Potential gains: Comparison of a mono-isocentric three-dimensional conformal radiotherapy (3D-CRT) planning technique to hybrid intensity-modulated radiotherapy (hIMRT) to the whole breast and supraclavicular fossa (SCF) region
Abstract
Introduction
Until late 2018, standard of practice at the Northern Sydney Cancer Centre (NSCC) for breast and nodal treatment was a conformal mono-isocentric technique. A planning study comparing an existing mono-isocentric three-dimensional conformal radiotherapy (3D-CRT) planning technique to a hybrid intensity-modulated radiotherapy (hIMRT) approach for the whole breast and supraclavicular fossa (SCF) region was undertaken with the aim to improve plan quality by improving dose conformity/homogeneity across target volumes and reducing hotspots outside the target.
Methods
A cohort of 17 patients was retrospectively planned using the proposed hIMRT technique, keeping the same planning constraints as the original treated breast and SCF 3D-CRT plan and normalising the 3D-CRT plans to achieve minimum breast/SCF target coverage to compare organs at risk (OARs). Normal tissue index (NTI) and homogeneity index (HI) were compared for plan quality as well as for evaluating OARs.
Results
The hIMRT technique showed statistically significant improvements in NTI and HI, as well as improvement in maximum brachial plexus and thyroid doses. There was a negligible increase in maximum oesophagus dose which could be improved if used in optimisation. Other OAR doses in the irradiated region were comparable to the 3D-CRT plans, however maximum doses were reduced overall.
Conclusion
The hIMRT planning technique maintained clinically acceptable doses to OARs and reduced normal tissue dose while maintaining equivalent dose coverage to breast and SCF planning target volumes with improved conformity and homogeneity. The reduction in maximum doses promotes a favourable toxicity profile, with potential benefit of improved long-term cosmesis.
Introduction
Breast cancer is one of the most commonly diagnosed cancers in women over 40 in Australia, with 1 in 8 women at risk of developing it before the age of 85.1 Women diagnosed with early stage breast cancer benefit from adjuvant therapy which combines breast conserving surgery and whole breast radiotherapy to reduce local recurrence.2 In women with higher risk breast cancer, regional nodal irradiation as well as adjuvant systemic therapies may be needed to provide improved loco-regional control and overall survival.3-5 The large variance in patient anatomy dictates the balance between target coverage and normal tissue sparing. Awareness and prevention of late toxicity is particularly pertinent in radiotherapy for breast cancer given the excellent long-term survival outcomes.6
The clinical benefits of treating whole breast alone using intensity-modulated radiotherapy (IMRT) have been published in contemporary literature.7, 8 The dose homogeneity achieved by IMRT as compared to three-dimensional conformal radiotherapy (3D-CRT) has been demonstrated to be a reliable surrogate measure for long-term outcomes, particularly improved cosmesis.7, 9 A ten year follow-up study concluded that late side effects were significantly correlated with acute toxicities in patients who had poor dose distributions.6 Thus, there is a need to focus on improved dose homogeneity to reduce acute side effects and offer long-term benefit.
For patients requiring breast alone treatment, dose benefits have been shown using a hybrid IMRT (hIMRT) planning technique consisting of IMRT fields with a conformal beam contribution.6, 9 In a planning study comparing a 3D-CRT technique to a 50% IMRT, 50% conformal hIMRT technique, improved dose homogeneity was demonstrated with no increase to organ at risk (OAR) dose.10
While traditional 3D-CRT field-based plans provide necessary nodal coverage, they have been shown to be inadequate or inferior to volumetric-based IMRT techniques.11, 12 Studies analysing a single-isocentric full IMRT breast and supraclavicular fossa (SCF) technique have been published but have limitations that include greater low dose wash to normal tissues.13-16 A 2-4 field IMRT technique for left sided breast cancer combined with an ipsilateral arc field approach reported reduced doses to lungs, heart and contralateral breast but resulted in higher doses to the oesophagus, thyroid and humeral head compared to a conventional 3D-CRT approach.17
The aim of this study was to build on both the whole breast tangential IMRT18 and whole breast hybrid IMRT10 solutions to create a technique that improves dose conformity and homogeneity to the SCF target while maintaining or potentially improving OAR doses in the surrounding region. By developing a modulated hIMRT approach using the existing field-based beam arrangements, the technique would then be compared to the current departmental mono-isocentric 3D-CRT breast SCF technique. The uniqueness of this technique lies in the use of a hybrid approach applied in the supraclavicular area rather than applying either a conformal or a fully dynamic approach.
Methods
Ethics approval was granted by the Human Research Ethics Committee of Northern Sydney Local Health District for our low and negligible risk study (LNR/18/HAWKE/55). Site-specific assessment authorisation for our single site study was also granted by the Research Governance Office (LNR/18/HAWKE/55).
Patient selection criteria and sample size
Twenty patients who previously received treatment to their left or right breast and SCF nodal region between December 2016 and October 2017 were randomly selected for this study. All patients were breast nodal cases with no patients being chest wall nodal. Of the 20 patients, three were excluded from the study because of the presence of internal mammary chain volumes.
Patient characteristics
Patient characteristics for the 17 patients in the study are presented in Table 1. The average age of the cohort was 64 years with ages ranging from 45 to 88 years and a similar number of right sided and left sided tumours. The mean planning target volume (PTV) breast volume was 829 cm3 and ranged from 415 to 1514 cm3. Breast separations measured along the posterior edge of the tangential field border ranged from 17.0 to 31.9 cm with a mean separation of 24.1 cm (SD = 4.4). Mean SCF depth, measured from the patient’s anterior surface to the maximum depth of the SCF PTV was 7.2 cm, and ranged from 4.1 to 10.2 cm (SD = 1.5).
| Patient | Age | Laterality | Staging | Breast Separation (cm) | PTV Breast (cm3) | SCF AP/PA Separation (cm) |
|---|---|---|---|---|---|---|
| 1 | 50 | Left | T2 N1 M0 G2 | 22.0 | 498 | 7.2 |
| 2 | 51 | Right | T1c pN1mi M0 G2 | 21.8 | 1055 | 5.9 |
| 3 | 52 | Right | T1c pN1a M0 G2 | 20.2 | 454 | 6.0 |
| 4 | 69 | Right | T2 pN1a M0 G3 | 27.6 | 850 | 8.1 |
| 5 | 66 | Right | T2N1aG2 | 27.6 | 1151 | 10.2 |
| 6 | 66 | Left | T2N1aG2 | 22.7 | 728 | 7.2 |
| 7 | 45 | Right | T1cpN1G2 | 18.8 | 505 | 4.1 |
| 8 | 88 | Left | T1N1G3 | 17.0 | 515 | 4.9 |
| 9 | 75 | Right | T2N2G2 | 27.9 | 649 | 8.6 |
| 10 | 81 | Right | T2N1G3 | 19.7 | 415 | 7.1 |
| 11 | 51 | Right | T2N2G3 | 28.8 | 1381 | 8.5 |
| 12 | 46 | Left | T0N0G3 | 20.2 | 500 | 6.4 |
| 13 | 72 | Left | T3N0 | 31.9 | 1406 | 9.0 |
| 14 | 63 | Left | T1cN1G2 | 26.2 | 886 | 5.8 |
| 15 | 74 | Left | T0N1G3 | 30.2 | 1515 | 7.9 |
| 16 | 62 | Left | T1cN1aG3 | 23.3 | 1028 | 7.6 |
| 17 | 80 | Right | T2N1G3 | 23.8 | 559 | 7.5 |
| Mean | 64 | -- | -- | 24.1 | 829 | 7.2 |
- PTV, planning target volume; SCF, supraclavicular fossa; AP, anteroposterior; PA, posteroanterior.
Contouring
All patients were previously scanned with a slice thickness of 2mm on a Phillips Brilliance Big Bore CT (Philips Medical Systems, Cleveland, OH, USA) scanner lying on a AccessTM Supine Breast and Lung board (Qfix®, Avondale, PA, USA) at a standard angle of 5 degrees, and immobilised in a T-shaped vac bag on the jig. Scanning was done in free breathing as per standard protocol for breast nodal patients. Breast and SCF PTVs, previously contoured by a radiation oncologist (RO) on the treated 3D-CRT plans using the Trans-Tasman Radiation Oncology Groups STARS guidelines19, were used to plan the new technique.
OARs (heart, lungs, contralateral breast, ipsilateral humerus, oesophagus and spinal cord) had been contoured by the planning radiation therapist (RT), and the thyroid and brachial plexus added by an RO. Contouring directly onto the planning CT scan followed the STARS guidelines19 and RT’s contours were reviewed by another RT as well as a physicist and RO at the plan evaluation stage.
The breast normal tissue (BNT) contours were created for calculating the normal tissue index (NTI) (see dosimetric indices). These volumes were created by subtracting the PTV Breast or PTV SCF from the boundary of the respective radiation portals defined by the 50% isodose line, which inherently includes any beam divergences throughout the patient. Overlapping OARs were also included in the BNT structure (Fig. 1).
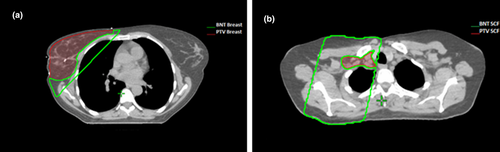
 ) created on a right sided breast patient to calculate the NTI. This is a subtraction of the PTV Breast (
) created on a right sided breast patient to calculate the NTI. This is a subtraction of the PTV Breast ( ) from the boundary of the radiation portal. (B) Transverse slice showing the BNT SCF contour (
) from the boundary of the radiation portal. (B) Transverse slice showing the BNT SCF contour ( ) with subtraction of the PTV SCF (
) with subtraction of the PTV SCF ( ) for calculation of the NTI.
) for calculation of the NTI.Planning
All plans were created using the AAA algorithm on version 13.6.23 of the Varian Eclipse treatment planning system (Varian Medical Systems, Palo Alto, CA, USA) for treatment on a Varian Trilogy with MillenniumTM 120-leaf multi-leaf collimator (MLC) system. For this system, leaf width at isocentre for each of the central 80 leaves is 5 mm, and for all others 10 mm. The breast was prescribed to 50 Gy in 25# with a minimum coverage of D95% = 47.5 Gy. For the SCF, the department adhered to STARS guidelines19 which specifies a minimum covering isodose of 95% of 45 Gy (42.75 Gy) to 95% of the target volume. OAR constraints also adhered to the same STARS guidelines.19 This minimal coverage was chosen to enable a direct comparison between techniques. The hIMRT technique was used to re-plan all volumes to the current European Society for Radiotherapy and Oncology (ESTRO) guided dosing standards (min coverage of D98% 45 Gy).20
The 3D-CRT plans were originally produced using medial and lateral opposed tangential fields to the breast junctioned with a single anterior oblique SCF field angled 150 towards the contralateral neck to keep dose off the spinal cord. Fields were asymmetrically configured to minimise the projection of the heart, lung and contralateral breast in the beam’s eye view, with collimators positioned to allow optimal wedging orientation and MLCs used to shield out lungs, heart and unnecessary normal tissue. Plans contained 6 and 18 MV beams as necessary to achieve optimal dose coverage of the PTVs. A portion of 18MV was employed for patients with a maximum tangential separation >22cm to reduce hotspots, with all tangent separations being measured at the central axis of the patient superior-inferior and along the posterior edge of the tangential fields. This cut-off value is based off historical departmental criteria where conformal plans would require the addition of 18 MV.
An internal plan comparison project was undertaken to determine the optimal beam energy and arrangement for comparison to the 3D-CRT plans. In producing plans for each patient, a hybrid mono-isocentric technique was found to be the optimal with regards to target coverage, plan homogeneity and doses to OARs. The aim was to maintain similar field arrangements to the 3D-CRT plans and in doing so, keeping the primary jaws off the contralateral side and the underlying lung and heart (Fig. 2). To achieve this, the tangent portion of the plan (breast region) was converted to a full IMRT. For the SCF portion of the plan, a hybrid approach was used with both a 6MV IMRT and an 18MV conformal SCF field positioned at the gantry angle of 15 degrees to the contralateral side. The conformal SCF field was treated as a base plan with a standard weighting of 0.45. This aimed to give better dose homogeneity from the static portion of the plan.
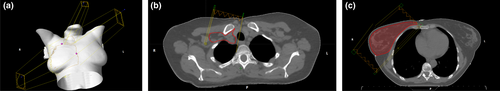
For the tangent portion of the plan when the maximum tangent separations were above 22 cm, a 60–80% portion of 18MV was used, converting the tangent to a hIMRT as well. The angle of the SCF fields were chosen to ensure dose was kept off the spinal cord by using the primary jaw. Patients with larger separations (>22 cm for the maximum antero-posterior separation) required an 18 MV post-axilla contribution of 25% of the base plan.
Optimisation objectives were kept simple and included only the planning PTV Breast and SCF volumes as well as the spinal cord. While lung and heart are important OARs for the breast cohort, they were not included in the optimisation objectives as the tangential approach of the IMRT fields meant that any added heart and lung objectives would degrade the minimum breast target coverage. Due to the beam arrangement with primary jaws set to fixed boundaries that cover the SCF target and largely avoid surrounding OARs, it was not necessary to optimise off the oesophagus, thyroid, humerus and brachial plexus.
Plan comparison
The hIMRT plans were optimised to give the same minimum D95% target coverage constraints as the normalised 3D-CRT plans for direct OAR comparison. As per ICRU 50/62 recommendations, PTV dose coverage values were collected for D98%, D50% and D2% (for both breast and SCF volumes).21, 22 For OAR values, volumetric(VD) and mean(Dmean) doses as well as maximum (Dmax) doses currently utilised in our department protocols were also collected and analysed. Total Monitor Units (MUs) for all plans were also recorded across both techniques to determine time considerations for treatment.
Dosimetric indices
Two indices were used to compare plan quality of the 3D-CRT and hIMRT plans. The NTI and homogeneity index (HI) were utilised to evaluate both the minimisation of absorbed dose to the irradiated volume and dose variation between the 3D-CRT and hIMRT techniques.10
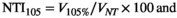
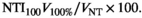
Where V105% is the volume of normal tissue receiving 105%, V100% is the volume of normal tissue receiving 100% and VNT is the volume of normal tissue as defined by the BNT contour. Using a percentage enables a more standardised comparison, independent of the patient’s treated volume, providing a meaningful and clinically useful comparator between plans.10 A value of zero indicates the hot spot is constrained to the target volume. For the breast region, where the covering dose is 95%, an NTI105 is calculated. In the SCF region, the covering isodose is 95% of 45 Gy therefore it is important to assess the amount of normal tissue that receives both 100% and 105%, so both NTI100 and NTI105 are calculated.
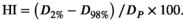
Where D2% is the dose received by 2% of the target volume; D98% is the dose received by 98% of the target volume; Dp is the prescription dose. A value of zero indicates that the absorbed dose distribution is almost homogeneous.23, 24
Statistical analysis
In comparing both target and OAR doses between the 3D-CRT and hIMRT plans, Welsh’s t-test for unequal variances was performed using GraphPad Prism version 5.03 for Windows, GraphPad Software (La Jolla CA, USA). In making the comparison, a P-value ≤ 0.05 was considered statistically significant.
Results
Minimum D95% target dose coverage was equal within experimental uncertainty (1 standard deviation, SD) for both planning techniques due to the optimisation and normalisation process used for plan comparison (Table 2). This was accompanied by a statistically significant reduction in D50% for breast (3D-CRT 102.2% vs. hIMRT 100.3%, P < 0.001) and SCF (3D-CRT 95.0% vs. hIMRT 90.3%, P < 0.001) as well as a D2% maximum for breast (3D-CRT 107.2% vs. hIMRT 102.4%, P < 0.001) and SCF (3D-CRT 101.7% vs. hIMRT 94.4%, P < 0.001). Correspondingly, a statistically significant improvement in HI for both PTV Breast (3D-CRT 11.8 vs. hIMRT 7.8, P < 0.001) and PTV SCF (3D-CRT 22.9 vs. hIMRT 11.3, P < 0.001) was observed, as well as a statistically significant improvement in NTI105% for PTV Breast (3D-CRT 3.6 vs. hIMRT 0.5, P < 0.01) and PTV SCF (3D-CRT 1.7 vs. hIMRT 0.2, P < 0.05). As Figure 3 indicates, the 105% hotspot was removed in a number of plans for both breast and SCF regions.
| Target Structure | DVH Goal | 3D-CRT, mean (SD) | hIMRT, mean (SD) | P-value |
|---|---|---|---|---|
| PTV Breast | D95% > 95% | 97.2% (1.0%) | 96.5% (1.2%) | 0.07 |
| D2cc (max) < 110% | 108.3% (1.2%) | 103.2% (2.0%) | <0.001 | |
| D98% | 95.4% (1.6%) | 94.6% (3.0%) | 0.36 | |
| D50% | 102.2% (1.2%) | 100.3% (0.9%) | <0.001 | |
| D2% | 107.2% (1.2%) | 102.4% (1.6%) | <0.001 | |
| HI: (D2% - D98%)/DP × 100 | 11.8 (1.4) | 7.8 (3.1) | <0.001 | |
| NTI105%: V105%/VNT × 100 | 3.6 (3.9) | 0.5 (0.9) | <0.01 | |
| PTV SCF | D95% > 85.5% | 85.7% (0.5%) | 85.8% (0.4%) | 0.53 |
| D0.1cc (max) < 107% | 102.6% (3.3%) | 95.6% (2.4%) | <0.001 | |
| D98% > 80% | 81.1% (4.8%) | 84.1% (1.7%) | <0.05 | |
| D50% | 95.0% (2.3%) | 90.3% (1.3%) | <0.001 | |
| D2% | 101.7% (3.0%) | 94.4% (2.2%) | <0.001 | |
| HI: (D2% - D98%)/DP × 100 | 22.9 (8.1) | 11.3 (2.9) | <0.001 | |
| NTI100%: V100%/VNT × 100 | 7.5 (4.9) | 2.4 (2.4) | <0.001 | |
| NTI105%: V105%/VNT × 100 | 1.7 (2.8) | 0.2 (0.4) | <0.05 |
- 3D-CRT = three-dimensional conformal radiotherapy; hIMRT = hybrid intensity-modulated radiotherapy; DVH = dose-volume histogram; SD = standard deviation; PTV = planning target volume; HI = homogeneity index; D2% = dose received by 2% of the target volume; D98% = dose received by 98% of the target volume; Dp = prescription dose; NTI = normal tissue index; V105% = volume of normal tissue receiving 105%; V100% = volume of normal tissue receiving 100%; VNT = volume of normal tissue as defined by the breast normal tissue contour; SCF = supraclavicular fossa.
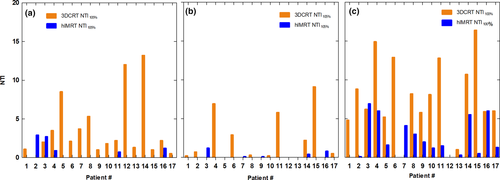
Comparative transverse slices through the right sided SCF region showing a dose wash of 42.75 Gy demonstrates visually the improved homogeneity and conformity in the hIMRT plan (Fig. 4A) versus 3D-CRT plan (Fig. 4B). Comparative slices through the right breast of the 47.5 Gy dose wash in the hIMRT plan (Fig. 4C) versus 3D-CRT plan (Fig. 4D) look similar.
In comparing the average total plan MUs and standard deviations between the two techniques, the hIMRT technique required almost 50% more MUs than the 3D-CRT technique, 651(42) compared to 443(15).
OAR dose comparison
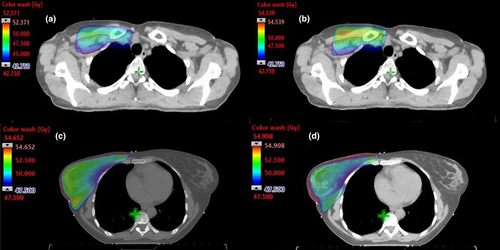
In comparing the doses between hIMRT and 3D-CRT plans, the OARs neighbouring the breast within the tangent portals (including heart, lung and contralateral breast) were within 1SD (Table 3). Meanwhile, when assessing planning goals to OARs neighbouring the SCF target volume, the maximum doses to both thyroid (3D-CRT 49.5 Gy vs. hIMRT 47.5 Gy, P < 0.05) and brachial plexus (3D-CRT 45.1 Gy vs. hIMRT 41.4 Gy, P < 0.05) were statistically significantly lower. This is visibly evident in the plot of population mean dose-volume histogram (DVH) where the hIMRT technique displays dose reduction in the brachial plexus (Fig. 5A, P < 0.05) and thyroid (Fig. 5B, P < 0.05). As Table 3 indicates, no significant dose difference was observed for humeral heads P = 0.46 (Lt), P = 0.71 (Rt), oesophagus (P = 0.70) or spinal cord (P = 0.18).
| OAR | DVH Goal | 3D-CRT, mean (SD) | hIMRT, mean (SD) | P-value |
|---|---|---|---|---|
| Heart (Lt-sided lesion) | Dmean < 4 Gy | 2.7 Gy (1.5 Gy) | 2.7 Gy (1.6 Gy) | 1.00 |
| V2.5 Gy < 40% | 16.3% (8.3%) | 16.3% (8.3%) | 0.99 | |
| V5 Gy < 10% | 7.2% (5.4%) | 7.5% (6.1%) | 0.93 | |
| V10 Gy < 5% | 4.5% (3.9%) | 4.9% (4.7%) | 0.86 | |
| LAD LCA (Lt-sided lesion) | D0.04cc (max) < 45 Gy | 32.0 Gy (19.2 Gy) | 30.1 Gy (20.2 Gy) | 0.85 |
| Heart (Rt-sided lesion) | D2.0cc (max) < 3 Gy | 2.9 Gy (0.7 Gy) | 3.1 Gy (0.8 Gy) | 0.76 |
| Lung Ipsilateral | V5 Gy < 50% | 41.2% (5.8%) | 41.9% (5.8%) | 0.71 |
| V10 Gy < 35% | 27.7% (4.9%) | 28.9% (4.7%) | 0.49 | |
| V20 Gy < 25% | 21.7% (4.8%) | 22.7% (4.5%) | 0.54 | |
| Dmean < 12 Gy | 11.4 Gy (1.8 Gy) | 11.4 Gy (1.8 Gy) | 0.93 | |
| Lung Contralateral | V2.5 Gy < 15% | 0.0 Gy (0.1 Gy) | 0.0 Gy (0.1 Gy) | 0.61 |
| Lung Combined | V20 Gy < 15% | 10.9% (2.2%) | 11.4% (2.3%) | 0.47 |
| V30 Gy < 10% | 9.1% (1.9%) | 9.5% (2.2%) | 0.65 | |
| Dmean < 8 Gy | 5.8 Gy (0.9 Gy) | 5.9 Gy (1.0 Gy) | 0.87 | |
| Breast Contralateral | D2cc (max) < 5 Gy | 3.3 Gy (1.3 Gy) | 3.2 Gy (1.8 Gy) | 0.89 |
| Humerus (Lt-sided lesion | D0.1cc (max) < 27 Gy | 14.7 Gy (13.7 Gy) | 9.8 Gy (12.0 Gy) | 0.46 |
| Humerus (Rt-sided lesion) | D0.1cc (max) < 27 Gy | 16.6 Gy (16.2 Gy) | 13.9 Gy (13.4 Gy) | 0.71 |
| Thyroid | V30 Gy < 62.5% | 36.8% (12.8%) | 41.1% (11.7%) | 0.32 |
| D0.04cc (max) < 50 Gy | 49.5 Gy (2.9 Gy) | 47.5 Gy (2.2 Gy) | <0.05 | |
| Oesophagus | D0.1cc (max) < 30 Gy | 17.3 Gy (13.7 Gy) | 19.2 Gy (14.7 Gy) | 0.70 |
| Dmean < 17 Gy | 2.1 Gy (1.1 Gy) | 2.5 Gy (1.8 Gy) | 0.46 | |
| Brachial Plexus | D2.0cc (max) < 54 Gy | 45.1 Gy (2.7 Gy) | 41.4 Gy (5.8 Gy) | <0.05 |
| Spinal Cord | D0.1cc (max) < 45 Gy | 2.9 Gy (1.0 Gy) | 3.6 Gy (1.9 Gy) | 0.18 |
- OAR, organ at risk; DVH, dose-volume histogram; 3D-CRT, three-dimensional radiotherapy; hIMRT, hybrid intensity-modulated radiotherapy; SD, standard deviation; Lt, left; Rt, right; LAD LC, left anterior descending left coronary artery.
Discussion
Application of the hIMRT technique, when normalised to meet minimum (D95%) target dose constraints, provided statistically significant improvements in NTI and HI for both PTV Breast and PTV SCF. The corresponding reduction in hotspot outside the target volume in particular could lead to a potential improvement in long-term cosmesis given the correlation between late side effects and acute toxicities in patients having had poor dose distribution.7 However, the steep dose drop-off associated with the hIMRT plans did reduce the D50% SCF coverage from 95% (47.5 Gy) for the 3D-CRT plans to 90.3% (45.1 Gy) which is above the minimum specified 45 Gy prescription.
Maximum OAR dose values were improved with the hIMRT technique for most organs in the SCF region, with a statistically significant reduction to the thyroid and brachial plexus. However, a small increase was observed in oesophagus and spinal cord, being well within the DVH goals. The hIMRT plans used for comparison only utilised optimisation off the spinal cord, so there is potential to further reduce OAR doses while maintaining target coverage. This could be a meaningful comparative study to undertake. Other studies have shown that an IMRT approach is beneficial in ipsilateral lung sparing and improved conformity but not without a slight increase in low dose wash to normal tissue.11, 23
In a study comparing IMRT and 3D-CRT for whole breast irradiation, Yim et al.10 proposed that the NTI represented the most clinically relevant tool to evaluate a breast IMRT plan and recommended the use of V105 as the most meaningful parameter. This led to the assessment of the NTI and HI for plan quality and OAR values as they met the department protocols at the time of planning. As presented in Table 2, the HI and NTI105% and NTI100% values indicate a lower percentage of normal tissue irradiated within the treatment portal and a reduced dose variation within the PTVs, showing an overall improvement in plan quality using the hIMRT technique. Studies have also been published showing NTI improvements using IMRT by the reduction of dose to normal tissues while maintaining reasonable target coverage.10, 24 The hIMRT technique was still a preferred technique for patients with larger separations (>22 cm) due to the ability to add an 18MV portion to all IMRT fields. IMRT field size limitations in this cohort can also be addressed by opening up the zero jaw along the posterior edge of the tangent. It should be noted here that the trade-off for this is more contributing dose to OARs from a divergent edge. While this technique resulted in improvements when compared to 3D-CRT for the previously stated reasons, it should be noted that clinically acceptable plans can still be achieved using the 3D-CRT technique. Alternatively, use of volumetric modulated arc therapy (VMAT) may be considered for difficult patient anatomies such as pectus excavatum, and limited neck and arm mobility. This would help improve target coverage and OAR sparing, but at the expense of increased low dose wash (and potential for increased secondary cancer risk).25 Given the significant increase in MUs for the hIMRT technique, considerations should be taken with regard to machine treating time and a patient’s ability to breath hold multiple times throughout their treatment.
Further to the restricted use of OARs for optimisation and increased treatment time, another limitation of this study is the retention of the junctioned fields between the breast and nodal portion of the plan. This type of technique calls for a separation between the breast and nodal volumes due to the cooler dose at the junction. This has contributed to the need to begin development of a more sophisticated technique (VMAT) for attaining dose coverage for contiguous breast and nodal volumes.
The development of this hIMRT technique enabled the NSCC breast unit to move from a field-based planning method (STARS)19 to a volume-based approach (ESTRO recommended target volumes)20 with a minimum dose coverage constraint to the PTV SCF of D95% > 45 Gy without violating the OAR constraints.4 This new standard of care lends itself to the development of more sophisticated techniques required to treat complex target volumes, particularly in patients with unfavourable anatomies.
Conclusion
The application of a mono-isocentric hybrid IMRT treatment planning technique for patients requiring breast and SCF adjuvant irradiation was superior to 3D-CRT in terms of the validated key parameters of HI and NTI. PTV coverage was similar for both techniques with a reduction of maximum doses to the breast, non-target normal tissue, brachial plexus and thyroid. Improvements in dose homogeneity may well result in reducing the risk of breast lymphoedema and poor cosmesis. The improved plan quality with the hIMRT technique has allowed us to deliver a minimum therapeutic dose to the SCF of 45 Gy in 25 fractions in this varied body habitus cohort while respecting OAR tolerances. Improvements in dose homogeneity may well result in reducing the risk of breast lymphoedema and poor cosmesis.
Conflict of interest
There are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.




