Reduced dose to small bowel with the prone position and a belly board versus the supine position in neoadjuvant 3D conformal radiotherapy for rectal adenocarcinoma
Abstract
Introduction
No consensus exists regarding the optimal treatment setup for neoadjuvant radiotherapy of rectal cancer using a 3D conformal (3D CRT) technique. Positioning the patient prone with a belly board aims to reduce the amount of small bowel irradiated.
Methods
Twenty-five patients with locally advanced rectal cancer underwent computed tomography (CT) planning for neoadjuvant chemoradiotherapy. Patients were simulated prone with a belly board and then in the supine position. Questionnaires rating the comfort of each position were completed. 3D CRT plans were generated for both positions to a prescribed dose of 50.4 Gy in 1.8 Gy daily fractions. Dose–volume parameters in 5 Gy increments for small bowel, large bowel and bladder wall were compared.
Results
Small bowel V5 Gy, V10 Gy, V15 Gy and V20 Gy values were significantly higher in the supine position (398, 366, 245, 151 cm3 for supine vs. 243, 213, 161, 122 cm3 for prone respectively; P < 0.001, <0.001, <0.001 and 0.025). Large bowel V5 Gy, V10 Gy and V15 Gy values were significantly higher in the supine position (266, 209, 147 cm3 supine, 175, 139, 108 cm3 prone respectively; P = 0.001, <0.001, 0.003). There was a significant difference in comfort scores favouring the supine position (P = 0.015).
Conclusion
A significant increase in small and large bowel dose was seen in the supine plans. Treatment in the prone position with a belly board may reduce toxicity when using a 3D CRT technique. Whilst both setup positions were tolerable the supine was more comfortable.
Introduction
Adjuvant radiotherapy is a well-established component of curative intent therapy for selected stage II and III rectal adenocarcinoma and a large body of evidence supports neoadjuvant versus postoperative timing.1-3 The small bowel is the dose-limiting structure when irradiating the pelvis and the minimisation of small bowel dose is associated with improved treatment tolerability, reduced late toxicity and safer dose escalation.4, 5
The dose to small bowel can be reduced with manipulation of bladder filling and through patient positioning. The adoption of the prone position with belly board (PBB) is one such method. The anterior abdominal wall and mobile peritoneal contents inclusive of small bowel are coerced superiorly and away from the direct paths of the pelvic radiotherapy fields. Issues identified with the PBB technique included increased setup complexity, increased setup time requirements, increased setup error and patient discomfort.6-8
There are no existing studies comparing small bowel dosimetry in the supine position with a full bladder to the PBB position exclusively in a long course, neoadjuvant radiotherapy setting using a 3D conformal technique (3D CRT) for adenocarcinoma of the rectum. The primary aim of this study was to compare dose–volume histograms (DVH's) generated for the small bowel in the supine versus PBB position. Secondary objectives were to compare the DVH's of large bowel and bladder wall, and to assess the tolerability of the prone position.
Methods
Ethics approval for this study was granted through the Austin Health Human Ethics Committee. Patients were recruited prospectively and considered eligible if they had American Joint Committee on Cancer (AJCC) TNM stage II or III adenocarcinoma of the rectum and were suitable for long course radiotherapy. Exclusions were made if the patient was unable to give informed consent, or unable to assume both positions.
Twenty-five patients were consented and enrolled between 2006 and 2012. The patient characteristics are summarised in Table 1. Each patient underwent two separate simulations performed using the General Electric Medical Systems LightSpeedTM Radiotherapy (CE0459) computed tomography (CT) scanner and couch. A presimulation bladder protocol was utilised to achieve a comfortably full bladder which required complete void 30 min prior to simulation followed by ingestion of 500 mL of water.
| n | % | |
|---|---|---|
| Age | ||
| Median | 64 | |
| Range | 45–79 | |
| Gender | ||
| M | 18 | 72 |
| F | 7 | 28 |
| Tumour position | ||
| Upper | 0 | 0 |
| Mid | 8 | 32 |
| Low | 17 | 68 |
| TNM stage | ||
| II | 10 | 40 |
| III | 15 | 60 |
The prone simulation was performed first and used an in-house foam belly board construction with a rectangular aperture of 20 × 25 cm with head supports and customised ankle pillows for stability. The belly board was positioned and clipped into the couch with the lower level of the aperture at the L4/5 vertebral level. A helical CT data set was acquired of 2.5-mm-thick transverse slices at 512 × 512-pixel resolution from the L2/3 level to 10 cm below the ischial tuberosities.
The supine position simulation was performed second. Stabilisation was achieved with the Hip-FixTM device (MED TEC Inc., Hillsborough, NC) and an individualised foam support from iliac crest to mid-thigh. Indexed foot stocks and a 10 cm forehead sponge were used for comfort. A CT of similar specifications was acquired.
Patients were asked to complete a questionnaire regarding the comfort of the simulation process on the same day. Patients scored both positions on a scale of 1–10 spanning comfortable to very uncomfortable respectively. A score above 5 was considered to be potentially intolerable for a conventionally fractionated, 5 1/2-week treatment course.
Acquired CT images were transferred directly to the treatment planning software (XiO v4.70, Elekta, Stockholm, Sweden). All treatment targets were defined on CT by a single radiation oncologist with assistance from endoscopy reports, examination notes and fused pelvic magnetic resonance images (MRI) in accordance with definitions outlined by the International Commission of Radiation Units and Measurements (ICRU) reports 50 and 62.9 The clinical target volume (CTV) consisted of the gross tumour volume (GTV), mesorectum and regional lymphatics. Planning target volume to 45 Gray (PTV_45) consisted of a uniform 1 cm expansion on CTV. A CTV2 boost volume to the highest risk area comprised of the mesorectum at the level of the GTV and any involved nodes with an additional 1 cm superiorly and inferiorly. A further 1 cm isotropic expansion formed the PTV_50.4. The prescription dose was 45 Gy to the PTV and 50.4 Gy to PTV2. All small and large bowel loops within the simulation scan were contoured as organs at risk (OAR), excluding the volume of bowel within CTV's and verified by a single, consultant radiologist. The entire small and large bowel were not imaged within the limits of the simulation scan. Femoral heads and bladder wall were also contoured.
An optimised treatment plan was produced in both positions by radiation therapists. A typical plan consisted of 3–5 coplanar beams of between 6 and 10 MV energy. Plans were optimised for homogeneity and sparing of OAR dose through modifications to beam weightings, energies, field shapes and wedge use. An acceptable plan covered the PTV by a minimum 95% of the prescribed dose with no hotspots of greater than 107%. Femoral head dose was constrained to a V35 Gy <50% and the maximum dose to 2 cc of small bowel was required to be less than 50.4 Gy but there were no other specified small or large bowel constraints. All patients were treated in the supine position with a comfortably full bladder.
The volume of small bowel, large bowel and bladder wall receiving at least 5–50 Gy in 5 Gy increments, and the maximum dose to 2 cc of small bowel were generated from the treatment planning system (TPS) and cumulative DVHs produced. The paired volume differences between the two setup positions were analysed for each dose increment, assessed for normality and then for a statistical significant difference using a P < 0.05 cut-off. All statistical tests were two-sided and performed using IBM SPSS software v22 (Armonk, New York).
Results
The patient and tumour characteristics of the 25 patients are summarised in Table 1. Of note, all patients had low or mid rectal tumour defined as the lowermost tumour extension within 0–4 and 4.1–8 cm from the anorectal junction, respectively, on endoscopic examination.
The mean bladder volume was 234 mL in the supine position and 167 mL in the prone position with the difference between means reaching statistical significance (P < 0.001). There were no other statistically significant differences regarding the volumes of structures contoured between the two positions including the small bowel.
The mean small bowel, large bowel and bladder wall volumes receiving doses in 5 Gy increments in the two positions are represented in Table 2 along with the mean difference between positions and P-values. Small bowel V5 Gy, V10 Gy, V15 Gy and V20 Gy values were significantly higher in the supine position (398 cm3 vs. 244 cm3, 366 cm3 vs. 213 cm3, 245 cm3 vs. 161 cm3 and 151 cm3 vs. 122 cm3 for supine and prone respectively; P < 0.001, <0.001, <0.001 and 0.025). This equated to an extra 154, 153, 84 and 29 cm3 on average of small bowel receiving at least 5, 10, 15 and 20 Gy, respectively, in the supine position. There were no statistically significant differences regarding higher dose increments or the maximum dose to 2 cm3 of small bowel. Figure 1 demonstrates the cumulative DVHs for small bowel, large bowel and bladder wall in the two positions.
| Dose (Gy) | Small bowel | Large bowel | Bladder wall | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean volume (cc) | Mean volume (cc) | Mean volume (cc) | ||||||||||
| Supine | Prone | Difference | P value | Supine | Prone | Difference | P value | Supine | Prone | Difference | P value | |
| 5 | 398 | 243 | 154 | <0.001 | 257 | 175 | 82 | 0.001 | 52 | 49 | 3 | 0.42 |
| 10 | 366 | 213 | 153 | <0.001 | 209 | 139 | 71 | <0.001 | 52 | 49 | 3 | 0.42 |
| 15 | 245 | 161 | 84 | <0.001 | 146 | 108 | 38 | 0.003 | 51 | 49 | 2 | 0.62 |
| 20 | 151 | 122 | 29 | 0.025 | 96 | 85 | 11 | 0.14 | 36 | 39 | −3 | 0.25 |
| 25 | 120 | 103 | 17 | 0.10 | 75 | 72 | 3 | 0.58 | 28 | 30 | −2 | 0.25 |
| 30 | 102 | 89 | 13 | 0.15 | 62 | 60 | 2 | 0.63 | 25 | 27 | −2 | 0.31 |
| 35 | 85 | 74 | 11 | 0.19 | 46 | 44 | 1 | 0.72 | 23 | 25 | −2 | 0.36 |
| 40 | 72 | 63 | 8 | 0.25 | 35 | 37 | −2 | 0.67 | 21 | 23 | −2 | 0.45 |
| 45 | 48 | 42 | 6 | 0.33 | 26 | 26 | 0 | 0.89 | 18 | 19 | −1 | 0.75 |
| 50 | 8 | 7 | 1 | 0.79 | 6 | 8 | −2 | 0.43 | 7 | 8 | −1 | 0.87 |
- Bold font indicates a statistically significant difference (P < 0.05).
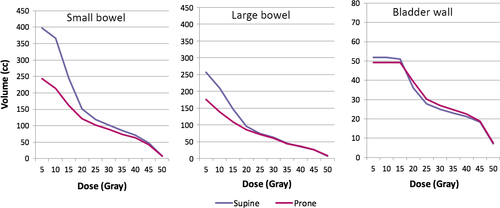
Large bowel V5 Gy, V10 Gy and V15 Gy values were significantly higher in the supine position (266 cm3 vs. 175 cm3, 209 cm3 vs. 139 cm3, 146 cm3 vs. 108 cm3 for supine and prone respectively; P = 0.001, <0.001, 0.003). There were no statistically significant differences regarding dose to bladder wall at any dose levels.
Comfort analysis favoured the supine position although on the whole both positions were tolerable. The supine position averaged a score of 2.18 (range 1–5) as opposed to a score of 3.88 (range 1–9) for PBB. The difference of the means reached statistical significance (P = 0.02). Three of 25 patients (12%) experienced a potentially intolerable score in the PBB with the remainder scoring 5 or less.
Discussion
Our study confirms a reduction in dose to small and large bowel at a number of dose levels in the prone position with a belly board relative to the supine position when using long course, neoadjuvant 3D CRT for rectal cancer. These results are important as the PBB would be expected to improve tolerability of treatment through reduced acute toxicity and improve quality of life through reduced late toxicity. This is particularly relevant in a disease where long-term survival is the expectation.
Acute small bowel toxicity may include symptoms of nausea, vomiting, diarrhoea, abdominal pain and anorexia which at their worst can necessitate admissions, treatment breaks and even the early cessation of therapy.4, 5, 10 Late effects include stricture formation, chronic diarrhoea, malabsorption, bowel perforation, chronic pain and second malignancy.11, 12 The Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) recommendation for 3D CRT are to limit the volume of small bowel receiving 15 Gy or more to under 120 cc (V15 < 120 cc) if contouring individual loops or a V45 < 195 cc if contouring the in-field peritoneal cavity. Both predict for a risk of grade 3 toxicity of under 10%.4, 10 The former constraint is most relevant to this study and was met in 12% of plans in the supine position versus 60% in the PBB position. The risk of grade 3 or worse acute toxicity has been estimated to be as high as 70% with a V15 > 300 cc.5 This target was breached in 40% of cases in the supine position compared to just 10% in the prone position. Research regarding severe late toxicity has suggested a <5% risk of grade 3 or worse toxicity if the V50 is <5% of the total small bowel volume.4 This was achievable in both positions but may have been more problematic with dose escalation beyond 50.4 Gy.
Although dose–volume relationships regarding large bowel toxicity outside of the rectum are not well documented both increasing dose and volume irradiated are likely to correlate with toxicity. Hence, the PBB position would be expected to reduce toxicity.
Mean bladder wall volume was significantly different between the two groups with the supine position associated with a fuller bladder. This is almost certainly due to the supine CT simulation occurring second and hence more time for bladder filling. A full bladder has been demonstrated to reduce small bowel irradiated.13 This has likely reduced the magnitude of difference in small bowel irradiated between supine and PBB position in our study. There were no high rectal tumours in this cohort which could also have further reduced the differences between the two positions as small bowel would be expected to be most at risk with the boost volume closer to the pelvic peritoneal reflection.
Our conclusions seem consistent with other studies assessing the use of a belly board and/or prone positioning in pelvic radiotherapy although there are no others specific to this context.7, 8, 13-15 Most recently a large randomised controlled trial was conducted comparing the supine position to prone exclusively in the neoadjuvant, long course radiotherapy setting for rectal cancer and hence, the same context as our study. Importantly, the study did not use a belly board as part of the prone setup and did not find any statistically significant dosimetric improvements to small bowel DVH's.6
Our study did not use intensity modulated radiotherapy (IMRT) technology. The addition of which may offset some of the dosimetric benefits of treating in the PBB although there is already some existing evidence to the contrary. Kim et al. found reduced small bowel volume irradiated in all dose levels between 20 and 100% of the prescribed dose when comparing neoadjuvant radiotherapy for rectal cancer in the prone versus prone position with belly board.14 Synergies between IMRT and the PBB position may represent an opportunity for safer dose escalation when investigating rectal preservation approaches or involved pelvic node boost assuming daily setup accuracy.16
Whilst this study confirmed reduced comfort in the PBB position only a minority of patients graded it in the uncomfortable domain. This is consistent with a previous study reporting both prone and supine positions to be equally tolerable during radiotherapy to the male pelvis and that position may be reasonably determined without consideration of comfort.17 It is conceivable that a degree of patient discomfort can be tempered with more individualised supports and belly board apertures or that whilst a small proportion may not tolerate, most will and can hence derive the benefits.
Our centre, like many in Australia and New Zealand has adopted the supine position for neoadjuvant rectal cancer radiotherapy but has retained the relevant equipment for PBB and uses it routinely in the postoperative radiotherapy setting where in-field small bowel is more problematic. This study has forced a rethink although there has also been a shift towards IMRT.
Conclusion
The prone position with a comfortably full bladder and belly board appears to give superior small and large bowel DVHs relative to that of the supine position when utilising a 3D conformal radiotherapy planning technique in the setting of neoadjuvant, long course, radiotherapy for rectal cancer. This would be expected to reduce both acute and late toxicity of treatment and may have important implications for improving the safety of dose escalation. Whilst less comfortable on average, the vast majority of patients tolerated the prone position with belly board.
Conflicts of Interest
The authors declare no conflict of interest.




