Host sphingolipid biosynthesis is a promising therapeutic target for the inhibition of hepatitis B virus replication
Abstract
Serine palmitoyltransferase (SPT) catalyzes the first step in the sphingolipid biosynthetic pathway. Myriocin inhibits SPT and was shown to suppress the replication of hepatitis C virus (HCV) in vitro and in vivo. However, its effect on hepatitis B virus (HBV) replication is unknown. In this study, the HBV DNA levels in HuH7 cell culture supernatants were lowered successfully by using myriocin and it was found that the 50% inhibitory concentration of myriocin is approximately 5 µM. Myriocin and/or pegylated interferon (PEG-IFN) were also administered to chimeric mice for 2 weeks and the effects of these compounds on HBV DNA levels were determined. Myriocin alone did not reduce effectively the HBV DNA levels, whereas PEG-IFN alone reduced the DNA levels to 1/10th of the control levels. The combination of myriocin with PEG-IFN reduced the HBV levels to about 1/1,000th of the control levels and induced a 1.0 log reduction in the levels of the HBV surface antigen and core protein. This latter effect was not observed in the other treatment groups. In conclusion, the combination of myriocin with PEG-IFN represses synergistically HBV replication in vivo without inducing hepatotoxicity. J. Med. Virol. 83:587–593, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Infection with hepatitis B virus (HBV) is widespread and affects more than 350 million people worldwide. Chronic HBV infection may progress to complications such as cirrhosis and hepatocellular carcinoma [Lok, 2002]. Although there is no cure for HBV infection, several antiviral drugs are being used currently for treatment [Sorrell et al., 2009]. However, the poor response rate and development of resistant mutations warrant the search for alternative and the conventional therapeutic options [Liaw, 2001].
As rapidly mutating viruses represent a “moving target” for antiviral drugs, targeting of host proteins required for viral replication during the early stages of viral infection, can be an appropriate therapeutic strategy. This was demonstrated recently for NA255, a lipophilic long-chain base compound with a structure similar to that of myriocin that could inhibit efficiently replication of hepatitis C virus (HCV) in in vitro subgenomic replicon cell culture system [Sakamoto et al., 2005].
Myriocin is a specific inhibitor of serine palmitoyltransferase (SPT), which catalyzes the first step in the sphingolipid biosynthetic pathway (Fig. 1) [Fujita et al., 1994; Miyake et al., 1995]. The in vitro and in vivo replication of several viruses such as HCV is suppressed by myriocin, as observed in experiments involving severe combined immunodeficient mice transgenic for the urokinase-type plasminogen activator (uPA/SCID mice) which had undergone human hepatocyte transplantation (hereafter referred to as chimeric mice) [Umehara et al., 2006]. It is generally accepted that a complex of non-structural HCV proteins is associated with the lipid rafts on the Golgi apparatus and endoplasmic reticulum (ER) membranes, where HCV replication occurs. Myriocin-induced SPT inhibition is thought to eventually lead to disruption of the lipid raft assembly, because sphingomyelin is one of the integral components of this assembly [Umehara et al., 2006].
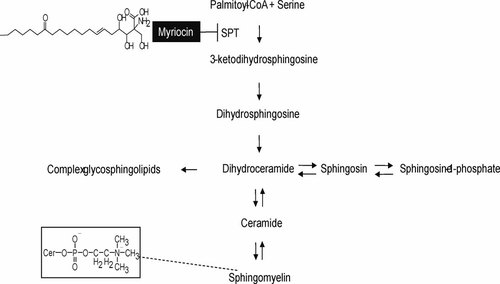
Sphingolipid biosynthetic pathway and the structures of myriocin and sphingomyelin.
It is not known however, whether an SPT inhibitor with a target upstream of the sphingolipid biosynthesis pathway suppresses replication of HBV. In the present study, the anti-HBV effect of myriocin alone or in combination with pegylated interferon (PEG-IFN) was determined experimentally using HuH7 cells transfected with HBV. The synergistic anti-HBV effect of the combination of myriocin and PEG-IFN in vivo was confirmed further on chimeric mice infected with HBV and hypothetical mechanisms of this effect are discussed.
MATERIALS AND METHODS
Plasmid Constructs for HBV DNA and Sequencing
A constructed [Sugiyama et al., 2006] HBV genotype C (HBV/C) clone containing 1.24-fold of the HBV genome (C_JPNAT) was used in this study. The HBV DNA clone was confirmed to possess the intended sequence by using the Prism Big Dye (Applied Biosystems, Foster City, CA) in an ABI 3100 automated sequencer. This clone possessed no G1896A mutations in the precore region or A1762T/G1764A mutations in the basic core promoter. These mutations interfere with the expression of HBV e antigen (HBeAg) and the efficiency of pregenome encapsidation for replication.
Cell Culture and Transfection
After 16 hr of culture, HuH7 cells were transfected with 5 µg of the DNA construct by using Fugene 6 reagent (Roche Diagnostics, Indianapolis, IN) and were harvested 3 days later. The transfection efficiency was measured by cotransfection with 0.5 µg of a reporter plasmid expressing secreted alkaline phosphatase (SEAP) and by estimating SEAP enzymatic activity in the culture supernatant.
Inhibition Assay of Replication in HuH7 Cells Transfected With HBV
Myriocin extracted from natural products [Kluepfel et al., 1972] was added to the HuH7 cells transfected with HBV/C at final concentrations of 0, 0.1, 1, 5, 7.5, 10, 15, and 20 µM. After 72 hr of incubation, the supernatant was collected from each 10-cm dish by centrifugation at 22,000g for 5 min, and a 100 µl sample was treated with 200 µg/ml DNaseI at 37°C for 30 min. The reaction was terminated by the addition of EDTA at a final concentration of 10 mM, and the mixture was incubated at 65°C for 10 min. HBV DNA was extracted using microspin columns (QIAamp Blood kit; Qiagen K.K., Tokyo, Japan). For real-time detection PCR (RTD-PCR), 5 µl of the eluted sample was amplified in a 25 µl mixture containing 2× TaqMan Universal Mastermix (Applied Biosystems, Foster City, CA), a forward primer (HBV-S190F), a reverse primer (HBV-S703R), and a TaqMan probe (HBSP2) (for details, see Supplementary Information).
Measurement of Cell Viability Using TetraColor One (WST-8) Assay
Myriocin was added to HuH7 cells transfected with HBV/C as described above. After 72 hr of incubation, cell viability was measured using the TetraColor One kit (Seikagakukougyo, Tokyo, Japan), according to the manufacturer's instructions.
Inoculation of Chimeric Mice
Chimeric mice were purchased from Phoenix Bio Co. Ltd. (Hiroshima, Japan). Human hepatocytes were obtained from BD Biosciences (San Jose, CA). The levels of human serum albumin in the chimeric mice were measured using commercial enzyme-linked immunosorbent assay kits (Eiken Chemical, Tokyo, Japan). It was ensured that all mice had very similar serum levels of human albumin and body weights in order to obtain reliable comparisons. Animals were housed in clean area, and cared for in accordance with Act on Welfare and Management of Animals (act no. 105 of October 1, 1973) and Guidelines for Proper Conduct of Animal Experiments (Science Council of Japan. June 1, 2006). Experimental protocols were reviewed and approved by the Animal Welfare Committee of Phoenix Bio Co., Ltd.
Assuming that a mixture of immature virions can be present in the Huh7 cell supernatants transfected with HBV plasmids [Yuan et al., 1999; Chua et al., 2005], Direct use of the supernatants was avoided in the experimental mice. Initially, “donor mice” were infected with the culture media and after successful infection was established, the sera obtained from the donor mice were used to infect experimental mice. Thirteen mice were thereby infected successfully with HBV/C recovered from sera of the donor mice as described in the previous report [Sugiyama et al., 2006].
Administration of Myriocin and/or PEG-IFN to Chimeric Mice Infected With HBV/C
Injection of myriocin and/or PEG-IFN (Chugai, Tokyo, Japan) were administered to the mice infected with HBV/C, and blood samples were then collected according to the protocol shown in Table I.
| Day | −1 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Collection of blood | B | B | B | B | B | ||||||
| Control (DMSO) | D | D | D | D | D | D | D | D | |||
| PEG-IFN | I | I | I | ||||||||
| Myriocin | M | M | M | M | M | M | M | M | |||
| Myriocin + PEG-IFN | M/I | M | M | M/I | M | M | M/I | M |
- *B, D, I, or M indicates that each manipulation was performed as required, and the administration of reagents was started from day 0. PEG-IFN was subcutaneously injected at 30 µg/kg. The amount of myriocin intraperitoneally injected was adjusted according to the body weights of the mice. The initial dose was 1 mg/kg, and upon a 10% reduction in body weight, the dose was reduced to 0.5 mg/kg.
Measurement of Serum Levels of HBV DNA, HBsAg, and HBV Core-Related Antigen
HBV DNA was extracted using microspin columns (QIAamp Blood kit; Qiagen K.K.) and quantified using RTD-PCR according to a method described previously [Abe et al., 1999]. The forward primer HBSF2 (5′-CTT CAT CCT GCT GCT ATG CCT-3′ [nt: 406–426]), the reverse primer HBSR2 (5′-AAA GCC CAG GAT GAT GGG AT-3′ [nt: 646–627]), and the TaqMan probe HBSP2 (5′-ATG TTG CCC GTT TGT CCT CTA ATT CCA G-3′ [nt 461–488]) with an additional G at the 3′-end were used for RTD-PCR [Tanaka et al., 2004]. The detection limit of RTD-PCR was 1,000 copies/ml. The levels of HBsAg and HBV core-related antigen (HBcrAg) were determined using a chemiluminescence enzyme immunoassay (Fujirebio, Tokyo, Japan) as described previously [Kimura et al., 2002].
Immunofluorescent and Histologic Staining of Chimeric Mouse Liver Tissue
Freshly prepared liver tissue samples were snap-frozen in isopentane that was precooled in liquid nitrogen. Isolated epithelia of bovine cornea were embedded in OCT compound and frozen immediately. Specimens (thickness, 5–6 µm) were cut with a cryostat, mounted on glass slides, air-dried, and fixed in 100% acetone at room temperature for 10 min. Sections were blocked with antibody diluent (Dako, Tokyo, Japan), incubated with rabbit anti-HBc (Dako) at room temperature for 1 hr, washed in phosphate-buffered saline, and then incubated with goat anti-rabbit IgG conjugated with Cy3 (Chemicon International, Temecula, CA) or goat anti-human albumin labeled with fluorescein isothiocyanate (Bethyl Laboratories, Montgomery, TX). Finally, the sections were washed with phosphate-buffered saline and observed under a fluorescent microscope (Eclipse E800M; Nikon, Tokyo, Japan). For histologic analysis, the liver tissues were fixed in buffered formalin, embedded in paraffin, and stained with hematoxylin-eosin and Masson's trichrome.
RESULTS
Anti-HBV Effect of Myriocin in HuH7 Cells
Myriocin inhibited the SPT activity in HuH7 cells and reduced downstream sphingomyelin production [Umehara et al., 2006]. It decreased HBV DNA levels in HuH7 cells transfected with HBV/C in a dose-dependent manner, without affecting cell viability or growth. The maximum inhibition rate was 80% in the presence of 20 µM of myriocin (Fig. 2), whereas the 50% inhibitory concentration (IC50) was approximately 5 µM. On the other hand, the inhibition rate was only 30% in the presence of 1,000 IU of IFNα-2b. When the combination of 1,000 IU of IFNα-2b with 5 µM of myriocin was added to the HuH7 cells transfected with HBV/C, HBV DNA was reduced by around 60% in the combination therapy, suggesting that these two drugs might have additive effect in vitro.
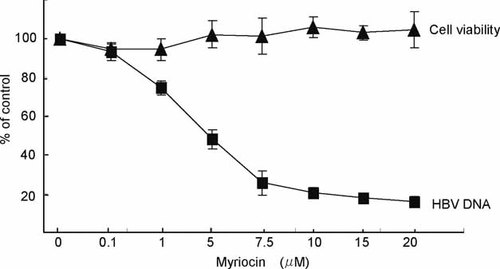
Anti-HBV effect of myriocin in HBV transfected cells. HBV DNA and cell viability of HuH7 cells in the presence of myriocin. The experiments were conducted independently at least three times.
HBV Localization in Chimeric Mice
In order to determine HBV localization, the livers of chimeric mice infected with HBV/C were examined using immunofluorescent microscopy for HBV core antigen (HBcAg) by using Cy3-labeled anti-HBc antibody (Fig. 3A). The staining for HBcAg was confined to areas where the mouse liver had been replaced with human hepatocytes; the same areas also stained with antibodies against human albumin (Fig. 3B). Co-localization of HBcAg and human hepatocytes was confirmed using double staining for HBcAg and human albumin (Fig. 3C).
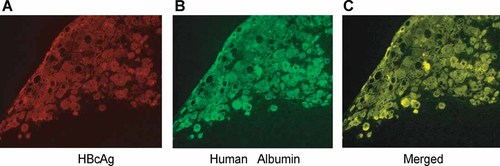
Co-localization of HBcAg and human albumin in the livers of chimeric mice infected with a clone of genotype C (C_JPNAT) and examined 24 weeks later. Liver sections were stained for HBcAg (A), human albumin (B), or double-stained for both (C). Note that HBcAg was detected exclusively in cells expressing human albumin.
Anti-HBV Effect of Myriocin and PEG-IFN in Chimeric Mice Infected With HBV
In chimeric mice infected with HBV/C, the serum HBV DNA levels plateaued (around 108 copies/ml) 8 weeks after myriocin administration. Myriocin alone did not reduce sufficiently the serum levels of HBV DNA (0.5 log reduction), and HBsAg and HBcrAg (no reduction), because 5 µM of myriocin (IC50) is relatively high concentration in vivo, and then the concentration in serum would not be achieved in vivo. PEG-IFN alone reduced the serum HBV DNA levels by 1.3 log copies of the levels observed 14 days before the treatment (Fig. 4A), but did not effectively reduce the HBsAg and HBcrAg levels (Table II). Interestingly, myriocin in combination with PEG-IFN reduced the HBV DNA levels by 2.5 log copies, the HBsAg levels by 1.2 log copies, and the HBcrAg by 1.0 log copies as compared to the control levels. Then, the levels of HBsAg and HBcrAg were recovered after the treatment of myriocin and IFN. Concurrently, the concentration of human albumin was monitored and no significant reductions were found in any treatment group (Fig. 4B), indicating little depletion of human hepatocytes by the treatment.
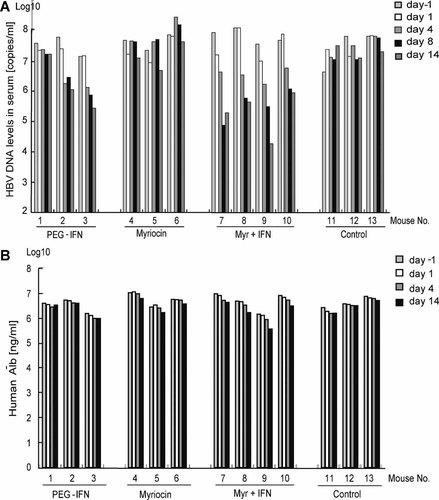
A: Anti-HBV effect of myriocin in chimeric mice infected with HBV genotype C. HBV DNA levels in the sera of chimeric mice. B: Anti-HBV effect of myriocin in chimeric mice infected with HBV genotype C. Human albumin concentrations in the sera of chimeric mice.
| Myriocin | Peg-IFN | Peg-IFN + myriocin | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HBsAg | HBcAg | HBsAg | HBcAg | HBsAg | HBcAg | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Day 0 | 0.0 | 0.2 | 0.0 | 0.2 | 0.0 | 0.1 | 0.0 | 0.1 | 0.0 | 0.1 | 0.0 | 0.0 |
| Day 1 | 0.0 | 0.2 | 0.0 | 0.2 | 0.1 | 0.1 | −0.1 | 0.3 | 0.0 | 0.0 | −0.3 | 0.0 |
| Day 4 | −0.1 | 0.2 | 0.0 | 0.2 | 0.1 | 0.3 | −0.3 | 0.3 | −0.2 | 0.1 | −0.5 | 0.1 |
| Day 8 | −0.1 | 0.1 | −0.1 | 0.2 | 0.0 | 0.4 | 0.1 | 0.4 | −1.0 | 0.2 | −1.1 | 0.2 |
| Day 14 | −0.2 | 0.1 | −0.3 | 0.2 | 0.2 | 0.4 | −0.1 | 0.3 | −1.2 | 0.2 | −1.0 | 0.1 |
| Day 20 | −0.2 | 0.3 | −0.2 | 0.3 | 0.1 | 0.3 | 0.0 | 0.4 | −0.9 | 0.3 | −0.8 | 0.2 |
| Day 28 | −0.3 | 0.3 | −0.2 | 0.2 | 0.0 | 0.3 | −0.1 | 0.4 | −0.7 | 0.3 | −0.6 | 0.2 |
| Day 35 | −0.2 | 0.3 | −0.2 | 0.2 | 0.0 | 0.4 | −0.1 | 0.4 | −0.5 | 0.2 | −0.3 | 0.2 |
Histologic analysis was performed of the livers obtained from the untreated control (188-4), myriocin-treated (188-6), and myriocin and PEG-IFN-treated (188-14) mice. No significant morphologic differences were observed between the tissues of the 188-4, 188-6, and 188-14 mice (Fig. 5). Thus, myriocin did not induce hepatocyte damage in the chimeric mice to any biologically significant degree. These results indicate that the dose of myriocin used in this study had little or no influence on host cell viability (Fig. 2) and caused little liver damage in vivo (Fig. 5).
H&E staining of liver tissue from chimeric mice. A: No treated control, (B) myriocin treated, (C) myriocin with PEG-IFN treated chimeric mice. Primary human hepatocytes were observed in these mice, and these hepatocytes displayed no significant morphologic changes.
DISCUSSION
This is the first report to show that myriocin, a specific SPT inhibitor, inhibits HBV replication. In the present study, uPA/SCID mice that had been transplanted with human hepatocytes were used to evaluate the anti-HBV effect of this SPT inhibitor. It was found that the combination of myriocin and PEG-IFN repressed synergistically HBV replication in vivo. SPT inhibitors have been shown to inhibit specifically HCV replication in vitro and in vivo [Umehara et al., 2006]. The present study, demonstrating that SPT inhibition inhibits HBV, implies that sphingolipid synthesis is important for virus maturation and is a promising target for treatment of HBV infection.
Several reports have shown that lipid rafts are important at various stages of the HIV-1 replication cycle [Campbell et al., 2001] as well as in HCV replication. Myriocin decreases the concentrations of intercellular sphingomyelin and its intermediates dihydrosphingosine, sphingosine, ceramide, and sphingosine-1-phosphate [Umehara et al., 2006], and thereby inhibits viral replication.
Hepadnaviruses acquire their envelope proteins in the ER and are then presumably secreted through the constitutive secretion pathway [Funk et al., 2008]. Membrane trafficking and sorting processes are governed by the lipid composition and organization within membranes. The membranes of eukaryotic cells contain specialized microdomains such as lipid rafts enriched in cholesterol and sphingolipids, with caveolae being the prototype [Moffett et al., 2000; Pelkmans, 2005]. The functional role of cholesterol and lipid rafts has been studied through cholesterol depletion or sequestration experiments [Pralle et al., 2000; Nichols et al., 2001]. Lovastatin, a competitive inhibitor of the enzyme 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase involved in the de novo synthesis of mevalonate and cholesterol, inhibits HBsAg secretion [Lin et al., 2003], suggesting that cholesterol plays an important role in the assembly of the HBV envelope.
Detailed examination of the HBV envelope has revealed that its cholesterol content is relatively high compared to the contents of other incorporated lipids [Gavilanes et al., 1982; Diminsky et al., 1997; Satoh et al., 2000]. Its lipid composition thus clearly diverges from that of the ER membrane [Lange and Steck, 1985] and suggests an important function for cholesterol in the envelope. A recent report showed that the depletion of cholesterol from the envelope of both duck HBV (DHBV) and human HBV reduced significantly viral infectivity, regardless of the presence of lipid rafts. Cholesterol depletion increases the density of viral particles and leads to ultrastructural changes in the virus envelope, resulting in endosomal entrapment and presumably proteolytic clearance, which in turn leads to abrogation of HBV infection [Funk et al., 2008]. Hence, myriocin may influence ER membrane synthesis and reduce the cholesterol content of the ER and envelope, resulting in a reduction in viral infectivity.
Thus, the depletion of cholesterol and sphingomyelin from the membrane destabilizes lipid rafts and is one of the mechanisms underlying the inhibition of HBV replication by SPT inhibitors such as myriocin. Antiviral activity of myriocin or IFN-alpha was weak in vivo, however, their combination showed relatively strong anti-HBV activity. IFN-alpha inhibits human T-cell leukemia virus assembly by preventing the interaction of GTPase-activating protein (GAP) with lipid rafts. IFN treatment of cells does not affect viral protein synthesis but decreases the level of released virus [Feng et al., 2003]. Leaphart et al. [2008] hypothesized that Cx43 was present on lipid rafts in enterocytes, and that IFN inhibited enterocyte migration by displacing Cx43 from lipid rafts. Such a mechanism could account for the rapid re-distribution of Cx43 from the surface of enterocytes after exposure to IFN, as well as the reversibility of the effect. Thus, mechanism of actions of myriocin and IFN-alpha may be relevant a synthetic antiviral activity through membrane fluidity on lipid rafts in vivo. This might explain the discrepancy of viral dynamic between in vitro and in vivo. In conclusion, the results suggest that SPT is an effective target for drugs designed to inhibit HBV replication. Further study is needed to confirm the proposed mechanism of the inhibition; SPT inhibitors together with IFN may produce antiviral effects by the destabilization of lipid rafts.




