunosuppressive therapy regimen used consisted of tacrolimus + mycophenolate mofetil + steroids and patient 3 therapy consisted of cyclosporine A + mycophenolate mofetil + steroids. In the patient 1, tacrolimus was replaced with cyclosporine A and this patient successfully diminished the detectable level of viral loads during follow-up. In patient 2, mycophenolate mofetil was stopped resulting in full viral clearance during follow-up. In patient 3, cyclosporine A was replaced with sirolimus and mycophenolate mofetil was withdrawn resulting in successful viral clearance during follow-up.
| P01 | P02 | P03 | |
|---|---|---|---|
| Age/gender | 44 y/female | 55 y/male | 60 y/male |
| ESRD | polycystic kidney | Grawitz tumor | DN |
| BKVN after Tx (days) | 92 | 147 | 94 |
| BK viremia at the BKVN+ biopsy finding | 1.1 × 103 copies/ml | 2.2 × 103 copies/ml | 7.1 × 107 copies/ml |
| BK viruria at the BKVN+ biopsy finding | 1.3 × 109 copies/ml | 1.6 × 106 copies/ml | 1.2 × 103 copies/ml |
| BKVN+ biopsy finding | BKVN, ACR I B | BKVN, ACR I A | BKVN, DN transfer |
| Cr at BKVN diagnosis | 85 | 218 | 135 |
| No. of biopsies before BKVN | 0 | 1 | 0 |
| Finding biopsy before BKVN | 0 | Normal | 0 |
| No. of biopsies after BKVN+ | 1 | 0 | 0 |
| First biopsy after BKVN+ | BKVN, ACR I B | Borderline, IFTA | |
| Outcome | Functioning graft | Functioning graft | Functioning graft |
| Initial therapy | Tac, MMF, P | Tac, MMF, P | CsA, MMF, P |
| Therapy at the BKVN diagnosis | Tac, MMF, P | SIR, MMF, P | CsA, MMF, P |
| Therapy after BKVN diagnosis | Switched Tac to CsA | MMF withdrawal | Switched CsA to sirolimus and withdrew MMF |
- ESRD, end-stage renal disease; GN, glomerulonephritis; NC, nephrocalcinosis; IS, immunosuppressive therapy; HR, humoral rejection; Tac, Tacrolimus, CsA, cyclosporine; P, prednisone; MMF, mycophenolate mofetil; SIR, sirolimus; Cr, Creatinine, BKVN, BK virus nephropathy; DN, diabetic nephropathy; ACR, acute cellular rejection, Tx, transplantation; IFTA, interstitial fibrosis/tubular atrophy.
Patients with BK Viremia >104 Copies/ml Serum
Six patients exhibited viremia (>104 copies/ml serum) and BKVN was confirmed by biopsy in 1/6 patients, 3 patients presented with transient viruria on day one (n = 2) and 12 months later (n = 1). One patient presented with sustained viremia and viruria by the time he was treated for acute grade IIa cellular rejection and cytomegalovirus disease, but BKVN could not be determined following histologic analysis. One patient was clinically stable with sustained BK viruria (>108 copies/ml) and viremia (>104 copies/ml) following four consecutive measurements during the follow-up. Compared to the control group, patients with viremia >104 were dialyzed for longer time periods before transplantation and had higher levels of peak panel reactive antibodies (P < 0.05) (Table III).
| 107 Urine (n = 31) | 104 Serum (n = 6) | Controls (n = 85) | P | |
|---|---|---|---|---|
| Age (yrs) | 55 (27–77) | 54 (30–72) | 54 (18–75) | n.s. |
| Gender (male, %) | 20 (65%) | 5 (83%) | 57 (64%) | n.s. |
| Donor Age (yrs) | 52 (18–77) | 52 (33–58) | 53 (22–77) | n.s. |
| Living donor (%) | 4 (40%) | 1 (17%) | 19 (21%) | n.s. |
| CIT (hours) | 17 (0–24) | 18 (13–21) | 18 (0–26) | n.s. |
| BMI (kg/m2) | 25 (19–33) | 27 (23–30) | 25 (17–34) | n.s. |
| HLA mismatch (avg ± SD) | 3 (0–5) | 3 (2–5) | 4 (0–6) | n.s. |
| First graft (n, %) | 23 (74%) | 5 (83%) | 74 (83%) | n.s. |
| Peak level of PRA | 4 (0–98) | 6 (4–90) | 2 (0–98) | *a |
| CMV serology | n.s. | |||
| D−/R− | 10% | 0% | 9% | n.s.ns |
| D−/R+ | 19% | 17% | 20% | n.s.ns |
| D+/R− | 13% | 17% | 12% | n.s. |
| D+/R+ | 58% | 67% | 60% | n.s. |
| Immunosuppression | n.s. | |||
| Induction | 38% | 33% | 36% | n.s. |
| Tacrolimus | 80% | 67% | 73% | n.s. |
| Cyclosporin A | 17% | 33% | 18% | n.s. |
| Duration of dialysis (weeks) | 37 (0–155) | 53 (31–155) | 21 (0–83) | *a,b |
| Serum creatinine at 12 M | 111 (68–170) | 161 (125–170) | 124 (65–244) | n.s. |
| Proteinuria g/24 h at 12 M | 0.17 (0.0–2) | 0 (0–2) | 0.1 (0–2) | n.s. |
| eGFR at 12 M | 0.93 (0.65–1.47) | 0.71 (0.65–0.75) | 0.77 (0.4–1,78) | *b |
| Rejections (Mean ± SD) at 3 M | 0.35 ± 0.6 | 0.67 ± 0.52 | 0.38 ± 0.49 | n.s. |
| Rejections (Mean ± SD) at 12 M | 0.44 ± 0.71 | 0.83 ± 0.75 | 0.55 ± 0.78 | n.s. |
- Data are shown median (minimum–maximum) values unless otherwise indicated. ESRD, end-stage renal disease; CIT, cold ischemia time; eGFR, estimated glomerular filtration rate; PRA, panel reactive antibody; BMI, body mass index; CMV, cytomegalovirus; D, donor; R, recipient; *(P < 0.05); **(P < 0.01); a, the difference between 104 in serum and normal; b, the difference between 107 in urine and normals; n.s., not significant.
Patients with BK Viruria >107 Copies/ml Urine
The 2005 recommended guidelines for the quantitative BKV urine cutoff loads are >107 copies/ml in urine [Hirsch et al., 2005]. Thirty-one patients had detectable BK-DNAuria >107 copies/ml. Compared to the control group, patients with viruria >107 were treated longer by dialysis and had impaired graft function one-year post transplantation (P < 0.05) (Table II).
Sustained and Transient Viruria
Thirteen patients (10.8%) were positive consistently for two or more consecutive BKV positive urine samples and 18 (15%) patients presented with urine samples that were transiently positive, i.e., 1 positive urine sample. Median BKV loads in patients with sustained and transient viruria were 3.33E + 07 (range 1.02E + 06–5.83E + 08) and 5.27E + 08 (range 6.44 E + 07–1.39E + 10), respectively [P < 0.05]). BKV loads in the serum of patients with sustained and transient viruria showed marginal differences, i.e., 0.00E + 00 (range (0.00E + 00–1.08E + 03) and 5.59E + 03 (range 0.00E + 00–1.14E + 05) (P = 0.08).
BKV Reactivation and its Association with Acute Rejection
Transplant patients presenting with sustained viruria exhibited a higher rate of acute rejection within one-year post Tx compared to patients without BKV reactivation (P < 0.05). Graft rejection in patients with transient viruria was not statistically different from the control group (Fig. 1, P = 0.1). No statistical differences in the highest level of panel reactive antibodies, presence of induction therapy or the number of HLA mismatches were observed between groups.
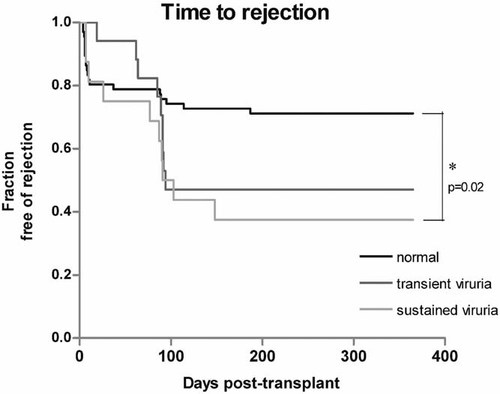
Kaplan–Meier survival analysis for time-to-rejection in patients with sustained and transient viruria. The fraction of patients exhibiting acute rejection within one year post Tx were significantly higher than in patients without BKV reactivation (controls) (P < 0.05). Graft rejection in the group of patients with transient viruria did not reach significant levels (P = 0.1) compared to the control group.
Immunosuppressive Therapy as a Risk Factor for BKV Replication in Urine
The immunosuppressive therapy administered to 90/120 patients consisted of combinations of tacrolimus + mycophenolate mofetil + steroids and from this subset, 2 patients developed BKVN, and BKV replication was detected in 25 patients, i.e., >107 copies in urine samples. The immunosuppressive therapy administered to 22 patients consisted of cyclosporine A + mycophenolate mofetil + steroids (considered a safer treatment modality). One of these patients developed BKVN and four presented with BKV replication in urine, i.e., >107 copies in urine samples. No statistical differences between the different immunosuppressive protocols used and the development of BKNV were observed.
Cut-Off Points for BK Viremia and Viruria
A receiver operating characteristic curve analysis was used to assess the accuracy of quantitative viral load measurements used for BKVN screening that revealed a cut-off point of 103 BKV copies/ml serum and a cutoff point of 6.7 × 107 BKV copies/ml urine (Fig. 2). Thirty-four patients exhibited viremia that was >103 and <104 copies/ml serum and two patients were diagnosed with BKVN, with the remaining patients not demonstrating any clinical or histopathological signs of BKVN. In total, 40/120 patients examined were positive for BK viremia using the redefined cut-off point.
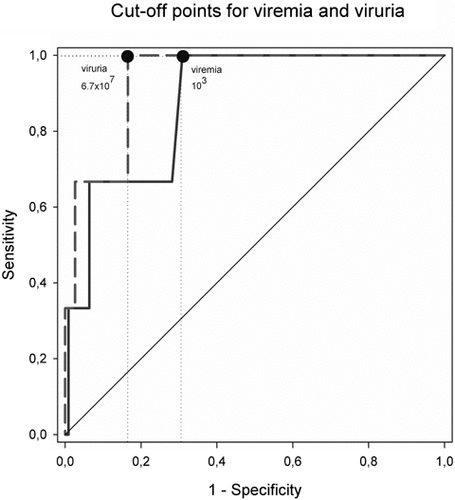
Receiver operating characteristic (ROC) curve analysis for BK viremia and viruria cutoffs used in BKVN screening. The difference in DNA levels between patients with and without BKVN were significant (P < 0.01). In the kidney transplant population studied, BKV viral loads in serum >103 copies/ml showed sensitivity of 100% and specificity of 69% for BKVN. BKV viral loads in urine >6.7 × 107 copies/ml showed sensitivity of 100% and specificity of 83% for BKVN.
DISCUSSION
The aim of this prospective study was to evaluate the incidence of post-transplant polyoma BK viral reactivation by detecting BKV in either urine or blood. The incidence rate described supported data collected from similar studies, that is, viremia and viruria were detectable in 20–40% of patients during their first year after kidney transplantation with a BKVN incidence of 2.5% [Tong et al., 2004; Nickeleit and Mihatsch, 2006; Costa et al., 2008]. Also paralleling other studies, [Koukoulaki et al., 2009] the highest incidence of BK viruria and viremia was assessed during the third month after treatment, with most patients never developing BKVN and clearing their viral load.
Several risk factors have been associated with increased viral replication and nephropathy progression, including warm ischemia and reperfusion injury [Hariharan, 2006], cytomegalovirus co-infection [Vasudev et al., 2005], the level of panel reactive antibodies, duration of dialysis and the type of induction therapy used [Hirsch et al., 2005; Eash et al., 2006; Hariharan, 2006]. In this study, the clinical data corresponding to patients with serum BK viral loads >104 copies/ml and BK viral loads >107 copies/ml urine were identified and compared to the clinical parameters obtained for patients in the control group. This analysis revealed that patients with increased viral loads remained on dialysis for longer time periods before transplantation, had impaired one-year graft function and had a higher level of peak panel reactive antibodies.
Immune suppression is considered the primary risk factor associated with BKV replication [Koukoulaki et al., 2009]. It remains unclear whether specific immunosuppressive agents or their respective doses are critical to BKVN development, however, steroid maintenance therapy, full dose thymoglobulin [Dadhania et al., 2008], increased doses of tacrolimus and mycophenolate mofetil [Mengel et al., 2003; Herman et al., 2004] are considered significant risk factors [Dharnidharka and Gupta, 2009] and some studies have identified relationships between treatment regimens that combined tacrolimus and mycophenolate mofetil with heightened viruria compared to viruria observed in patients treated with cyclosporine and mycophenolate mofetil [Brennan et al., 2005; Hirsch et al., 2005].
The results of this study did not identify statistical differences between immunosuppressive protocols and patients who developed BKVN or who presented with viral reactivation. It has been shown that viremia preceded BKVN by a median of 12 weeks and that viremia levels correlated with emergence of BKV-associated disease [Hirsch et al., 2002; Randhawa et al., 2004], however, these observations were not confirmed by data obtained during the course of this study since two patients had detectable viral loads 8 weeks before diagnosis of BKVN diagnoses and one did not. Quantification by qPCR remains the defining methodology used to establish threshold values for BK DNA-viruria and BK DNA-viremia with 100% specificity [Babel et al., 2009].
The 2005 guidelines defining the recommended quantitative cut-offs for BKV loads in urine were >107 copies/ml and >104 copies/ml in serum [Hirsch et al., 2005] compared to 104 copies/ml of BKV in serum described for the diagnosis of BKVN in a separate study [Boudreault et al., 2009]. Hoffman et al. [2008] uncovered discrepancies in the BKV loads measured by different assays, which partly explains the differences in viral loads described in the context of BKVN presentation. Similarly, in this study, the cut-offs for BKV loads in urine were 6.75 × 107 copies/ml and 103 copies/ml in serum.
In this study, sustained viruria was found to be associated with acute rejection. Similarly, Babel et al. showed that acute rejection was associated with BKV reactivation. In about 50% of patients, acute rejection occurred simultaneously or preceded viral reactivation, thus an increase in immunosuppressive therapies combined with inflammatory responses could lead to BKV reactivation [Babel et al., 2009]. It still remains a matter of debate whether modification to immunosuppressive therapies affects BKV replication resulting in an increased risk of acute rejection. In conclusion, polyoma BK viremia and viruria were identified frequently in kidney transplant recipients, suggesting that monitoring BKV loads in this patient group is a means of preventing graft failure and progression to BKVN.
EXPERIMENTAL ETHICS
The study protocol was approved by the Ethics Committee of the Institute for Clinical and Experimental Medicine in Prague and all patients provided written informed consent prior to participation in the study.
Acknowledgements
We are grateful to R. Polackova for her excellent technical assistance, the patients and nurses from the ward for their cooperation and help.




