The use of dried blood spots for assessing antibody response to hepatitis A virus after natural infection and vaccination†
Financial support: Finep, CNPq.
Abstract
During recent years, vaccination against hepatitis A has been implemented in several countries. It is expected that the increase in mass vaccination against hepatitis A will eventually result in a decreased prevalence of anti-HAV antibodies in the general population. For this reason, a suitable clinical sample for diagnosis of hepatitis A must be sufficiently sensitive to enable detection of lower antibodies titers. In this study, the feasibility of using dried blood spots (DBS) was assessed for the detection of anti-HAV antibodies after a natural infection and vaccination. Seventy-four DBS and paired plasma samples were obtained from a group of college students for a cross-sectional hepatitis A seroepidemiological study. Forty-six students seronegative for anti-HAV were selected randomly and immunized with an inactivated hepatitis A vaccine using an 0–6 month schedule. Seroconversion was monitored in paired plasma and DBS samples 6 months after the first dose followed by a period of 8 and 24 months after the second dose. A strong correlation between OD/CO rates of paired plasma and DBS samples for the detection of anti-HAV was observed. The sensitivity and specificity of the DBS compared with plasma for the detection of anti-HAV antibodies after natural infection was 100%. The sensitivity of DBS in samples collected 24 months after the second dose of hepatitis A vaccine was 95.4%. The results showed that DBS samples can be used for the detection of anti-HAV antibodies both after natural infection or vaccination. J. Med. Virol. 83:208–217, 2011. © 2010 Wiley-Liss, Inc.
INTRODUCTION
Worldwide, there has been an increase in the number of individuals susceptible to hepatitis A virus (HAV) infection due to improved standards of living, which increases the importance of hepatitis A vaccination. Vaccination programs for hepatitis A in childhood have been introduced in several countries including Argentina, Australia, Israel, Italy (Puglia), Spain (Catalonia), and the United States. The effects of these vaccination programs have been confirmed by a substantial reduction in HAV incidence, outbreaks, mortality rates, and hospitalization [Hendrickx et al., 2008]. However, any strategy of implementing a hepatitis A vaccination program should take into account factors such as the level of endemicity, socio-economic development and sanitation, and the risk of outbreaks. Epidemiological data are often obtained from seroepidemiological surveys of antibody prevalence, which provide useful information on the immunity of populations. The potential of serosurveillance used on large-scale surveys could be hampered due to the invasive nature of blood collection procedures, associated with both ethical and technical issues. The development of less invasive methods for sample collection in non-clinical settings may be particularly useful when such procedures are carried out in developing countries, where poor health services, inadequate laboratory infrastructure, and logistical obstacles associated with sample collection and transportation may hamper health-surveillance [Boerma et al., 2001]. The use of dried blood spots (DBS), drops of whole blood collected on filter paper from a finger prick, has emerged as an alternative to venipuncture. The advantages of using DBS samples include the relative ease and low cost of sample collection, transport, and storage. Disadvantages include requirements for development and validation of assays as well as small volume of samples collected [Parker and Cubit, 1999]. Since its first implementation for the diagnosis of phenylketonuria in newborns in the 1960s [McDade et al., 2007], DBS has been used for the diagnosis of a number of metabolic disorders and for the analysis of a wide range of biomarkers [McDade et al., 2007], including antibodies raised against several viruses, such as the human immunodeficiency virus [Major et al., 1991; Castro et al., 2008], rubella virus [Punnarugsa and Mungmee, 1991; Neto et al., 1995; Helfand et al., 2001, 2007; Karapanagiotidis et al., 2005], measles virus [Helfand et al., 2001; Ridell et al., 2002], HTLV [Parker et al., 1995], Epstein-Barr virus [Fachiroh et al., 2008], and hepatitis viruses [Villa et al., 1981; Gil et al., 1997; Tappin et al., 1998; De Almeida et al., 1999; McCarron et al., 1999; Desbois et al., 2009]. However, the use of DBS for the detection of antibodies to hepatitis A virus (anti-HAV) has been limited [Gil et al., 1997; De Almeida et al., 1999; Desbois et al., 2009], and there are no studies which describe the use of DBS for assessing the humoral response after hepatitis A vaccination. Serological testing for detection of immunoglubulin M (IgM) antibody to the capsid proteins of HAV (IgM anti-HAV) is required to confirm the diagnosis of acute HAV infection. In the majority of individuals, serum IgM anti-HAV becomes detectable 5–10 days before the onset of symptoms [Liaw et al., 1986; Bower et al., 2000]. IgG anti-HAV, which appears early in the course of infection, remains detectable throughout life, and provides lifelong protection against the infection. Total anti-HAV testing is used in epidemiological studies to determine the prevalence of previous infection or by physicians to determine whether a person with an indication for pre-exposure prophylaxis is already immune. Four inactivated vaccines are available [Center for Disease Control and Prevention, 2006]. Vaccination induces protective anti-HAV antibody levels in 94–100% of individuals one month after the first dose. A full primary (i.e. two-dose) schedule confers protection in terms of antibody persistence for at least 5 years in children and 10 years in adults [Werzberger et al., 1998; Van Herck et al., 2004; Rendi-Wagner et al., 2007]. However, vaccination in terms of a long-term protection and alternative schedules (e.g. one-dose at 12 months of age as adopted in Argentina), should be investigated further. This study describes a comparison of results from paired plasma and DBS samples for the detection of anti-HAV by EIA. The results showed that DBS can be a reliable method for detecting anti-HAV antibodies both after natural infection or vaccination.
METHODS
Studied Group
This cross-sectional hepatitis A seroepidemiological study was conducted in a group of students from a public medical university in Rio de Janeiro, Brazil (Instituto Biomédico, Universidade Federal Fluminense). For this purpose, paired venous and capillary blood samples were collected from recruited individuals. Protocols for collection and use of specimen were submitted and approved by the Human Research Committee of Oswaldo Cruz Foundation, Health Ministry, Rio de Janeiro, Brazil (protocol number: 407/01).
On recruitment day (13th March, 2007), a total of 80 students of the same Virology class were invited to take part in the study, and 74 of them (mean age 20.6 ± 1.9 years old) agreed to participate by signing a formal consent form. Demographic data such as age, gender, family income, parents' educational status, previous history of hepatitis and vaccination against hepatitis A, and housing sanitary conditions were collected. The socioeconomic status of participants was recorded using an index adapted from Toukan et al. (1990) was used.
HAV Vaccination
Forty-six students seronegative for anti-HAV were selected at random and immunized with an inactivated hepatitis A vaccine (Merck Sharp & Dohme, West Point, USA) using a 0–6 months schedule. The vaccine contained 50 U/1.0 mL of inactivated hepatitis A antigen adsorbed to 0.45 mg aluminum hydroxide (adult dose). The vaccine was administered intramuscularly in the deltoid region of the left arm. To investigate the post-vaccination humoral immune response, vaccinated individuals were monitored four times throughout: time 0 (T0), before the 1st dose of vaccine; time 1 (T1), 6 months after the 1st dose; time 2 (T2), 8 months after the 2nd dose; and, time 3 (T3), 24 months after the 2nd dose. All vaccinated individuals were monitored for a period of 14 months. 22 individuals out of 46 were followed for a period of 30 months (Fig. 1).
Study design. All vaccinated students were monitored for a period of 14 months and 22 out of 46 students were followed for a period of 30 months.
Sample Collection and Processing
To validate DBS as a reliable clinical sample for detecting anti-HAV after natural HAV infection and vaccination against hepatitis A, DBS, and venous blood samples were collected from each individual during the study and after vaccination against hepatitis A.
To find a reliable parameter for DBS sample collection and processing, several variables were evaluated before testing the samples. Issued such as the type of filter paper, the size of blood spot, storage conditions of DBS spots, the type and volume of elution buffer, and the quantity of DBS eluate to be used for immunoassay were considered.
Using universal precautions, venous and capillary blood samples were collected by venepuncture (VaccuntainerTM, Becton Dickinson, Franklin Lakes, New Jersey, USA) and finger stick (BD Lancet Device, BD Consumer Healthcare, New Jersey, USA), respectively. Capillary blood drops were applied to Whatman Protein Saver 903 Filter Paper Card (Whatman Inc., Piscataway, New Jersey, USA) until filling a preprinted circle of 12.5 mm diameter. When filled to its border, each circle contained approximately 80 µL of whole blood, which is equivalent to approximately 26 µL of serum [Steger et al., 1990; McDade et al., 2007]. Blood spots were dried at room temperature for a minimum of 4 hr, then placed in zip-closure bags with a desiccant pack and stored at −20°C. Blood samples were placed in microtainer tubes, centrifuged (224 g), and plasma was separated and frozen at −20°C.
Elution of DBS Samples
The whole circle of 12.5 mm diameter was punched from each filter paper card using a paper hole punch (TedPella®, Redding, California, USA). The card was then placed in plastic tubes with 350 µL of phosphate-buffered saline containing 0.2% Tween 20 and 5% bovine plasma albumin (PBST-BSA), shaken at room temperature for 30 min, followed by an overnight incubation at 4°C, and again shaken at room temperature for 15 min before centrifugation (224 g). Filter papers were removed with a disposable stick, and eluates were stored at −20°C until tested.
Laboratory Tests
All technical procedures were carried out at Oswaldo Cruz Institute (Laboratório de Desenvolvimento Tecnológico em Virologia), Rio de Janeiro, Brazil. DBS eluates and plasma samples were assayed for total anti-HAV by a commercial competitive enzyme-linked immunosorbent assay (Bioelisa HAV-EIA, Biokit, Barcelona, Spain). The detection limit of lots used in the study of Bioelisa HAV EIA was 75 mUI/ml (lot L-0407) and 67 mIU/ml (lot I-3308). DBS eluates were tested using a modified protocol. According to manufacturer's protocol, 10 µL of plasma was incubated with 100 µL of monoclonal anti-HAV antibodies conjugated with peroxidase in a well coated with HAV. For DBS, 100 µL of the eluate was incubated with the same volume of anti-HAV peroxidase conjugate. The tenfold increase in sample volume was applied to compensate the dilution of serum after elution of DBS. Despite this change in protocol, the input of serum in the EIA reaction remained 2.6 times smaller for DBS. This is because after elution in 350 µL of PBST-BSA, the volume of serum contained in 80 µL of DBS was initially diluted 1/13. After incubation with the same volume of anti-HAV peroxidase conjugate, the final dilution in the reaction was 1/26 compared with plasma samples, which are diluted 1/10. In addition to the required number of kit control samples, each test run included negative and positive DBS-controls. To evaluate if the value of cut-off (CO) established for plasma samples would be suitable for DBS eluates, negative and positive control sera were diluted 1/10 and 1/26 for CO calculation when samples obtained from the cross-sectional study were assayed. The comparison of signal-to-CO ratios (i.e., optical density [OD]/CO) obtained from each of the 74 DBS eluates using both control dilutions did not show any significant difference between them (t = 0.355). For this reason, the CO value established for plasma samples was applied for DBS eluates at the subsequent reactions. EIA results were read according to OD/CO ratios, which were positive when OD/CO ≤ 1.0, negative when OD/CO > 1.1, and indeterminate when OD/CO > 1.0 ≤ 1.1.
Statistical Analysis
A logistic regression test was used to establish a correlation between the socio-economic data and OD/CO ratios from samples collected before and after vaccination against hepatitis A. The following variables were analyzed: age, gender, socioeconomic level, parents' educational status, number of adults/children in the household, number of rooms at the household, sewage facilities in the household, previous history of hepatitis, vaccination against hepatitis A, and contact with an icteric patient.
Variance analysis (Kruskal-Wallis, software GraphPad Prisma®) was used for comparing OD/CO ratios from plasma and DBS samples obtained during the follow-up period (T0, T1, T2, and T3).
Sensitivity, specificity, positive and negative predictive values, and test efficiency were calculated using the plasma results as reference standards. Pearson's correlation test was employed additionally to investigate if there was a linear correlation between OD/CO ratios from paired plasma and DBS eluates.
RESULTS
Socioeconomic Characteristics and Anti-HAV Prevalence in the Studied Group
A total of 74 students who signed the informed consent form were included in the study. Of these, 43 (58.2%) were female and 31 (41.8%) were male. The analysis of demographic variables showed that students came from very similar socioeconomic background, most of them belonging to middle to high socioeconomic levels (70/74, 94.6%) with parents with university level of education (57/74, 77%), and living in houses with access to piped water and sewage system (74/74, 100%) (Table I).
| Variables | Total cohort (N = 74) | Pa |
|---|---|---|
| Average age standard deviation (years) | 20.6 ± 1.9 | 0.399 |
| Gender ratio (male/female) | 31/43 | 0.098 |
| Socioeconomic level, n (%) | ||
| Low | 4 (5.4) | 0.996 |
| Middle | 20 (27) | 0.470 |
| High | 50 (67.6) | 0.999 |
| Number of subjects in the household, n (%) | ||
| 1–3 | 15 (20.3) | 0.904 |
| 4–6 | 57 (77) | 0.749 |
| 7–10 | 2 (2.7) | 0.995 |
| Number of children in the household, n (%) | ||
| None | 58 (78.4) | 0.615 |
| 1–2 | 13 (17.6) | 0.839 |
| >2 | 3 (4) | 0.995 |
| Parents' educational levelb, n (%) | ||
| None | 1 (1.4) | 1.000 |
| Primary school | 6 (8.1) | 0.996 |
| Secondary school | 10 (13.5) | 0.995 |
| College or university | 57 (77) | 0.595 |
| Sewage facilities, n (%) | ||
| Yes | 74 (100) | >0.05 |
| No | 0 (0) | >0.05 |
| Previous contact with icteric patient, n (%) | ||
| Yes | 6 (8.1) | 0.997 |
| No | 68 (91.8) | 0.995 |
| Past history of hepatitis, n (%) | ||
| Yes | 1 (1.4) | 0.995 |
| No | 73 (98.6) | 0.998 |
- a Logistic regression test: calculated by software MedCalc® Setup version 11.1.1.0 (Demo version).
- b Educational level of senior family member.
The overall prevalence for anti-HAV antibodies estimated in both plasma and DBS samples was 10.8% (8/74). It was not detected any statistical association between anti-HAV prevalence and demographic characteristics such as parents educational level, socioeconomic status, number of people living in the household, number of children in the residence, and housing sanitary conditions (P > 0.05). Other variables were also not statistically associated with anti-HAV including age, gender, contact with icteric patient, and previous history of hepatitis (P > 0.05). However, female gender was strongly associated with humoral response after hepatitis A vaccination (P < 0.0001), as shown below.
Seroconversion Rate After Hepatitis A Vaccination
The OD/CO ratios of each plasma sample collected before and after vaccination for anti-HAV detection are shown in Figure 2. Induction of HAV-specific antibodies was monitored up to 24 months after the last dose of vaccine. Within 6 months after the first dose (T1), 69.6% (32/46) of the vaccinees were found seropositive with an average OD/CO ratio significantly lower (0.794) than the one observed before vaccination (1.34) (P < 0.001). After the second dose (T2), all vaccinees were seropositive and presented a significant fall on the average of OD/CO ratio (0.111) (P < 0.001), which showed a slight increase after 24 months (0.285) but without statistical significance in relation to T2 (P > 0.05). When vaccinees were separated by gender, it was possible to observe that from the total of 32 subjects that seroconverted after the first dose, 23 (72.7%) were female and 9 were male (27.2%) (Fig. 3). The variance analysis was used to compare OD/CO ratios of plasma samples collected before and after vaccination. Although samples collected at T0, T2, and T3 presented similar OD/CO ratios independently of the gender (difference among the sum of OD/CO averages: 7.78 (T0),—29.69 (T2), and −42.49 (T3), P > 0.05), there was a significant difference in OD/CO ratios at T1 when gender was considered (difference among the sum of OD/CO averages:−83.91, P < 0.05).
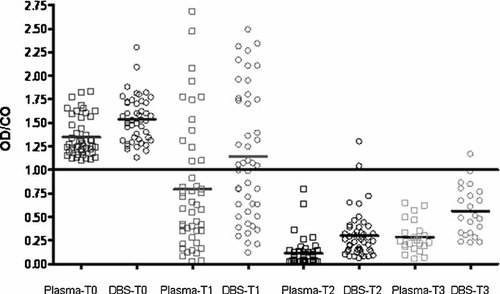
OD/CO ratios of each plasma or DBS samples are plotted according to time of collection: T0, before vaccination; T1, 6 months after first dose; T2 and T3, 8 months and 24 months after second dose, respectively. Samples with OD/CO ratios below 1.0 are considered positive for anti-HAV. Averages of OD/CO ratios at T0, T1, T2, and T3 time points for plasma and DBS samples were the following: 1.349/1.537, 0.794/1.141, 0.111/0.302, and 0.285/0.659.
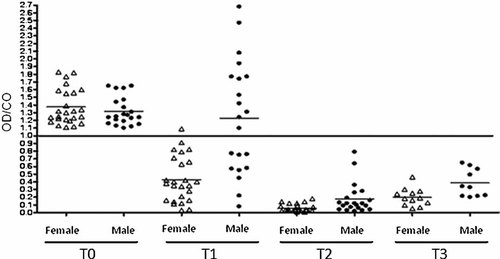
OD/CO ratios of each plasma or DBS samples separated by gender are plotted according to the time of collection: T0, before vaccination; T1, 6 months after first dose; T2 and T3, 8 months and 24 months after second dose, respectively. Samples with OD/CO ratios below 1.0 are considered positive for anti-HAV. Averages of OD/CO ratios at T0, T1, T2, and T3 time points for female and male gender were the following: 1.374/1.319, 0.429/1.228, 0.055/0.178, and 0.201/0.385.
Validation of DBS Eluate as a Clinical Sample for Anti-HAV Detection
A pilot study was performed previously to test the likely success of using the protocol developed for detecting anti-HAV from DBS eluates using the Bioelisa HAV-EIA, Biokit. DBS samples were obtained from anti-HAV seropositive (n = 21) and anti-HAV seronegative (n = 10) individuals. All 21 samples identified previously as anti-HAV seropositive were also positive on DBS eluates. The other 10 samples were negative for anti-HAV in DBS eluates.
Cross-sectional Study
The results of plasma and DBS samples collected initially for the analysis of the anti-HAV prevalence (T0) using the Bioelisa anti-HAV tests are shown in Table I. No false positive or false negative results were observed with DBS samples. A strong correlation among OD/CO rates of paired plasma and DBS samples was observed (r2 = 0.6262; IC = 0.8133−1.175; P < 0.001) (Fig. 4a).
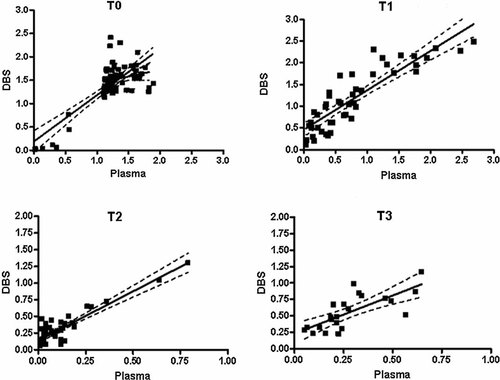
Correlation of OD/CO ratios for plasma/DBS sample pairs. The solid line represents a perfect correlation of 1.00, and the diagonal dashed lines represent the trend lines. (a) Results obtained with plasma/DBS sample pairs collected before vaccination, and at three time points after immunization: (b) 6 months after first dose; (c) 8 months, and (d) 24 months after second dose.
Detection of Anti-HAV After Hepatitis A Vaccination
Seventy percent (32/46) of samples collected 6 months after the first dose (T1) were concordant for anti-HAV testing (Table II). Of the 32 anti-HAV positive plasma samples, 20 were positive, 6 were indeterminate, and 6 were negative for DBS testing. After second immunization for hepatitis A, there was an impressive increase in the concordance of results obtained for plasma and DBS samples. Agreement was observed in 95.6% (44/46) and 95.4% (21/22) of samples collected at T2 and T3, respectively. The ability to detect the presence of anti-HAV in DBS obtained from vaccinees could be increased if indeterminate and positive results were combined, especially for samples with lower anti-HAV titer, such as after administration of the first dose (T1). The addition of indeterminate to positive results at T1, for example, increased the rate of anti-HAV detection from 62.5% (20/32) to 81.2% (26/32). A strong correlation among OD/CO rates of paired plasma and DBS samples could be observed at Figure 4 at all three different periods after vaccination against hepatitis A: T1 [r2 = 0.7695; IC(99%) = 0.757−1.059; P < 0.0001)], T2 [r2 = 0.8242; IC(99%) = 1.269−1.683; P < 0.0001], and T3 [r2 = 0.5522; IC(99%) = 0.4680−0.8869; P < 0.0001]. The association of female gender and faster humoral response was also detected when samples of DBS were analyzed (difference among the sum of OD/CO averages: 4.16 (T0, P > 0.05),−87.31 (T1, P < 0.05),−39.81 (T2, P > 0.05), and −30.18 (T3, P > 0.05).
| DBS samples | Plasma samples | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-HAV detection after hepatitis A vaccination | ||||||||||||||||
| Anti-HAV detection after HAV natural infection | 6 months after 1st dose (T1) | 8 months after 2nd dose (T2) | 24 months after 2nd dose (T3) | |||||||||||||
| Pos | Ind | Neg | Total | Pos | Ind | Neg | Total | Pos | Ind | Neg | Total | Pos | Ind | Neg | Total | |
| Positive | 8 | 0 | 0 | 8 | 20 | 0 | 0 | 20 | 44 | 0 | 0 | 44 | 21 | 0 | 0 | 21 |
| Indeterminate | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 6 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Negative | 0 | 0 | 66 | 66 | 6 | 2 | 12 | 20 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 |
| Total | 8 | 0 | 66 | 74 | 32 | 2 | 12 | 46 | 46 | 0 | 0 | 46 | 22 | 0 | 0 | 22 |
- Pos, positive; Neg, negative; Ind, indeterminate.
Table III shows estimates of sensitivity, specificity, positive and negative predictive values, and test efficiency of DBS compared to plasma. The point estimated of the DBS sensitivity varied from 76.9% to 100%. The higher sensitivity value (100%) was observed with DBS samples obtained from naturally HAV infected persons, and the lower one (76.9%) with DBS samples collected 6 months after the 1st dose of vaccination against hepatitis A. Counting DBS indeterminate results as positive increased the lower point estimative of DBS sensitivity from 76.9% to 81.2%. The manufacturer quotes a sensitivity of 99.8% and specificity of 99.5% when the kit is used with plasma. The DBS point estimated of specificity for T0 and T1 was always 100%, as there were no negative plasma samples that were DBS-positive. The specificity could not be calculated for T2 and T3 because there were no negative anti-HAV samples presented after the second dose of vaccination against hepatitis A. The positive predictive value of DBS was always 100% irrespectively of time of sample collection. The point estimated of the negative predictive value for T0 and T1 were 100% and 64.7%, respectively. Test efficiency ranged between 84.2 (T1) and 100% (T0).
| Anti-HAV detection | Anti-HAV in DBS for ELISA % (95% CI) | ||||
|---|---|---|---|---|---|
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | Test efficiency | |
| Natural infection | 100 (84.6–100) | 100 (83–100) | 100 (84.6–100) | 100 (83–100) | 100 (94.9–100) |
| Vaccine response (1st dose) | 76.9 (68.4–85.4) | 100 (87.4–100) | 100 (91.5–100) | 64.7 (56.5–77) | 84.2 (77.1–91.3) |
| Vaccine response (8 months after 2nd dose) | 97.7 (91.3–99.5) | a | 100 (93.6–100) | a | 97.8 (91.4–99.8) |
| Vaccine response (24 months after 2nd dose) | 95.4 (86.2–100) | a | 100 (90.5–100) | a | 95.5 (86.3–96) |
- a Not calculated because all plasma samples were anti-HAV positive.
DISCUSSION
Epidemiological studies have a critical importance for surveillance and control of infectious diseases. However, the lack of resources as well as logistical constraints associated with the collection and testing of clinical samples have been considered as significant impediments for performing studies of this nature [McDade et al., 2007]. DBS have been used for the diagnosis of infections and for sentinel surveys in many countries worldwide [Parker and Cubit, 1999; McDade et al., 2007]. Nevertheless, because this sample is considered less sensitive than the testingof plasma, it has been little used in epidemiological studies for hepatitis A [Gil et al., 1997; De Almeida et al., 1999; Desbois et al., 2009]. Data from this study show that DBS can be a reliable sample not only for the diagnosis of past HAV infection with an equivalent sensitivity of serological diagnosis, but also for assessing the humoral response after vaccination against hepatitis A. The importance of this investigation is that with the crescent introduction of mass vaccination against hepatitis A in several countries, there will be a decrease in the levels of anti-HAV antibodies in the general population. This is expected because vaccination induces titers of anti-HAV that are lower than those produced after a natural infection and below the detection level of some commercially available diagnostic assays [Wasley et al., 2006]. Titers of antibody achieved after passive transfer by IG or active induction by vaccination ranges from 10- to 100-fold lower than those produced after a natural infection [Lemon et al., 1997]. A suitable clinical sample for the diagnosis of hepatitis A has to be sufficiently sensitive to enable the detection of lower antibodies titers.
A strong correlation among OD/CO rates of paired plasma and DBS samples for detecting anti-HAV was observed. This result suggests that the commercial assay for anti-HAV detection used in this study can be adapted for DBS testing without the need to recalculate the cut-off values. To compensate plasma dilution after the elution process, an increase of 10X in the volume of sample was necessary. The inclusion of indeterminate results with positive results can also be used as an alternative to adjust for a slightly lower OD values obtained from DBS in relation to those obtained from sera. This simple adjustment method has been used by other authors enabling obtainment of a good correlation between sera and DBS results [Karapanagiotidis et al., 2005; Helfand et al., 2007]. In this study, no indeterminate results for plasma and DBS samples collected before vaccination for the cross-sectional study were found. For the analysis of antibody response after vaccination, counting of DBS indeterminate results as positive increased the lower point estimative of DBS sensitivity from 76.9% to 81.2%. Once these results were obtained from vaccinated individuals that were seronegative previously, these few equivocal samples would be considered to be positive with lower anti-HAV titer still under the detection limit of the assay. In fact, all subjects who presented indeterminate results for DBS after the first dose (T1) seroconverted for anti-HAV at the following sample collection (T2).
The use of DBS for the investigation of HAV infection is beneficial for several reasons. Firstly, it can be used for both diagnosis of hepatitis A as well as for detection of HAV. Desbois et al. (2009) have detected successfully HAV-specific IgM from sera spotted onto filter paper of laboratory confirmed acute hepatitis A cases with a sensitivity and specificity of 100%. They were also able to amplify HAV RNA sequences in 23 of 26 HAV RNA-positive sera samples, which after sequencing were shown to be identical to those found in paired plasma samples. Secondly, the collection of samples of blood from finger stick is often easier to obtain and more acceptable for use with infants and young children. According to Neto et al. (1995), the percentage of individuals who refuse to take part in surveys based on venopuncture procedures can reach 13%. Finally, samples can be stored up to one month at room temperature or at 37°C with no need of processing [Desbois et al., 2009], which provides flexibility when managing the collection of samples in field settings.
In this study, a lower rate of anti-HAV seroconversion was detected after the first dose of hepatitis A vaccine (69.6%) which differs from those found by other authors (93.5–100%) [Center for Disease Control and Prevention, 2006]. It has been described by several investigators that commercially available immunoassays for total anti-HAV antibodies are in general relatively non-sensitive, and may not detect protective antibody responses after a single dose of inactivated HAV vaccine [Lemon, 1997; Lemon et al., 1997; Nainan et al., 2006]. Unless they are modified, commercial tests for total anti-HAV are not sufficiently sensitive to detect antibody concentrations in a significant proportion of immunized individuals [Lemon, 1993; Center for Disease Control and Prevention, 2006]. Although the protocol for administration of the vaccine against for hepatitis A involves two doses, any investigation concerning the humoral response after vaccination will consider results obtained after the application of the second dose. By this time, the sensitivity of DBS was almost the same as presented in plasma samples, at a rate of 97.7%.
Data obtained in this study are based on a limited group of subjects. The number of subjects in studies involving the evaluation of immune response after vaccination against hepatitis A varies in general from 10 to more than 100 individuals when they were monitored for a total period of 7 months (last samples collected 1 month after the second dose taken at 6th month) [Cederna et al., 2000; Burgess et al., 2001; Guptan et al., 2002; Schmidtke et al., 2005; Nyström et al., 2008]. In this study, 46 subjects were monitored for a period of 24 months after they have taken the second dose. Since this study dealt with a rather small number of participants, a group of individuals with very similar socioeconomic backgrounds was selected intentionally, which led to a homogenous group. This last characteristic was highlighted by the analysis of those variants according to the anti-HAV prevalence, which was not significant with none other analyzed (Table I).
In agreement with other investigators [Wolters et al., 2003; Van Der Wielen et al., 2006; Weissman et al., 2006; Höler et al., 2007], a significant (P < 0.05) effect of gender on the humoral response was found after vaccination against hepatitis A. Sex-difference in antibody response, female greater than male, was also observed with other types of vaccines such as influenza, hepatitis B, rubella, measles, diphtheria, tetanus, brucella, and rabies [Green et al., 1994; Mitchell, 1999; Moore et al., 2003; Cook et al., 2006; Van Der Wielen et al., 2006]. In this study, the rate of seroconversion in females was roughly twice as high in comparison to males after the first dose. Beyond that, females also developed a higher anti-HAV titer at this point in time observed by the analysis of the difference between OD/CO ratios when gender was considered (P < 0.05). The genesis of the sex-difference in antibody response with vaccines is still uncertain. Cook (2008) carried out an extensive analysis of the literature and retrieved 94 studies that showed different humoral responses from viral and bacterial vaccines in females and males. Cook (2008) observed that this difference is not entirely related to gonadal hormones (differences were observed in pre-pubertal and post-menopausal subjects not on hormone replacement therapy) or female sex (males showed greater serological response to several other types of vaccines), but probably reflects an specific antigen interaction with the immune system via mechanism as yet to be defined.
A comparison between seropositivity observed in this study with data obtained in previous seroprevalence studies carried out at the same university with college students during the last 20 years showed a marked decline in the anti-HAV prevalence (54% to 10.8%, P < 0.0001) [Oliveira et al., 1991; Vitral et al., 1998; Guedes et al., 2007]. The results of seroprevalence studies conducted in several regions in Brazil have shown an increase in the number of susceptible individuals for HAV infections [Vitral et al., 2006, 2008]. Although vaccination against hepatitis A is still not included in the Brazilian National Program for Immunization, there is a formal project being evaluated by the Ministry of Health for its future inclusion at the childhood immunization program.
In conclusion, results of this study showed that DBS samples can be used for detecting anti-HAV antibodies both after natural infection or vaccination. The collection of blood by finger prick in preprinted 12.5 mm circles of filter paper would represent a very practical manner for obtaining blood samples for epidemiological studies or large-scale screening projects of HAV infection.




