Breast Tissue Chemistry Measured In Vivo In Healthy Women Correlate with Breast Density and Breast Cancer Risk
Gorane Santamaría and Natali Naude are co-first authors
Abstract
Background
The relationship of tissue chemistry to breast density and cancer risk has not been documented despite breast density being a known risk factor.
Purpose
To investigate whether distinct chemical profiles associated with breast density and cancer risk are identified in healthy breast tissue using in vivo two-dimensional correlated spectroscopy (2D COSY).
Study Type
Prospective.
Population
One-hundred-seven participants including 55 at low risk and 52 at high risk of developing breast cancer.
Field Strength/Sequence
3 T/ axial/ T1, T2, 2D COSY.
Assessment
Two radiologists defined breast density on T2. Interobserver variability assessed. Peak volumes normalized to methylene at (1.30, 1.30) ppm as internal shift reference.
Statistical Tests
Chi-squared/Mann–Whitney/Kappa statistics/Kruskal Wallis/pairwise analyses. Significance level 0.05.
Results
Ten percentage were fatty breasts, 39% scattered fibroglandular, 35% heterogeneously dense, and 16% extremely dense. Interobserver variability was excellent (kappa = 0.817). Sixty percentage (64/107) were premenopausal. Four distinct tissue chemistry categories were identified: low-density (LD)/premenopausal, high-density (HD)/premenopausal, LD/postmenopausal, and HD/postmenopausal. Compared to LD, HD breast chemistry showed significant increases of cholesterol (235%) and lipid unsaturation (33%).
In the low-risk category, postmenopausal women with dense breasts recorded the largest significant changes including cholesterol methyl 540%, lipid unsaturation 207%, glutamine/glutamate 900%, and choline/phosphocholine 800%.
In the high-risk cohort, premenopausal women with HD recorded a more active chemical profile with significant increases in choline/phosphocholine 1100%, taurine/glucose 550% and cholesterol sterol 250%.
Data Conclusion
Four distinct chemical profiles were identified in healthy breast tissue based on breast density and menopausal status in participants at low and high risk. Gradual increase in neutral lipid content and metabolites was noted in both risk groups across categories in different order. In low risk, the HD postmenopausal category exhibited the highest metabolic activity, while women at high risk exhibited the highest lipid content and metabolic activity in the HD premenopausal category.
Level of Evidence
2
Technical Efficacy Stage
3
Epidemiological studies report breast density as an independent risk factor for cancer1-3 with high density (HD) conferring a 4 to 6-fold increased risk of breast cancer.4 Only age and BRCA mutation status are associated with a higher risk.5 The most widespread method to qualitatively assess breast density is the breast imaging reporting and data system (BI-RADS) classification.6 BI-RADS evaluates parenchymal patterns and distributions and classifies the breast density into four categories.6 Stroma is the major tissue component of the breast and is composed of stromal cells and extracellular matrix proteins.7 Dense breast tissue contains higher amounts of stroma and less fat than nondense breasts8 and the increase in breast density is mainly associated with an augmentation in the deposition of collagen.9
One-dimensional MR spectroscopy was reported to identify variations in water and lipid ratio of breast tissue related to age, breast density, and menopausal status.10 A more modern approach using in vivo MR two-dimensional correlated spectroscopy (2D COSY) reported the chemical species unambiguously assigned using a second magnetic frequency.11, 12 This 2D COSY method was used to evaluate breast tissue chemistry in women carrying the BRCA1 or BRCA2 gene mutations.11 Neutral lipid content was reported to differ between the BRCA1, BRCA2, and healthy cohorts. Recent advances in MR hardware, including magnet stability, coil technology, and the capacity for radiographers to operate the scanner with precision in spectroscopy mode, have resulted in an improved signal-to-noise ratio.
Cell models of tumor development and progression paved the way to evaluate the role of lipid chemistry and metabolism in tumor development and progression. In vitro cell lines were examined and the MR spectral changes were correlated with specific biological and genetic characteristics.13-15 The earliest biomarkers of the premalignant state(s) were molecules active on the MR timescale and included lipids and cholesterol.15 In parallel, the causes of the aberrant choline phospholipid metabolism in breast cancer were documented.16
Our hypothesis is that the lipid chemistry and metabolites, recorded in well-defined cell models of tumor development and progression, could now be measured in vivo in a clinical scanner and that there is an association between the tissue chemistry, the breast density, and the risk of breast cancer. Therefore, the evaluation of breast tissue chemistry might provide an objective evaluation of both breast density and biomarkers of risk for cancer.
This study aims to investigate whether distinct chemical profiles associated with breast density can be identified in the tissue of healthy women at low and high risk of breast cancer using in vivo 2D COSY.
Material and Methods
Patient Cohort and Inclusion Criteria
Institutional review board approval and written informed consent was obtained from all participants. A cross-sectional study with prospective data collection was carried out between October 2017 and August 2019 at three hospitals. Sixty-four healthy women at low risk and 89 at high risk according to the National Institute for Health and Care Excellence guidelines17 (Table 1) were consecutively recruited. None of them was pregnant or taking hormone replacement therapy. The lifetime risk was calculated for each patient according to the international breast cancer intervention study (IBIS) score using the Tyrer–Cuzick model18 (version 8.0b). The American Cancer Society recommends women with a lifetime risk of cancer of 20% or greater as being high risk.19 According to these guidelines, participants with an IBIS score ≤ 20% were selected from the low-risk cohort. Women with a score > 20% were chosen from the high-risk category. Therefore, the study group included 107 participants (mean age, 44.07 years; SD, 11.48 years; range: 20–72), including 55 at low risk with an IBIS score ≤ 20% and 52 at high risk with no known pathogenic mutations and an IBIS score > 20%.
| Low Risk | Medium Risk | High Risk |
|---|---|---|
No family history of breast cancer One first-degree relative with breast cancer >40 One second-degree relative with breast cancer at any age Two first- or second-degree relatives with breast cancer >50 (on different sides of the family) |
One first-degree relative with breast cancer <40 Two first- or second-degree relatives with breast cancer at an average age > 50 Three first- or second-degree relatives with breast cancer at an average age > 60 |
Two first- or second-degree relatives with breast cancer <50 Three first- or second-degree relatives with breast cancer <60 Four relatives with breast cancer at any age Two first- or second-degree relatives with breast or ovarian cancer plus any of the following:
One member of a family where a breast cancer gene has been identified |
MR Imaging
Participants underwent MR imaging of the breast and in vivo MR 2D COSY between days 6 and 14 of the menstrual cycle, where relevant. The data were collected on a 3-T Magnetom Prisma or a 3-T Magnetom Vida scanner (Siemens AG, Erlangen, Germany) using either an 18-channel (Siemens AG) or a 16-channel (RAPID Biomedical, Germany) breast coil.
Breast MRI consisted of 1) localizer sequence (repetition time [TR]:6 msec, echo time [TE]: 2.61 msec, slice thickness: 7 mm, field of view [FOV]: 400 x 400 mm2); 2) axial T1-weighted three-dimensional (3D) flash (TR: 5.43 msec, TE: 2.46 msec, flip angle: 20°, slice thickness: 2 mm, FOV: 320 x 320 mm2, matrix: 448 × 448 mm2); and 3) axial T2-weighted TSE sequence (TR: 4280 msec, TE: 97 msec, slice thickness: 2 mm, FOV: 300 x 300 mm2, matrix: 448 × 448 mm2).
A dynamic axial 3D fat suppressed T1-weighted gradient-echo sequence was added to the protocol in women at high risk (TR: 4.51 msec; TE: 2.03 msec; flip angle: 10°; slice thickness: 1.2 mm; FOV: 320 × 320 mm2; matrix: 448 × 448 mm2; in-plane resolution: 0.7 × 0.7 mm2, acquisition time 91.5 seconds). Images were obtained prior to a rapid bolus injection and five times after injection of contrast material, with a resulting total imaging time of 9 minutes 9 seconds. The bolus injection consisted of 0.1-mmol gadobutrol (Gadovist; Bayer, Berlin, Germany) per kilogram of body weight and a 20-mL saline flush, delivered through an intravenous cannula. Automated subtraction of the appropriate precontrast and postcontrast images and multiplanar reconstruction of data sets were performed.
Using the T2-weighted sequence, two radiologists (G.S. and T. L.) with 20 years and 10 years of experience in breast imaging independently assessed the breast density based on BI-RADS classification6: category a (fatty breast), category b (scattered density), category c (heterogeneous density), and category d (extremely dense breast). For analysis purposes, a and b made up the low-density (LD) group, whereas c and d made up the HD group. Interobserver variability was assessed. For discrepancies in assessing the amount of fibroglandular tissue (low vs. high), the radiologists agreed on the breast density type that would be recorded for further analysis.
Two-Dimensional Correlated Spectroscopy
A 3D T1-weighted sequence was used to position a voxel of 20 × 20 × 20 mm3 in the left breast. To get a reliable description of the breast tissue chemistry related to a specific category of breast density, the voxel was placed in a region that mainly included fatty tissue whenever the overall breast density was type a; a mixture of fat and fibroglandular tissue in participants with type b or type c (predominantly fat in type b and fibroglandular tissue in type c) and fibroglandular tissue in those participants with type d (Fig. 1).
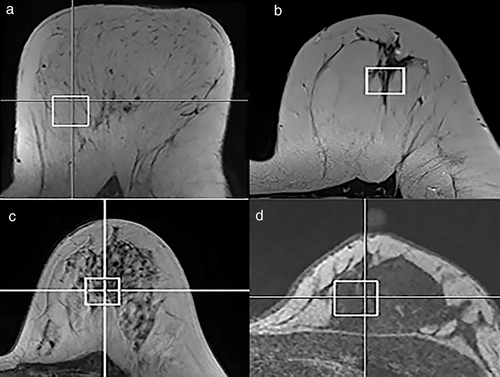
Localized shimming was performed using the automatic B0-field mapping technique Siemens auto-shimming algorithm,20 followed by manual adjustment of zero order shim gradients to achieve a width of the water peak at half maximum of ≤65 Hz.
The 2D COSY sequence parameters were TR of 2000 msec, TE initial of 30 msec, 96 t1 increments at 0.8 msec, 6 averages per increment, f2 bandwidth of 2000 Hz, vector size of 1024 points, and RF offset frequency set on 3.2 ppm. “WET” water suppression21 was applied. The total acquisition time was 19 minutes. Processing was undertaken as reported by Ramadan11 and participants were instructed to remain still.
For the measurement of cross peak and diagonal peak volumes, all peak volumes were normalized to the methylene peak at (1.30, 1.30) ppm as the internal chemical shift reference. Next, a box was prescribed on top of each peak, and lastly, the Felix 2D/ND software (Felix NMR, San Diego, California, USA) was used for off-line data processing, spectral visualization, and analysis of high-resolution NMR data measuring the volume of signal under these boxes. Peak assignments were noted along (F2, F1) in ppm and made as previously reported by Ramadan and Thomas in vivo11, 12 and by Sitter et al from breast tissue extracts.22
Standard spectral analysis utilized a level multiplier of 1.4, whereas for metabolite regions, we utilized a level multiplier of 1.05 to make it possible identify the diagonal peaks of metabolites.
Statistical Analysis
Statistical analysis was undertaken using IBM SPSS Statistics 25.0 (IBM, Armonk, New York, USA). Age, MRI BI-RADS category of breast density, menopausal status, body mass index (BMI), IBIS risk score, measured volume of various lipid diagonal peaks and cross peaks, metabolites, and cholesterol were collected for each participant.
Chi-squared test was used to compare categorical variables. Mean comparison between low- and high-risk groups of developing breast cancer and between low and high breast density categories was performed using Mann–Whitney test. Interobserver variability was assessed by kappa statistics for qualitative data. Kruskal–Wallis test was used for mean comparison across several categories and Bonferroni correction for pairwise comparison of the means. A two-sided P-value of <0.05 was considered statistically significant.
Results
Clinical Features
The demographics of the cohort are listed in Table 2. No significant differences in age (P = 0.632), menopausal status (P = 0.221), or BMI (P = 0.622) were recorded between the low- and high-risk groups. Sixty percentage (64/107) of participants were premenopausal and the remaining 40% (43/107) postmenopausal.
| Low Risk (N = 53) | High Risk (N = 54) | P-Value | |
|---|---|---|---|
| Age, mean (SD) | 43.6 (12.0) | 44.6 (11.0) | 0.632 |
| Menopausal status, N (%) | 0.221 | ||
| Premenopausal | 36 (34) | 28 (26) | |
| Postmenopausal | 19 (18) | 24 (22) | |
| Breast density, N (%) | 0.026 | ||
| Fatty breast | 9 (8) | 2 (2) | |
| Scattered fibroglandular tissue | 24 (22) | 18 (17) | |
| Heterogeneously dense | 14 (13) | 23 (22) | |
| Extremely dense | 8 (7) | 9 (8) | |
| BMI, mean (SD) | 25.63 (5.20) | 26.08 (4.84) | 0.622 |
| IBIS score, mean (SD) | 10.83 (3.99) | 29.35 (7.91) | <0.001 |
- Mann–Whitney test used for mean comparison and chi-squared test for categorical variables.
- N = number of subjects; SD = standard deviation; BMI = body mass index; IBIS = international breast cancer intervention study.
The breast density distribution in this cohort was made up of 10% (11/107) type a, 39% (42/107) type b, 35% (37/107) type c, and 16% (17/107) type d. LD and HD categories included 53 and 54 participants, respectively. A significantly higher proportion of LD breasts was noted in the low-risk cohort in comparison with the high-risk group. The interobserver variability for the breast density assessment in terms of LD or HD was excellent (kappa coefficient = 0.817).
In Vivo 2D COSY of Healthy Human Breast Tissue
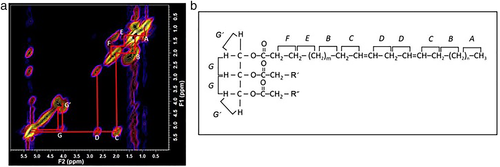
The improved capability of the 3-T scanners used in this study has resulted in new assignments. A representative 2D COSY spectrum of the region F2/F1: 0.00 ppm to 6.00 ppm, with the cross peaks assigned (A–G′), is shown for an HD/postmenopausal participant in Fig. 2a. The triglyceride molecule, with associated cross peaks labeled (A–G′), can be seen in Fig. 2b. The spin–spin coupling between adjacent hydrogen atoms are denoted. The G′ is seen in Fig. 2a as two clear cross peaks not previously reported in vivo in the human breast.
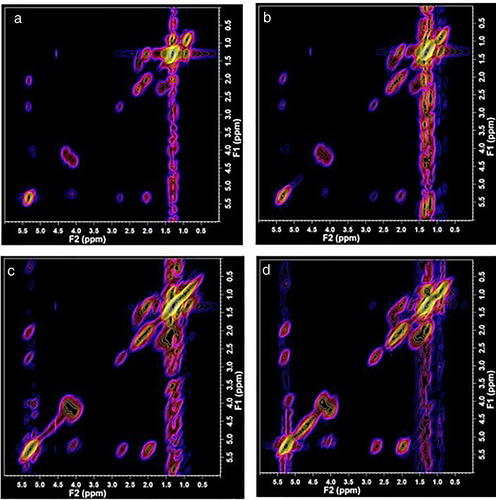
Typical spectra recorded from LD/premenopausal, LD/postmenopausal, HD/premenopausal, and HD/postmenopausal are shown in Fig. 3. An increase of signal can be observed in the series for lipid cross peaks C, D, G, and G′ in this series. The three-dimensional and 2D contour plots of the expanded spectral region (F2/F1: 3.00 ppm to 3.90 ppm), which contain the metabolites, are shown from the same participants in Fig. 4. Note the increase in signal intensity in high breast density participants (Fig. 4c and d).
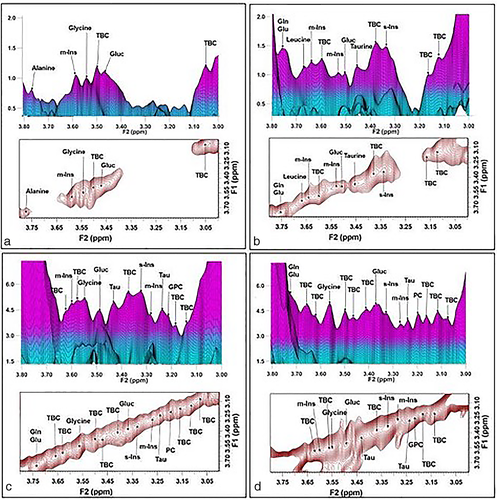
Correlation of Tissue Chemistry with Breast Density
The molecules that are mobile on the MR time scale have been measured and comparisons between low and high breast density are summarized in Table 3. In women with high breast density, there is a range of metabolites that increase by a factor between 224% and 900%; the cholesterol sterol increases by 135% and cholesterol methyl resonances by a factor of 235%.
| Chemical Shift (F2, F1) ppm | Chemical Species | Low-Density Tissue, N = 53 (Mean) | High-Density Tissue, N = 54 (Mean) | Percentage Difference | P-Value |
|---|---|---|---|---|---|
| Lipids | |||||
| 0.40, 0.40 | Cholesterol sterol | 0.00055 | 0.00130 | +136 | <0.001 |
| 0.70, 0.70 | Cholesterol methyl | 0.00109 | 0.00365 | +235 | <0.001 |
| 0.90, 0.90 | –CH3 | 0.08261 | 0.09342 | +13 | <0.001 |
| 0.90, 1.30 | Lipid cross peak A | 0.04267 | 0.03959 | −7 | <0.001 |
| 2.02, 2.02 | –CH2–CH=CH–CH2– | 0.04136 | 0.05205 | +26 | <0.001 |
| 2.02, 5.31 | Lipid cross peak C | 0.02212 | 0.02671 | +21 | 0.363 |
| 2.25, 1.59 | Lipid cross peak F | 0.01812 | 0.02257 | +25 | <0.001 |
| 2.77, 2.77 | =CH–CH2–CH= | 0.01182 | 0.01426 | +21 | 0.001 |
| 2.77, 5.31 | Lipid cross peak D | 0.00920 | 0.01228 | +33 | 0.043 |
| 4.10, 4.10 | –CH2–O–(C=O)–R | 0.04065 | 0.04756 | +17 | 0.147 |
| 4.10, 5.22 | Lipid cross peak G | 0.00736 | 0.00681 | −7 | 0.001 |
| 5.31, 5.31 | –HC=HC– | 0.08738 | 0.10623 | +22 | 0.695 |
| Metabolites | |||||
| 3.15, 3.15 | Unassigned | 0.00003 | 0.00022 | +633 | <0.001 |
| 3.19, 3.19 | Unassigned | 0.00001 | 0.00010 | +900 | <0.001 |
| 3.21, 3.21 | Choline, Phosphocholine | 0.00005 | 0.00030 | +500 | <0.001 |
| 3.22, 3.22 | Choline, glycerophosphocholine | 0.00001 | 0.00009 | +800 | <0.001 |
| 3.24, 3.24 | Taurine, glucose | 0.00002 | 0.00011 | +450 | <0.001 |
| 3.27, 3.27 | Myo-inositol | 0.00002 | 0.00010 | +400 | <0.001 |
| 3.34, 3.34 | Scyllo-inositol | 0.00006 | 0.00026 | +333 | <0.001 |
| 3.50, 3.50 | Glycine, myo-inositol | 0.00009 | 0.00030 | +233 | <0.001 |
| 3.55, 3.55 | Glycine | 0.00005 | 0.00020 | +300 | <0.001 |
| 3.70, 3.70 | Leucine | 0.00004 | 0.00023 | +475 | <0.001 |
| 3.90. 3.90 | Creatine, aspartate | 0.00042 | 0.00136 | +224 | <0.001 |
- Mann–Whitney test used for mean comparison between categories.
- F2, F1 = frequency coordinates that explain the position of each peak; N = number of subjects.
Correlation of Tissue Chemistry with Breast Density, Menopausal Status and Risk
Tables 4 and 5 summarize the differences in tissue chemistry according to the breast density adjusted by menopausal status for both the low- and high-risk cohorts.
| Premenopausal (N = 36) | Postmenopausal (N = 19) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Chemical Shift (F2, F1) ppm | Chemical Species | Low-Density, N = 19 (Mean) | High-Density, N = 17 (Mean) | P-Value | % Change High Density vs. Low Density | Low-Density, N = 14 (Mean) | High-Density, N = 5 (Mean) | P-Value | % Change High Density vs. Low Density |
| Lipids | |||||||||
| 0.40, 0.40 | Cholesterol sterol | 0.00057 | 0.00145 | <0.001 | +154 | 0.00063 | 0.00403 | 0.002 | +540 |
| 0.70, 0.70 | Cholesterol methyl | 0.00102 | 0.00416 | <0.001 | +308 | 0.00125 | 0.00735 | 0.005 | +488 |
| 0.90, 0.90 | –CH3 | 0.08617 | 0.08866 | 0.003 | +3 | 0.08668 | 0.17348 | 0.007 | +100 |
| 0.90, 1.30 | Cross peak A | 0.04289 | 0.03775 | 0.006 | −12 | 0.04199 | 0.03650 | 0.070 | −13 |
| 1.59, 1.59 | –CH2–CH2–COO– | 0.01789 | 0.06216 | <0.001 | +247 | 0.01823 | 0.08788 | 0.001 | +382 |
| 2.02, 2.02 | –CH2–HC=HC–CH2– | 0.04327 | 0.05088 | <0.001 | +18 | 0.04393 | 0.10904 | 0.001 | +148 |
| 2.02, 5.31 | Cross peak C | 0.01747 | 0.01564 | 0.876 | −10 | 0.01724 | 0.04320 | 0.087 | +151 |
| 2.25, 2.25 | –CH2–COO– | 0.02991 | 0.03244 | 0.011 | +8 | 0.02969 | 0.06695 | 0.005 | +125 |
| 2.25, 1.59 | Cross peak F | 0.03168 | 0.04118 | 0.001 | +30 | 0.03168 | 0.07422 | 0.001 | +134 |
| 2.77, 2.77 | =HC–CH2–HC= | 0.01295 | 0.01434 | 0.006 | +11 | 0.01283 | 0.03343 | 0.001 | +161 |
| 2.77, 5.31 | Cross peak D | 0.00789 | 0.00809 | 0.594 | +3 | 0.00761 | 0.01976 | 0.026 | +160 |
| 4.25, 5.22 | Cross peak G | 0.00834 | 0.00545 | 0.016 | −3 | 0.00754 | 0.01292 | 0.156 | +71 |
| 4.10, 4.25 | Cross peak G′ | 0.06470 | 0.08488 | 0.016 | +31 | 0.07442 | 0.18042 | 0.003 | +142 |
| 5.31, 5.31 | –CH=HC– | 0.09507 | 0.09536 | 0.363 | +0 | 0.09263 | 0.31415 | 0.001 | +207 |
| Metabolites | |||||||||
| 3.03, 3.03 | Creatine | 0.00013 | 0.00051 | <0.001 | +292 | 0.00015 | 0.00129 | 0.001 | +760 |
| 3.15, 3.15 | Unassigned | 0.00003 | 0.00022 | <0.001 | +633 | 0.00004 | 0.00034 | 0.003 | +750 |
| 3.19, 3.19 | Unassigned | 0.00001 | 0.00011 | <0.001 | +1000 | 0.00002 | 0.00016 | 0.001 | +700 |
| 3.22, 3.22 | Choline, phosphocholine | 0.00001 | 0.00009 | <0.001 | +800 | 0.00002 | 0.00015 | 0.005 | +650 |
| 3.24, 3.24 | Taurine, glucose | 0.00002 | 0.00013 | <0.001 | +550 | 0.00003 | 0.00020 | 0.005 | +567 |
| 3.27, 3.27 | Myo-inositol | 0.00002 | 0.00011 | <0.001 | +450 | 0.00002 | 0.00021 | 0.003 | +950 |
| 3.34, 3.34 | Scyllo-inositol | 0.00005 | 0.00031 | <0.001 | +520 | 0.00007 | 0.00053 | 0.002 | +657 |
| 3.42, 3.42 | Taurine | 0.00006 | 0.00027 | <0.001 | +350 | 0.00007 | 0.00046 | 0.010 | +557 |
| 3.50, 3.50 | Glycine, myo-inositol | 0.00009 | 0.00035 | <0.001 | +289 | 0.00010 | 0.00064 | 0.005 | +540 |
| 3.55, 3.55 | Glycine | 0.00006 | 0.00022 | <0.001 | +267 | 0.00006 | 0.00041 | 0.001 | +583 |
| 3.61, 3.61 | Myo-inositol | 0.00009 | 0.00042 | <0.001 | +367 | 0.00009 | 0.00066 | 0.001 | +633 |
| 3.64, 3.64 | Myo-inositol | 0.00003 | 0.00015 | <0.001 | +400 | 0.00003 | 0.00021 | 0.010 | +600 |
| 3.70, 3.70 | Leucine | 0.00003 | 0.00032 | <0.001 | +967 | 0.00004 | 0.00030 | 0.014 | +650 |
| 3.75, 3.75 | Glutamine, glutamate | 0.00003 | 0.00030 | <0.001 | +900 | 0.00005 | 0.00033 | 0.034 | +560 |
| 3.78, 3.78 | Alanine | 0.00004 | 0.00034 | <0.001 | +750 | 0.00006 | 0.00045 | 0.014 | +650 |
| 3.90, 3.90 | Creatine, aspartate, phosphocreatine | 0.00041 | 0.00172 | <0.001 | +320 | 0.00052 | 0.00278 | 0.005 | +435 |
- Mann–Whitney test used for mean comparison between categories.
- F2, F1 = frequency coordinates that explain the position of each peak; N = number of subjects.
| Premenopausal (N = 28) | Postmenopausal (N = 24) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Chemical Shift (F2, F1) ppm | Chemical Species | Low Density, N = 9 (Mean) | High Density, N = 19 (Mean) | P-Value | % Change High Density vs. Low Density | Low Density, N = 11 (Mean) | High Density, N = 13 (Mean) | P-Value | % Change High Density vs. Low Density |
| Lipids | |||||||||
| 0.40, 0.40 | Cholesterol sterol | 0.00050 | 0.00086 | 0.016 | +72 | 0.00047 | 0.00069 | 0.001 | +47 |
| 0.70, 0.70 | Cholesterol methyl | 0.00101 | 0.00353 | <0.001 | +250 | 0.00108 | 0.00175 | 0.134 | +62 |
| 0.90, 0.90 | –CH3 | 0.07757 | 0.08759 | 0.105 | +13 | 0.07543 | 0.07736 | 0.531 | +3 |
| 0.90, 1.30 | Cross peak A | 0.04392 | 0.03947 | 0.005 | −10 | 0.04213 | 0.04335 | 0.776 | +3 |
| 1.59, 1.59 | –CH2–CH2–COO– | 0.01678 | 0.04160 | 0.001 | +148 | 0.01925 | 0.02630 | 0.331 | +37 |
| 2.02, 2.02 | –CH2–HC=HC–CH2– | 0.03938 | 0.04639 | 0.016 | +18 | 0.03640 | 0.03996 | 0.252 | +10 |
| 2.02, 5.31 | Cross peak C | 0.01494 | 0.01385 | 0.410 | −7 | 0.01347 | 0.01347 | 0.424 | +0 |
| 2.25, 2.25 | –CH2–COO– | 0.02600 | 0.02972 | 0.037 | +14 | 0.02533 | 0.02678 | 0.569 | +6 |
| 2.25, 1.59 | Cross peak F | 0.02797 | 0.03441 | 0.033 | +23 | 0.02641 | 0.03074 | 0.119 | +16 |
| 2.77, 2.77 | =HC–CH2–HC= | 0.01295 | 0.01434 | 0.735 | +11 | 0.00945 | 0.01133 | 0.106 | +20 |
| 2.77, 5.31 | Cross peak D | 0.00645 | 0.00607 | 0.438 | −6 | 0.00554 | 0.00612 | 0.608 | +10 |
| 4.25, 5.22 | Cross peak G | 0.00663 | 0.00740 | 0.076 | +17 | 0.00605 | 0.00537 | 0.277 | −11 |
| 4.10, 4.25 | Cross peak G′ | 0.05874 | 0.09006 | 0.076 | +53 | 0.05281 | 0.05884 | 0.776 | +11 |
| 5.31, 5.31 | –CH=HC– | 0.07947 | 0.08077 | 0.699 | +2 | 0.07388 | 0.07767 | 0.494 | +5 |
| Metabolites | |||||||||
| 3.03, 3.03 | Creatine | 0.00009 | 0.00058 | <0.001 | +544 | 0.00013 | 0.00023 | 0.063 | +77 |
| 3.15, 3.15 | Unassigned | 0.00003 | 0.00029 | 0.001 | +867 | 0.00002 | 0.00007 | 0.011 | +250 |
| 3.19, 3.19 | Unassigned | 0.00001 | 0.00012 | <0.001 | +1100 | 0.00001 | 0.00003 | 0.041 | +200 |
| 3.22, 3.22 | Choline, phosphocholine | 0.00001 | 0.00012 | <0.001 | +1100 | 0.00001 | 0.00003 | 0.082 | +200 |
| 3.24, 3.24 | Taurine, glucose | 0.00002 | 0.00013 | <0.001 | +550 | 0.00002 | 0.00004 | 0.002 | +100 |
| 3.27, 3.27 | Myo-inositol | 0.00002 | 0.00011 | 0.005 | +450 | 0.00002 | 0.00003 | 0.022 | +50 |
| 3.34, 3.34 | Scyllo-inositol | 0.00005 | 0.00027 | 0.001 | +440 | 0.00005 | 0.00010 | 0.228 | +100 |
| 3.42, 3.42 | Taurine | 0.00006 | 0.00022 | 0.001 | +267 | 0.00005 | 0.00009 | 0.167 | +80 |
| 3.50, 3.50 | Glycine, myo-inositol | 0.00008 | 0.00029 | <0.001 | +263 | 0.00008 | 0.00009 | 0.093 | +13 |
| 3.55, 3.55 | Glycine | 0.00005 | 0.00022 | 0.002 | +340 | 0.00005 | 0.00007 | 0.331 | +40 |
| 3.61, 3.61 | Myo-inositol | 0.00009 | 0.00040 | 0.007 | +344 | 0.00007 | 0.00012 | 0.063 | +71 |
| 3.64, 3.64 | Myo-inositol | 0.00003 | 0.00013 | 0.007 | +333 | 0.00002 | 0.00004 | 0.030 | +100 |
| 3.70, 3.70 | Leucine | 0.00004 | 0.00022 | 0.001 | +450 | 0.00003 | 0.00007 | 0.055 | +133 |
| 3.75, 3.75 | Glutamine, glutamate | 0.00004 | 0.00022 | 0.002 | +450 | 0.00004 | 0.00008 | 0.072 | +100 |
| 3.78, 3.78 | Alanine | 0.00005 | 0.00022 | 0.002 | +340 | 0.00004 | 0.00010 | 0.055 | +150 |
| 3.90, 3.90 | Creatine, aspartate, phosphocreatine | 0.00038 | 0.00118 | <0.001 | +211 | 0.00034 | 0.00059 | 0.134 | +74 |
- Mann–Whitney test used for mean comparison between categories.
- F2, F1 = Frequency coordinates that explain the position of each peak; N = number of subjects.
Participants at Low Risk
PREMENOPAUSAL PARTICIPANTS
Lipid: Compared to LD/premenopausal participants, HD/premenopausal women showed significant increase up to 308% in cholesterol methyl (0.70, 0.70) ppm, up to 154% in cholesterol sterol (0.40, 0.40) ppm, and up to 247% in the resonance from lipid (–CH2–CH2–COO–) at (1.59, 1.59) ppm. This was accompanied by an increase in triglyceride of 31% as determined by the cross peak G′ from the triglyceride backbone (Table 4).
Metabolites: The metabolites on the diagonal all significantly increased in the HD/premenopausal women, with glutamine/glutamate (3.75, 3.75) ppm increased by 900%; the composite choline, phosphocholine (3.22, 3.22) ppm 800%; taurine, glucose (3.24, 3.24) ppm 550%; scyllo-inositol (3.34, 3.34) ppm 520%; myo-inositol (3.27, 3.27) ppm 450%, creatine, aspartate, phosphocreatine (3.90, 3.90) ppm 320%; and the composite glycine, myo-inositol (3.50, 3.50) ppm 289% (Table 4 and Fig. 4).
POSTMENOPAUSAL PARTICIPANTS
Lipid: Compared to LD/postmenopausal women, HD/postmenopausal participants recorded a significant increase in cholesterol methyl (0.70, 0.70 ppm) of 488%, cholesterol sterol (0.40, 0.40) ppm of 540%; lipid (–CH2–CH2–COO–) at (1.59, 1.59) ppm of 382%. Cross peak D (2.77, 5.31) ppm from the unsaturated acyl chain (=HC–CH2–CH=CH–) increased by 160% and triglycerides by 142%, as determined by the cross peak G′. Thus, HD/postmenopausal women recorded a large increase in cholesterol and triglyceride, with a concomitant increase in unsaturated fatty acyl chains.
Metabolites: All the metabolites increased. Some increments were considerably larger than those recorded for the premenopausal group (Table 4and Fig. 4).
PAIRWISE ANALYSIS ACROSS BREAST DENSITY/MENOPAUSAL STATUS CATEGORIES
Overall, the low-risk participants showed increase in intensity for cholesterol (0.70, 0.70) ppm, cross peak F (1.59, 2.25) ppm, and cross peak G′ (4.10, 4.25) ppm and in the average volume of a number of metabolites across the four categories in the following order: LD/premenopausal, LD/postmenopausal, HD/premenopausal, and HD/postmenopausal (Fig. 5 and Table 4). Specifically, pairwise analysis showed significant differences between 1) LD/premenopausal and HD/premenopausal, 2) LD/premenopausal and HD/postmenopausal, 3) LD/postmenopausal and HD/postmenopausal, and 4) LD/postmenopausal and HD/premenopausal.
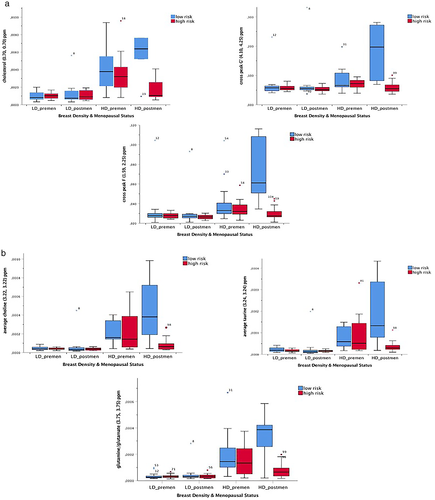
Participants at High Risk
PREMENOPAUSAL PARTICIPANTS
Lipid: In comparison with LD/premenopausal women, HD/premenopausal participants showed a 250% significant increase in the cholesterol methyl (0.70, 0.70) ppm, 72% increase in the cholesterol sterol (0.40, 0.40) ppm, and the resonance from lipid (–CH2–CH2–COO–) at (1.59, 1.59) ppm increased by 148% (Table 5).
Metabolites: The metabolites on the diagonal all increased significantly in the HD/premenopausal cohort, with the composite choline, phosphocholine (3.22, 3.22) ppm 1100%; taurine, glucose (3.24, 3.24) ppm 550%, glutamine/glutamate (3.75, 3.75) ppm up 450%, and leucine (3.70, 3.70) ppm 450% (Table 5).
POSTMENOPAUSAL PARTICIPANTS
Lipid: HD/postmenopausal women had a slight increase in cholesterol sterol (0.40, 0.40) ppm of 47% in comparison with LD/postmenopausal women (Table 5).
Metabolites: Changes recorded for the metabolites in the HD/postmenopausal women were overall smaller than the HD/premenopausal women (Table 5).
PAIRWISE ANALYSIS ACROSS BREAST DENSITY/MENOPAUSAL STATUS CATEGORIES
The high-risk participants recorded an increase in lipid (–CH2–CH2–COO–) (1.59, 1.59) ppm and cross peak F (1.59, 2.25) ppm across the four categories in the following order: LD/postmenopausal, LD/premenopausal, HD/postmenopausal, and HD/premenopausal (Fig. 5 and Table 5). The pairwise analysis showed that significant differences were present between LD/premenopausal and HD/premenopausal categories and between LD/postmenopausal and HD/premenopausal.
Discussion
Increasing neutral lipid and metabolite activity was recorded with increased breast density. There were four distinct chemical profiles recorded in the in vivo 2D COSY evaluation of breast tissue in healthy women. When correlated with the risk of developing cancer, in the low-risk group, the HD/postmenopausal women showed the most active metabolic and lipid profile compared to those with low breast density who were premenopausal. In the high-risk group, the HD/premenopausal participants recorded the most chemically active metabolic profile. Interestingly, participants with low dense tissue exhibited the less active profile in both groups. These results could offer new insight about why women at high risk with dense breasts develop cancer at a younger age and might provide metabolic biomarkers of cancer development based on the lifetime risk.
The changes in breast tissue chemistry recorded in this series include neutral lipids and a range of metabolites. Each needs to be considered separately. Triglycerides and cholesterol, as neutral lipids, have a natural affinity. They create neutral droplets or domains like those found in serum lipoproteins. Articles published from the 1980s describe the behavior of these neutral lipids and their spectral characteristics.23, 24 There are two possible locations of these increased levels of neutral lipids in postmenopausal and premenopausal dense breast tissue. The first is in the cytoplasm where they would provide the lipid pool for rapid doubling of cells when needed. The second is in the plasma membranes of activated or stimulated cells, such as macrophages or other inflammatory cells. A model was proposed whereby neutral lipid domains are intercalated with the bilayer lipid of the plasma membrane of activated, stimulated, or transformed cells.25 This model was contentious26 initially but verified many years later.27 The resonance at 0.70 ppm has previously been assigned to cholesterol C1823 and shown to have rapid molecular motion. As the triglyceride and cholesterol ester levels increase, the cholesterol becomes more mobile and can be measured.
King et al28 used 2D COSY to study murine macrophages and found that proliferation is not a prerequisite for acquisition of an “activated” high-resolution spectrum in cell models. There is evidence that dense breast tissue in postmenopausal women is associated with a pro-inflammatory microenvironment including cytokines and a significantly increased number of inflammatory cells.29 Additionally, increased levels of pro-inflammatory cytokines have been shown to stimulate triglyceride synthesis, cholesterol accumulation and de novo lipogenesis30 which would explain our findings in the low-risk group, with postmenopausal women with dense breasts exhibiting the highest increase in triglycerides and cholesterol. Likewise, high mammographic density has been reported to be associated with protumor inflammation.31 This pro-inflammatory microenvironment would underpin the active chemical profiles we identified in dense breasts. Importantly, the highest chemical activity shifts from postmenopausal to premenopausal women based on whether they pertain to the low-risk or to the high-risk group, respectively. This is an important observation as familial breast cancer is known to be of early onset.32 Our results in the low-risk cohort are in line with those reported by Advani et al33 on a large series of women aged 65 or older who underwent screening mammography. They reported that breast density was associated with increased breast cancer risk among women aged 65 years to 74 years regardless of BMI.
The gradual increase in metabolites through the series include glucose and taurine, choline, myo-inositol, and glutamine/glutamate. Morris et al34 reported a decrease in glucose metabolism in HD tissue collagen matrices and found an enhanced contribution of glutamine as a fuel source in these matrices. Similarly, others reported that glucose metabolism significantly increased in HD tissue, but was not affected by menopausal status.35
We recorded a steady increase in myo-inositol through this series. Myo-inositol has been shown to modulate inflammatory, oxidative, endocrine, and metabolic pathways.36 In parallel, taurine has been reported to be an antioxidant that exerts antineoplastic effects through downregulation of angiogenesis and suppressing cell proliferation.37 Thus, some of these changes recorded in the healthy breast are likely to be part of protective mechanisms. This requires further evaluation.
Limitations
Firstly, the data from premenopausal women were taken at the end of the follicular phase of their menstrual cycle. This may have an impact on the results as the lipid composition may change slightly during the menstrual cycle.38 Secondly, our results show an increase over all volumes of metabolites in dense breasts compared to nondense breasts. Note should be made that some of these volumes are composites of metabolites, which makes it difficult to confirm whether or not some metabolites of these composites could be decreased in relation to others. Lastly, it is likely that some resonances on the diagonal are composites. With future improvements in scanner capabilities, and increases in signal-to-noise ratio, they may be recorded with their associated cross peaks and thus assigned unambiguously.
Conclusion
Four distinct chemical profiles were identified in breast tissue based on breast density and menopausal status in healthy participants at low risk and high risk of breast cancer. Gradual increase in neutral lipid content and metabolites was noted in both risk groups across categories in different order. In women at low risk, the HD postmenopausal category exhibited the highest metabolic activity, while women at high risk with no known mutation exhibited the highest lipid content and metabolic activity in the HD premenopausal category.
In summary, our results indicate that high breast density is associated with a significantly more active tissue chemistry and might offer an explanation as to why postmenopausal women with dense breast in low-risk cohorts and premenopausal women with HD breast in high-risk cohorts are more likely to develop cancer.
From a clinical perspective, the capacity to monitor these changes in healthy breast tissue noninvasively by adding a 19-minute sequence on to a breast MR protocol may provide valuable biochemical information of metabolism in the breast tissue both in low-risk and high-risk cohorts. Although the metabolic profiles described need to be reproduced in larger series, they might be relevant for risk classification tasks.
Acknowledgments
The authors thank Juan Ramón Sabaté, César Garrido, Lidón Pages, Xavier Bargalló, and Sergi Ganau from Hospital Clinic de Barcelona; Randell Brown, Timothy King, and Jillian Borthwick from St Andrew's Hospital in Adelaide for their assistance in participant recruitment and data acquisition.
Open access publishing facilitated by Queensland University of Technology, as part of the Wiley - Queensland University of Technology agreement via the Council of Australian University Librarians.




