Retracted: Chest digital dynamic radiography to detect changes in human pulmonary perfusion in response to alveolar hypoxia
Abstract
Introduction
Hypoxic pulmonary vasoconstriction optimises oxygenation in the lung by matching the local-blood perfusion to local-ventilation ratio upon exposure to alveolar hypoxia. It plays an important role in various pulmonary diseases, but few imaging evaluations of this phenomenon in humans. This study aimed to determine whether chest digital dynamic radiography could detect hypoxic pulmonary vasoconstriction as changes in pulmonary blood flow in healthy individuals.
Methods
Five Asian men underwent chest digital dynamic radiography before and after 60 sec breath-holding at the maximal inspiratory level in upright and supine positions. Alveolar partial pressure of oxygen and atmospheric pressure were calculated using the blood gas test and digital dynamic radiography imaging, respectively. To evaluate the blood flow, the correlation rate of temporal change in each pixel value between the lung fields and left cardiac ventricles was analysed.
Results
Sixty seconds of breath-holding caused a mean reduction of 26.7 ± 6.4 mmHg in alveolar partial pressure of oxygen. The mean correlation rate of blood flow in the whole lung was significantly lower after than before breath-holding (before, upright 51.5%, supine 52.2%; after, upright 45.5%, supine 46.1%; both P < 0.05). The correlation rate significantly differed before and after breath-holding in the lower lung fields (upright, 11.8% difference; supine, 10.7% difference; both P < 0.05). The mean radiation exposure of each scan was 0.98 ± 0.09 mGy. No complications occurred.
Conclusions
Chest digital dynamic radiography could detect the rapid decrease in pulmonary perfusion in response to alveolar hypoxia. It may suggest hypoxic pulmonary vasoconstriction in healthy individuals.
Introduction
Hypoxic pulmonary vasoconstriction constitutes reflexive contraction of the pulmonary vascular smooth muscle following exposure to low alveolar oxygen tension. It optimises ventilation–perfusion matching by diverting blood flow from poorly ventilated areas of the lung.1 The underlying mechanism of hypoxic pulmonary vasoconstriction in humans remains unclear. This is partly because the majority of studies have been conducted in animals; however, this phenomenon might differ between animals and humans in the following: the size of constricting blood vessels, response of smooth muscle cells to reactive oxygen species and presence of hyperoxic vasodilation.2, 3 Thus, data on humans are lacking.3 Therefore, new methodologies and further studies are required to understand hypoxic pulmonary vasoconstriction in humans.4
Thoracic radiology may be a promising candidate for elucidating the mechanisms of hypoxic pulmonary vasoconstriction in humans. Computed tomography (CT), magnetic resonance imaging (MRI) and lung perfusion scintigraphy are important in evaluating pulmonary perfusion. New techniques such as right ventricle tissue characterisation with T1 mapping,5 four-dimensional flow of the right ventricle and pulmonary arteries,6 and CT lung perfusion imaging have paved the way for a new era of pulmonary perfusion imaging.7 However, these modalities are still limited in terms of spatial and temporal resolution and radiation exposure.
To balance high resolution and low radiation exposure, chest digital dynamic radiography (DDR) has been developed.8, 9 The DDR technology can capture sequential radiograph images at one time to evaluate the dynamic functional images of the lung. Chest DDR can detect the pixel value change synchronised with the cardiac cycle and generate pulmonary perfusion images. The DDR perfusion images were consistent with lung perfusion scintigraphy images in healthy individuals and patients with several pulmonary perfusion abnormalities.10-12 Specifically, occlusion of the main pulmonary artery trunk showed reduced blood flow signal in the whole lateral lung,11 whereas occlusion or stenosis of the area branch showed wedge-shaped blood flow signal deficits.12 DDR imaging can be completed within 6–15 s in upright and supine positions. It has fine pixel size (400 × 400 μm), short-cycle pulse irradiation (X-ray pulse, 4.0 ms) and does not require contrast media. Therefore, it was hypothesised that chest DDR may be beneficial for detecting hypoxic pulmonary vasoconstriction in humans.
To verify this hypothesis, this study aimed to determine whether chest DDR could detect hypoxic pulmonary vasoconstriction as the change in pulmonary blood flow in healthy participants in upright and supine positions.
Methods
Participants
Five healthy Asian men with no history of smoking participated in this study. The mean age was 29.8 ± 3.4 years. The mean body mass index was 23.4 ± 1.6, which was calculated from the body height (mean, 175.4 ± 2.7 cm) and body weight (mean, 72.0 ± 6.1 kg). The heart rate (mean, 74.4 ± 7.1 bpm) before imaging was in sinus rhythm. During chest DDR imaging, the participants had no arrhythmia, bundle block or atrioventricular block on electrocardiography.
Compliance with ethical guidelines
This study was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants prior to their participation. This study was approved by the Certified Review Board, Shiga University of Medical Science (Seta Tsukinowa-cho, Otsu, Shiga, Japan, approval number: CRB5180008) on 12 February 2019 and listed in the Japan Registry of Clinical Trials (https://jrct.niph.go.jp, approval number: jRCTs052180103) on 8 March 2019.
Examination protocol
All participants were instructed regarding the examination protocol (Fig. 1). In previous studies, the mean maximal breath-hold time at the maximal inspiratory position was 52.3–67.8 s in healthy participants13, 14 and the end-tidal CO2 was reported to increase to 46.6 mmHg.14 A 60 sec breath-hold was thus considered feasible, and the conditions were sufficient to cause hypoxic pulmonary vasoconstriction in humans. Participants were instructed not to perform the Valsalva manoeuvre because the increased intrathoracic pressure could affect cardiac output. In the current examination protocol, participants experienced hypoxia and hypercapnia. However, previous studies have demonstrated that hypercapnia does not affect pulmonary perfusion.15
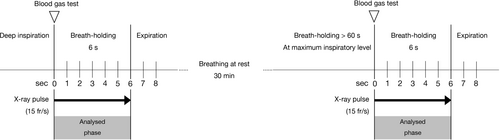
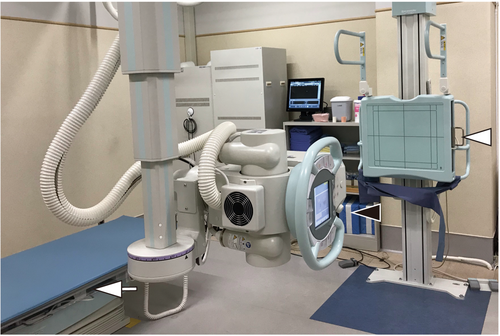
Chest DDR imaging
Chest DDR was undertaken by using a high-sensitive flat panel detector imaging system. The imaging equipment for DDR consists of following two sections: a high-sensitive flat panel detector, AeroDR fine (Konica Minolta, Inc., Tokyo, Japan) and a pulsed X-ray generator, RAD speed Pro (Shimadzu Corporation, Kyoto, Japan) (Fig. 2). A posteroanterior view in the upright position and anteroposterior view in the supine position were adopted.
Digital dynamic radiography imaging needs a minimum of 6 sec of breath-holding at deep inspiratory level (total time of exposure to radiation is at least 6 sec). The radiation exposure had the following parameters as described previously 16: tube voltage, 95 kV; tube current, 80 mA; duration of pulsed X-ray, 4.0 ms; source-to-image distance for upright position, 1.8 m; source-to-image distance for supine position, 1.5 m; and an additional filter, 0.2 mm of Cu. An additional filter is required to reduce the X-rays with low energy. With the introduction of the Cu filter the half-value layer increased from 3.96 to 6.58 mm A1. The estimated dose per pulse was 0.0074 mGy with a Cu filter and 0.0134 mGy without a Cu filter. The pixel size (400 × 400 μm), overall image area (424.8 × 424.8 mm), grey-level range of the images 65,536 (16 bits), and signal intensity were proportional to the incidental exposure of the X-ray detector. The data of the dynamic images were captured at 15 frames/s, which was synchronised with the pulsed X-rays. This was used to minimise radiation exposure.
Imaging analysis
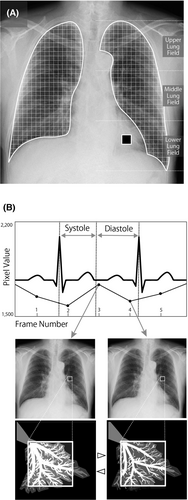
As shown in Figure 3, the temporal change in pixel values on sequential chest radiograph data was analysed by Workstation software (KINOSIS 1.00; Konica Minolta, Inc., Tokyo, Japan). The software automatically determined the edges of the lung fields and the region of interest (ROI; 10 × 10 mm) in the left ventricle in raw data of chest radiography. Because of technical limitations of the software, the lung fields behind the heart and below the diaphragm are not included in the analysis. A high-pitched increase and decrease in the pixel values of the left ventricle were considered as the diastole and systole phases, respectively. The software separated the lung fields at 5-mm intervals (Fig. 3A). The cross-correlation coefficients of the changes in each ROI in the lung fields and left ventricle were calculated. Throughout one cardiac cycle of each lung field block, the difference between the pixel values of the lung field and the left ventricle, representing the difference in blood volume relative to the mean state, was determined (Fig. 3B). From a technical point of view, the measurements should be performed for at least three cardiac cycles per image sequence. Therefore, the breath-hold protocol was set to 6 sec. The image analysis was based on the assumption that both ventricles were synchronised because the pulmonary perfusion is the result of the right ventricle beating.
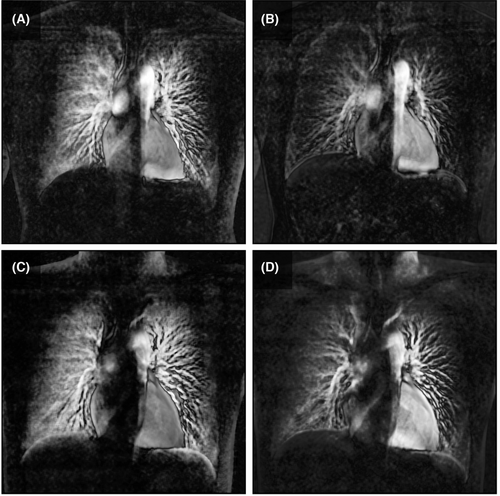
Colour-mapping images for visualising pulmonary perfusion were generated, described previously16 (Videos S1–S4). The difference in pixel values relative to the baseline was calculated sequentially for all frames and superimposed on the original image as a colour display. In the colour mapping image, the increase in blood volume was represented by a deeper red colour. Additionally, maximum-intensity projection (MIP) images were created from the colour-mapping images (Fig. 4).
Correlation rate and exposure to radiation
Blood gas tests and partial pressure of oxygen in the alveoli
Statistical analysis
The number of participants (n = 5) was determined using the following steps: previously, the mean correlation rate in patients with respiratory disease was shown to be 42%–44% (standard deviation [SD]: 8%–9%).16 Previous animal studies using X-ray TV systems demonstrated that the diameter of the muscular pulmonary arteries was reduced by 20%–30% in hypoxic pulmonary vasoconstriction.21 Therefore, with an alpha level of 0.05, statistical power of 0.8, difference to detect 20%, and SD 9%, the minimal required sample size was n = 4. Eight applicants applied in response to recruitment. Five were randomly selected as the final participants owing to concerns about technical failures in DDR imaging or blood gas examinations.
A paired t-test was used to compare the mean correlation rate before and after breath-holding. A P-value of <0.05 were considered to indicate statistical significance (all tests were two-tailed). JMP® 14.0.0 software (SAS Institute Inc., Cary, NC, USA) analysed data.
Results
The imaging conditions used during the experiments are provided in Table 1. It was found that 60 sec breath-holding caused a mean reduction of 26.7 ± 6.4 mmHg in PAO2. Participants whose PaO2 was higher than their PAO2 may have had hyperventilation.
| Participant | Atmospheric pressure† (mmHg) | Before 60 sec breath-holding | After 60 sec breath-holding | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | PaCO2 (mmHg) | PaO2 (mmHg) | HCO3− (mmol/L) | PAO2‡ (mmHg) | pH | PaCO2 (mmHg) | PaO2 (mmHg) | HCO3− (mmol/L) | PAO2‡ (mmHg) | ||
| Upright position | |||||||||||
| No. 1 | 754.0 | 7.45 | 37.5 | 90.3 | 25.6 | 104.8 | 7.39 | 47.2 | 57.6 | 27.7 | 76.3 |
| No. 2 | 754.8 | 7.45 | 34.9 | 103.0 | 23.9 | 108.0 | 7.40 | 43.8 | 42.5 | 26.3 | 81.6 |
| No. 3 | 754.9 | 7.45 | 36.3 | 101.0 | 25.0 | 99.0 | 7.37 | 46.0 | 67.6 | 26.2 | 78.1 |
| No. 4 | 749.8 | 7.46 | 32.8 | 108.0 | 22.7 | 110.5 | 7.36 | 46.7 | 82.1 | 25.7 | 77.0 |
| No. 5 | 749.8 | 7.43 | 36.7 | 95.6 | 23.9 | 105.8 | 7.38 | 41.9 | 65.2 | 24.2 | 84.5 |
| Mean ± SD | 752.7 ± 2.6 | 7.45 ± 0.01 | 35.6 ± 1.9 | 99.6 ± 6.8 | 24.2 ± 1.1 | 105.6 ± 4.3 | 7.38 ± 0.01 | 45.1 ± 2.22 | 63.0 ± 14.5 | 26.0 ± 1.3 | 79.5 ± 3.5 |
| Supine position | |||||||||||
| No. 1 | 751.2 | 7.41 | 37.7 | 93.5 | 23.3 | 104.6 | 7.36 | 46.4 | 61.3 | 25.8 | 77.5 |
| No. 2 | 750.7 | 7.45 | 36.8 | 98.6 | 25.1 | 105.7 | 7.41 | 42.8 | 63.1 | 26.3 | 83.1 |
| No. 3 | 750.6 | 7.47 | 33.6 | 110.0 | 24.0 | 109.5 | 7.36 | 47.6 | 65.7 | 26.4 | 75.6 |
| No. 4 | 750.4 | 7.52 | 26.0 | 130.0 | 21.0 | 118.7 | 7.38 | 43.2 | 96.2 | 25.1 | 82.5 |
| No. 5 | 750.8 | 7.42 | 38.0 | 99.9 | 24.1 | 104.2 | 7.40 | 40.0 | 81.8 | 24.1 | 87.5 |
| Mean ± SD | 750.7 ± 0.3 | 7.45 ± 0.04 | 34.4 ± 5.0 | 106.4 ± 14.5 | 23.5 ± 1.5 | 108.5 ± 6.0 | 7.38 ± 0.02 | 44.0 ± 3.03 | 73.6 ± 15.0 | 25.6 ± 1.0 | 81.3 ± 4.7 |
- PaO2: partial pressure of oxygen in arterial blood; PaCO2: partial pressure of carbon dioxide in arterial blood; PAO2: partial pressure of oxygen in the alveoli; SD standard deviation.
- † The institution's elevation was 120 m above sea level, and the mean room temperature was 25.9 ± 0.5° C.
- ‡ PAO2 was calculated from the values of atmospheric pressure and PaCO2.
The imaging duration and entrance surface dose are presented in Table 2. The mean entrance surface dose in each DDR imaging study was as follows: in upright position, 1.03 ± 0.14 mGy (before breath-holding), 0.96 ± 0.10 mGy (after breath-holding); in supine position, 0.98 ± 0.07 mGy (before breath-holding), 0.95 ± 0.05 mGy (after breath-holding).
| Participant | Before 60 sec breath-holding | After 60 sec breath-holding | ||
|---|---|---|---|---|
| Imaging time (s) | Entrance surface dose (mGy) | Imaging time (s) | Entrance surface dose (mGy) | |
| Upright position | ||||
| No. 1 | 9.7 | 1.08 | 9.9 | 1.10 |
| No. 2 | 11.2 | 1.24 | 9.1 | 1.01 |
| No. 3 | 8.1 | 0.90 | 8.5 | 0.94 |
| No. 4 | 8.5 | 0.94 | 7.5 | 0.83 |
| No. 5 | 8.8 | 0.98 | 8.4 | 0.93 |
| Mean ± SD | 9.3 ± 1.2 | 1.03 ± 0.14 | 8.7 ± 1.2 | 0.96 ± 0.10 |
| Supine position | ||||
| No. 1 | 9.1 | 1.01 | 8.3 | 0.92 |
| No. 2 | 8.5 | 0.94 | 9.1 | 1.01 |
| No. 3 | 9.5 | 1.05 | 8.4 | 0.93 |
| No. 4 | 7.9 | 0.88 | 8.1 | 0.90 |
| No. 5 | 9.2 | 1.02 | 8.9 | 0.99 |
| Mean ± SD | 8.8 ± 0.6 | 0.98 ± 0.07 | 8.6 ± 0.4 | 0.95 ± 0.05 |
- SD; Standard deviation.
The correlation rate was lower after 60 sec breath-holding than before 60 sec breath-holding (Table 3). In both upright and supine positions, chest DDR was able to detect a significant reduction in the correlation rate. In the lower lung fields, the correlation rates before and after breath-holding were markedly different. The correlation rate of the middle lung fields exhibited the highest values in both positions, and it reflected the influence of gravity most clearly, in this study. The areas of blood flow change were more extensive in the supine position than in the upright position.
| Mean correlation rate | Upright position | Supine position | ||||
|---|---|---|---|---|---|---|
| Before 60 sec breath-holding N = 5 | After 60 sec breath-holding N = 5 | P value | Before 60 sec breath-holding N = 5 | After 60 sec breath-holding N = 5 | P value | |
| Right | ||||||
| Upper lung field (%) | 41.3 ± 4.2 | 41.4 ± 6.7 | 1.0000 | 40.7 ± 6.0 | 40.1 ± 10.4 | 0.9124 |
| Middle lung field (%) | 57.7 ± 7.8 | 50.3 ± 4.5 | 0.1395 | 57.1 ± 6.5 | 51.7 ± 9.4 | 0.1157 |
| Lower lung field (%) | 56.5 ± 7.3 | 45.6 ± 5.7 | 0.0167* | 56.8 ± 7.2 | 46.5 ± 7.9 | 0.0295* |
| Total lung field (%) | 51.8 ± 9.5 | 45.8 ± 6.3 | 0.0218* | 51.5 ± 9.7 | 46.1 ± 9.5 | 0.0173* |
| Left | ||||||
| Upper lung field (%) | 42.5 ± 4.9 | 42.2 ± 8.2 | 1.0000 | 47.3 ± 7.8 | 43.8 ± 9.9 | 0.2610 |
| Middle lung field (%) | 56.1 ± 8.3 | 50.8 ± 8.4 | 0.3242 | 55.8 ± 7.1 | 50.2 ± 8.9 | 0.0337* |
| Lower lung field (%) | 55.1 ± 5.1 | 42.5 ± 7.1 | 0.0138* | 55.3 ± 9.0 | 44.4 ± 9.8 | 0.0006* |
| Total lung field (%) | 51.3 ± 8.4 | 45.2 ± 8.1 | 0.0460* | 52.8 ± 8.2 | 46.1 ± 9.0 | 0.0002* |
| Bilateral | ||||||
| Upper lung field (%) | 41.9 ± 4.3 | 41.9 ± 7.1 | 1.0000 | 44.0 ± 7.5 | 41.9 ± 9.7 | 0.3623 |
| Middle lung field (%) | 56.9 ± 7.7 | 50.6 ± 6.4 | 0.0608 | 56.4 ± 6.4 | 51.0 ± 8.7 | 0.0052* |
| Lower lung field (%) | 55.8 ± 6.0 | 44.0 ± 6.3 | 0.0002* | 56.1 ± 7.7 | 45.4 ± 8.5 | < 0.0001* |
| Total lung field (%) | 51.5 ± 9.1 | 45.5 ± 7.4 | 0.0002* | 52.2 ± 9.1 | 46.1 ± 9.5 | < 0.0001* |
- Values are presented as mean ± standard deviation unless otherwise indicated. All the results were analysed using paired t-test. *Indicates P value <0.05.
The chest DDR images of participant No. 2 are presented as representative example. Colour-mapping images of chest DDR were generated in both upright and supine positions (Videos S1–S4). After 60 sec breath-holding, the pulmonary perfusion of the bilateral lower lung fields became faint compared with that before 60 sec breath-holding. The regional branches of the pulmonary artery could be identified, but the peripheral pulmonary arteries were poorly depicted. Similar findings were recorded using the MIP images (Fig. 4).
Discussion
This study aimed to determine whether chest DDR could detect hypoxic pulmonary vasoconstriction in healthy participants in the upright and supine positions. It was found that 60 sec breath-holding caused a significant reduction in the PAO2, resulting in hypoxic pulmonary vasoconstriction. In both upright and supine positions, chest DDR was able to detect predetermined changes in pulmonary blood flow. To the best of our knowledge, this is the first study to assess hypoxic pulmonary vasoconstriction in humans using the DDR technology.
Chest DDR is attracting attention as an innovative imaging modality for pulmonary perfusion.8, 9 As an index, DDR can be used to quantitatively assess pulmonary blood flow using the correlation rate. Correlation rate is one of the analysing methods for DDR. The same calculation protocol has been used by Yamamoto et al for calculating the correlation rate16 and Tanaka et al. for P%(x,y)17 calculation. It has the advantage of being able to evaluate per minute changes in the pixel values associated with the cardiac cycle, but further validation studies are required because of the novelty. The commercial DDR system was released in 2018 and received approval from the US Food and Drug Administration in 2019. Currently, the DDR technology is available for use in clinical practice. Additionally, dual-energy CT iodine maps, lung perfusion single photon emission computed tomography/CT, arterial spin labelling in MRI and pulmonary angiography have been used to evaluate pulmonary perfusion in the human lung (Supporting Information: Comparison of the clinical imaging modalities for pulmonary perfusion). Dynamic contrast-enhanced MRI has also been utilised, although not validated, to evaluate blood flow in the peripheral pulmonary artery. Among these examination tools, DDR provides relatively fine pixel size and causes lower radiation exposure than pulmonary angiography.
The diameter of hypoxic pulmonary vasoconstriction in humans is still being explored. Although it is unclear whether hypoxic pulmonary vasoconstriction occurs in large diameter vessels (2–5 mm) in humans,2 it has been confirmed to occur in small diameter vessels (0.4–2 mm).3, 22 Thus, it is desirable to select the modality with the finest spatial and temporal resolutions. In our DDR perfusion images, it was clear that the subpleural blood flow reduced, whereas the central blood flow did not drastically change (Fig. 4). It is speculated that the most significant changes occurred in vessels of <2 mm in diameter; however, more data are required to confirm this speculation.
In general, hypoxic pulmonary vasoconstriction in humans appears to have three phases.23 The first acute phase begins within 5 min and lasts for about 3 min, followed by a plateau phase of ≥20 min after the first acute vasoconstriction.24 The second phase, also known as the sustained phase, starts after a latency period of 30 min and plateaus at 2 h.24 This is followed by the third chronic phase, which persists for ≥8 h.23 In this study, chest DDR would capture the first acute phase of hypoxic pulmonary vasoconstriction. DDR can capture all phases of hypoxic pulmonary vasoconstriction, from acute to chronic, in principle. This may constitute evaluating pulmonary perfusion in clinical practice. For example, chronic alveolar hypoxia leads to chronic hypoxic pulmonary vasoconstriction, which in turn, leads to pulmonary hypertension.25 Other examples include the use of DDR for hypoxic challenge flight assessments in patients with severe respiratory disease planning air travel or in patients with severe chest wall deformity or neuromuscular disease at risk for nocturnal hypoventilation.26
The ability to take pictures in two positions, upright and supine, is advantageous in the evaluation of pulmonary perfusion in a physiologically natural condition. Humans spend most of the day in upright or sitting position. Position significantly influences the pulmonary circulation. When upright from a supine position, gravitational forces pull the venous blood to the lower limbs. Because of the high compliance of veins, approximately 500 mL of blood can be redistributed to peripheral veins. This is known as venous pooling, which leads to an immediate drop in stroke volume by 40% and an overall drop in cardiac output by 20%.27 Chest DDR in upright position may be able to detect blood flow change with a shift in position, as evident from the increased correlation rate in the upper lung field in the supine position compared with the upright position (Table 3), but further studies are needed. Moreover, caution should be exercised while simply comparing the two positions, since the directions of radiation are different in the upright (posterior–anterior) and supine (anterior–posterior) positions.
Among the three lung fields (upper, middle, and lower), the correlation rates in the upper lung fields did not differ before and after 60 sec of breath-holding (Table 3). This phenomenon has been considered to involve capillary recruitment, in which blood flow shifts from the base of the lung to the apex when hypoxic pulmonary vasoconstriction occurs.28 The presence of capillary recruitment is important because in patients with pulmonary hypertension capillary recruitment may be absent.29 In the middle lung fields, the correlation rate tended to be high, as seen previously.16 Notably, the middle lung field most clearly reflected the influence of gravity in the present study. Furthermore, significant differences were found in the supine position that were not found in the upright position (Table 3).
Our study had several limitations. First, it was not able not to assess the velocity of the pulmonary blood flow or evaluate the pulmonary artery diameter. This is a technical challenge for the DDR technology. Since the DDR technology has only recently been approved for use in clinical practice, there have been few clinical reports with no comparisons with other modalities. It is expected that the evaluation of blood flow velocity and vessel diameter will become possible in the future as more data are accumulated. Second, as for technical limitation, the results for the left lower lung field may have been affected because the left posterior basal and left lateral basal arteries (to a lesser extent) were not included. The heartbeat also affected the left lower field. The assessment in this field was thus not robust. Third the intrathoracic pressure may have been increased due to breath-holding leading to decreased venous return and cardiac output. This may have affected the assessment of blood flow on DDR imaging. Fourth, just only healthy men were investigated, and healthy women were excluded. This is because early pregnancy could not be completely ruled out because of differences in the pregnancy tests. Moreover, in terms of bioethics and risk management, it is necessary to avoid radiation exposure for women of reproductive age.30 Finally, there were no available data for comparison with other modalities and this study included only healthy participants. Therefore, it was necessary to minimise the sample size, radiation dose, and invasiveness. For comparison with other modalities, further studies on patients with respiratory diseases or those experiencing hypoxia are needed.
In conclusion, the utility of a new imaging methodology was demonstrated for evaluating a decrease in pulmonary blood flow in humans in response to alveolar hypoxia. The combined use of DDR technology and the correlation rate could provide better understanding of pulmonary blood flow. Our results should contribute to the clinical investigation of hypoxic pulmonary vasoconstriction via radiological assessment.
Acknowledgements
We would like to thank all participants of this study. We acknowledge the valuable assistance from Kazunobu Hashida, M.D. and Satoshi Suda, M.D., from the Department of Radiology, Tokai University Hachioji Hospital. We are thankful to Aya Konno-Yamamoto, M.D., from the Department of Thoracic Oncology, National Cancer Centre Hospital for additional medical writing support. We would like to thank Editage (www.editage.com) for English language editing.
Financial/Nonfinancial Disclosures
None declared.
Conflict of Interest
The authors have no conflicts of interest.
Ethics Approval Statement
This study was approved by the Certified Review Board, Shiga University of Medical Science (Seta Tsukinowa-cho, Otsu, Shiga, Japan, approval number: CRB5180008) on 12 February 2019.
Patient Consent Statement
Written informed consent was obtained from all participants prior to their participation.
Clinical Trial Registration
This study was listed in the Japan Registry of Clinical Trials (https://jrct.niph.go.jp, approval number: jRCTs052180103) on 8 March 2019.
Open Research
Data Availability Statement
Data sets that are restricted and not publicly available. Due to confidentiality agreements, supporting data can only be made available to bona fide researchers subject to a non-disclosure agreement. Details of the data and how to request access are available from [email protected].




