Development of the visual system of anchovy larvae, Engraulis anchoita: A microanatomical description
Funding information: Consejo Nacional de Investigaciones Científicas y Técnicas; Instituto Nacional de Investigación y Desarrollo Pesquero
Abstract
During the early ontogeny of fish larvae, the accurate development of the visual system plays a key role, because it is involved in locating food, orientation, selection of favorable habitat, and evasion of predators. The structure of the eye of the fish is typical of vertebrates, with some modifications related to the aquatic environment. In the present work, we describe the development of the larval eye of Engraulis anchoita for the first time. Larvae were collected at the Permanent Station of Environmental Studies (EPEA) in coastal waters of the Southwestern Atlantic Ocean during research cruises in 2015 and 2016. We describe the histology of the retina layers, determine the beginning of the functionality of the eye, and discuss a possible synchronization with the development of the digestive tract. This study provides information about the biology of E. anchoita, the most abundant fish species in the southwestern Atlantic Ocean. Also, recent studies have shown responses of the retina and other tissues to the increase in environmental acidity. Therefore, results of this study are also discussed with respect to the possible effect of acidification on the larvae of this species. The continuity of the time series developed at the EPEA will allow monitoring the effect of long-term environmental and biological variables on the early ontogeny of anchovy in the context of climate change. The high commercial fishing potential of E. anchoita due to its high abundance, as well as its essential role in the trophic web of other commercially valuable fishing resources of Argentina, reinforce the need to continue deepening knowledge about this species.
Research highlights:
- Eyes of Engraulis anchoita larvae are functional from early larval stages.
- At hatching, the retina is formed by only few layers from which the other layers differentiates during ontogeny.
- Focal distance increases with larval growth.
1 INTRODUCTION
The Argentine anchovy, Engraulis anchoita Hubbs and Marini, 1935, is the most abundant and widely distributed clupeiform fish in the southwestern Atlantic, ranging from southern Brazil to San Jorge Gulf, Argentina. Two anchovy populations have been identified in the Argentine Sea: a northern population (34–41°S) and a southern population (41–48°S). Although the species spawns throughout the year, the largest spawning in the Buenos Aires region occurs during austral spring and summer (Sánchez, de Ciechomski, Lasta, & Guerrero, 1999). This species mainly feeds on zooplankton: adults consume medium to large planktonic crustaceans, and eventually phytoplankton and larvae feed mainly on copepod eggs and their naupliar stages (Sato, Hernández, & Viñas, 2011; Viñas & Ramírez, 1996).
As in most teleost fish, the larval stage of E. anchoita is a short and vulnerable milestone of life. Organs and tissues develop rapidly while changes occur in habitat, food resources, and behavior (Sabbah, Hui, Hauser, Nelson, & Hawryshyn, 2012). A rapid adaptation to environmental changes is indispensable for survival because it allows larvae to take advantage of the environmental resources and to protect against adverse factors (Miller & Kendall, 2009). During larval ontogeny, vision plays a key role in reducing the vulnerability because is involved in locating food, orientation, selection of favorable habitat, and the detection of predators (Bozzano & Catalán, 2002; Kawamura et al., 2003; Rodríguez & Gisbert, 2002; Yahaya et al., 2011). Because marine fish larvae are mostly visual predators, the light intensity is an important factor for the eyes to detect light stimuli, form images and allow the capture of prey (Morote, Olivar, Bozzano, Villate, & Uriarte, 2011; Rodríguez & Gisbert, 2002). From this perspective, we expect that the development of the visual system structures is synchronized with the development of the digestive system to optimize foraging success (Evans & Browman, 2004).
The ocular structure of the fish is typical of vertebrates, however, it has various structural modifications adapted to the aquatic environment. For example, the lens plays a key role in the refraction of light and the formation of the image in the retina. This structure has a gradient in its refractive index, with a maximum in the center of the lens decreasing toward the periphery (Mas-Riera, 1990). The cornea is optically ineffective due to immersion and the iris is a fixed structure that does not adjust to the light conditions (Bejarano-Escobar, Blasco, Martín-Partido, & Francisco-Morcillo, 2014; Yahaya et al., 2011). The visual capacity is conditioned by the structure and function of the nervous retina and the pigmented epithelium. For species where vision plays a central role, the development of the retina should be a good indicator of the strength of selection for the detection of prey and predators during their early life history (Evans & Browman, 2004).
The retina of adult fish is part of the nervous tunic of the eye and is the innermost layer of the eyeball. Histologically, the retina is organized into 10 layers that, from the most external to the most internal, are: (1) retinal pigmented epithelium (RPE); (2) photoreceptor (cones and rods) layer (PL); (3) outer limiting membrane (OLM); (4) outer nuclear layer (ONL); (5) outer plexiform layer (OPL); (6) inner nuclear layer (INL); (7) inner plexiform layer (IPL); (8) ganglion cell layer (GCL); (9) optic nerve fibers layer (ONFL), and (10) inner limiting membrane (ILM). In the retina, the cells differentiate into sensory cells and nerve cells and their nuclei are arranged in several layers; the outer layer give rise to the photoreceptor cells and the rest give rise to different nerve cells such as conducting neurons: bipolar and ganglion, association neurons: horizontal, and amacrine, and support cells: Müller cells, astrocytes, and microglia (Bejarano-Escobar et al., 2014; Bruel, Christensen, Tranum-Jensen, Qvortrup, & Geneser, 2015; Pawlina, 2015).
Due to their wide range of habitats, teleost fish have become a model for examining the relationship between ambient light and the spectral sensitivity of visual pigments (Shand et al., 2008). Structural differences have been observed in the retina of adult teleost fish indicating functional differences that allow adapting to the photic habitat and the fish visual orientation requirements (Lythgoe, 1979). Depending on the environment and the life history of the species, the retina displays topographic modifications related to the visual needs of the species (Collin & Shand, 2003; Evans & Browman, 2004). Cellular density can vary during the development of the individuals. The size, arrangement, and number of neurons can also vary depending on the ecology of the species (Bohórquez, Chavarro, Cortes, & Giraldo, 2009). Particularly, interspecific differences in the pigmented epithelium and photoreceptors cells have been observed. In general, the teleost retina is characterized by single cones, double cones and a combination of them in different patterns. Compound cones have been rarely observed (Heß, 2009). Compared to other teleosts, anchovies, or engraulidids possess a highly uncommon photoreceptor arrangement (Haacke, Heß, Melzer, Gebhart, & Smola, 2001). The polycone rows of adult anchovies are considered an adaptation to perceive polarized light (Fineran & Nicol, 1978; Heß, 2009).
Frommel et al. (2014, 2016) found alterations in the retina of the larvae associated with acidification. This could affect vision causing a variety of harmful effects in the larvae, for example, affecting light intensity perception and influencing larval orientation (Leis, 2018). Teleost fishes are especially vulnerable during the early stages of their life cycle because they lack specialized physiological mechanisms for pH regulation. Recent studies have shown pathological changes in different tissues as a consequence of the increase in environmental acidity. Exposure to high levels of CO2 produced a delay in the growth and development of cod larvae, a decrease in nutritional condition (RNA/DNA index), and severe tissue damage in many organs. Available research provides evidence that ocean acidification may act as an additional source of natural mortality, affecting fish populations, particularly those subject to fishing exploitation. Thus, natural mortality, climate change, and fishing are the two main processes affecting small pelagic fish populations (Checkley Jr, Asch, & Rykaczewski, 2017).
Pelagic fishes like anchovies are key components on the ecosystems and changes in their abundance may be accompanied by important changes in the ecosystem structure. A considerable plasticity in growth, survival and other life-history traits outlines their developmental dynamics. Small pelagic fishes are becoming of great interest to assess the effect of climate change on fisheries because they respond dramatically and quickly to changes in ocean chemical and physical composition (Alheit et al., 2012).
The Dynamics of Marine Plankton and Climate Change Project, initiated in 2000, is dedicated to studying long-term variations in the plankton community related to environmental factors at the Permanent Station for Environmental Studies (EPEA), which is a coastal point providing monthly samples of bacterioplankton, phytoplankton, zooplankton, and ichthyoplankton, as well as physicochemical variables, such as temperature, salinity, conductivity, dissolved gases, pH, carbonates, and nutrients. Data collected at EPEA constitute a wide marine time series that provide valuable information on natural seasonal variability and will allow detecting possible extraordinary events in the variables studied. Understanding the dynamics of environmental conditions and planktonic communities will permit to establish their link with climate change.
The general objective of this work was to study the development of the visual organ of larvae of the Argentine anchovy, Engraulis anchoita. We employed a histological and morphometrical approach. We analyzed the histological structure of the retina of the larva at different developmental stages and characterized the eye structural changes with respect to the development of the larval digestive system. Results of this work contribute to the knowledge of the biology of E. anchoita and represent baseline information necessary for future research, especially aimed at determining the possible effect of acidification on the larvae of this species.
2 MATERIALS AND METHODS
2.1 Study area
The EPEA is located at 38°28′S latitude and 57°41′W longitude (Figure 1), being at approximately 13.5 nautical miles from Miramar and 27 nautical miles from Mar del Plata. This coastal station is a monthly sampling point situated close to 50 m isobath but is dynamic in hydrographic terms because it may be influenced in some cases by the advection of the “Middle Shelf Waters” of Subantarctic origin or by the low salinity waters from the Río de la Plata (Lutz, Subramaniam, Negri, Silva, & Carreto, 2006). Anchovy eggs and larvae are found at the EPEA throughout the year, maximum densities during the spring (Pájaro, Diaz, Leonarduzzi, & Negri, 2009), being the main component of ichthyoplankton at the EPEA and Buenos Aires coastal area (Ciechomski, Ehrlich, Lasta, & Sánchez, 1981; Sánchez & Ciechomski, 1995).

2.2 Sample collection
Samples were obtained during three research surveys conducted by the Instituto Nacional de Investigación y Desarrollo Pesquero (INIDEP) at the EPEA (Figure 1) on board of the research vessel Dr. Bernardo Houssay. Sampling dates were: February 9, 2015; April 28, 2015; and April 22, 2016. The ichthyoplankton samples were collected performing oblique hauls from two meters above the bottom to the surface, employing a Bongo net with a 300 μm pore mesh. Anchovy larvae at different developmental stages were separated in situ, fixed in buffered formaldehyde 5% (pH 7) for two hours and subsequently preserved in 70% ethanol.
2.3 Sample processing
Whole larvae were processed following the routine histological procedure and embedded in paraplast according to the protocol developed for fish larvae by Cohen, Diaz, Díaz, Macchi, and Christiansen (2015). Serial cross-sections were cut with a Yamato Kohki sliding microtome using stainless steel blade (S35, FEATHER). Larvae smaller than 4 mm were cut in 3 μm thick sections, and larger larvae were cut in 5 μm thick sections. The obtained sections were processed according to the standard protocol and subsequently stained with Hematoxylin–Eosin for histological characterization.
2.4 Morphometric analysis
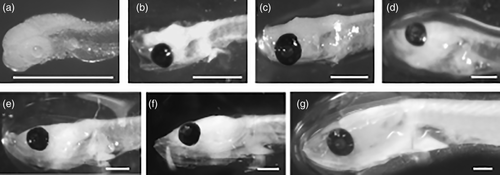
In the laboratory, the left side of 100 larvae was laying on their left side as well as left side photographed. Their standard length (SL) and eye diameter (ED) were measured to the nearest micrometer, using a Carl Zeiss binocular magnifier equipped with the Axio-Vision software. We did not correct for shrinkage due to fixation. From the observation of the histological preparations, developmental stages of larvae were determined (DS I to DS VII) according to the criteria established by Sieg (1998), presented in Table 1.
| DS I (2.22 ± 0.35) | Yolk sac stage. Yolk mass clearly detected or remnants in position anterior to the developing liver |
| DS II (4.01 ± 0.54) | No yolk detectable. Straight digestive tube, ventral jaw with a single layer of chondrocytes, first small gas bladder bulge |
| DS III (4.58 ± 0.29) | Digestive tube still straight. The height of the esophagus epithelium begins to increase. The pancreas extends to the cranial area. The ventral jaw is formed by more than one layer of chondrocytes. The gas bladder increases in size |
| DS IV (5.96 ± 0.45) | Mucosa of the digestive tube half folded in the ventral part, the dorsal part begins to fold, the posterior digestive tube without folding. The gas bladder continues to increase in size and can form a small cavity |
| DS V (7.63 ± 0.56) | Mucosa of the digestive tube folded dorsally and centrally, the posterior digestive tube begins to fold. Gas bladder with several layers of cells |
| DS VI (9.95 ± 0.86) | Posterior digestive tube completely folded. The height of the esophagus epithelium increases significantly, with different cell layers. The pancreas extends cranially and caudally. The gas bladder has a thin wall due to the first filling, it can be much extended |
| DS VII (14.47 ± 1.53) | Gas bladder completely functional. Increase in muscle layers. Ossification of fins and vertebrae. Great increase in size and weight |
- Note: Modified from Sieg (1998). Mean standard length ± SD of larvae employed in this study at each developmental stage are indicated.
The diameter of the lens (LD) was measured using a Nikon (NIKON 519CU) optical microscope (NIKON OPTIPHOT-2) equipped with the Micrometrics software, and a characterization of each of the layers forming the retina was carried out at each developmental stage. The measurements were made on the lens of greater diameter, using the 40× objective, where the slices were nearest through the lens centers. Both lenses of each larva were measured and two measurements on the same lens were made. The average diameter was calculated. The relationships of ED, LD, and FD with SL were described using standard linear regression (the complete data set is available in Data S1).
We report Fisher's statistic (F), probability value (p), adjustment coefficient (R2), and number of measurements employed in the analysis (N).
The visual system was considered functional when the RPE was observed. At this time, the GCL and the PL were present in the retina (Holt, 2011).
2.5 Allometric analysis
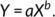
The allometric growth constant “b” is the slope of the logarithmically transformed equation. The value “b” was tested using a Student's t-test considering as null hypothesis as b = 1 (Sokal & Rohlf, 1987). b = 1 indicates isometric growth; b > 1 positive allometric growth, and b < 1 negative allometric growth (da Silva-Castiglioni & Negreiros-Fransozo, 2004).
2.6 Focal distance determination

3 RESULTS
3.1 Morphometric analysis



3.2 Allometric analysis
The allometric equation for the diameter of the lens dependent variable of standard length was: LD = 24.335 SL0.798; R2 = .8981 (allometry test LD/SL; b = .798; t = [abs (1 – b)]/sb; t = 13.95; p < .0001) where “R2” is the determination coefficient, “abs” is the absolute value and “sb” is the standard deviation of the slope.
The allometric equation for eye diameter as dependent variable of standard length was: ED = 105.67 SL0.6259; R2 = .9313 (allometry test ED/SL; b = .6259; t = [abs (1 – b)]/sb; t = 22.03; p < .0001). Because both allometric regressions were significantly different from 1 we rejected the hypothesis of isometric growth (b = 1). The value of the slope (b) is less than 1, indicating negative allometric growth of ED and LD with the SL.
3.3 Histological analysis
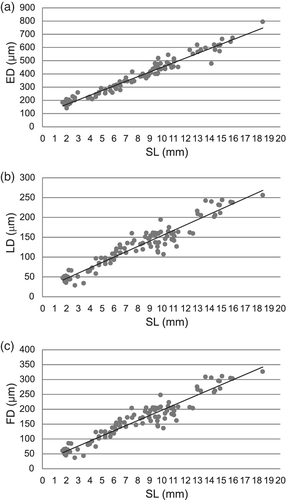
At the beginning of the larval development of E. anchoita (DS I), the majority of the individuals had nonpigmented eyes with a poorly differentiated retina (Figure 4a). The crystalline lens was distinguished by a fibrous translucent spherical structure covered by a cubic epithelium corresponding to the cornea (Figure 4b). The average diameter of the lens at this stage of development was 47.11 ± 10.05 μm. Generally, the retina was an undifferentiated array of radially arranged cells, without a clear stratification (Figure 4a). In a few larvae at DS I a slight differentiation was observed in the retina (Figure 4b) and some layers in formation were distinguished: ganglion cell, internal nuclear, and the internal and external plexuses showed little development. Only in a few specimens (Figure 4b), it was possible to differentiate the ONL, the pigmented epithelium, and the photoreceptor layer.
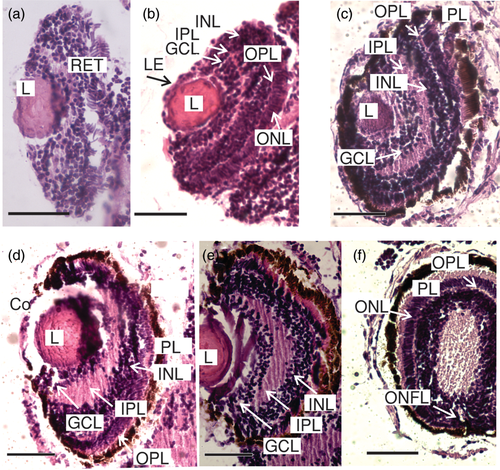
Anchovy larvae at DS II (Figure 4c) and DS III (Figure 4d–f) showed a more intensive pigmentation in the eye and some retina layers were also observed with greater distinction than at the end of DS I. The photoreceptor layer was more evident and the ONL (nuclei of photoreceptor cells) appeared like a row of nuclear segments of the cones in formation (Figure 4b,e,f). The INL was also visualized (Figure 4c–e). In these stages, the IPL acquired an important development (Figure 4c,e). However, the OPL was barely distinguishable in both stages (Figure 4c,d). In both stages, subtle axonal projections of the ganglion cells were observed, forming the fibers of the optic nerve that leave the retina toward the brain (Figure 4e).
Larvae at DS IV and DS V showed the aforementioned layers (Figure 5a–d). The OPL was thin, but more developed than in the previous stages (Figure 5b,d). The fibers of the optic nerve were also more evident, with the optic nerve now passing through the layers of the retina and projecting toward the brain (Figure 5c). At DS V, within the INL, it was possible to differentiate the nuclei of three types of cells: horizontal cells, bipolar cells and amacrine cells (Figure 5d).
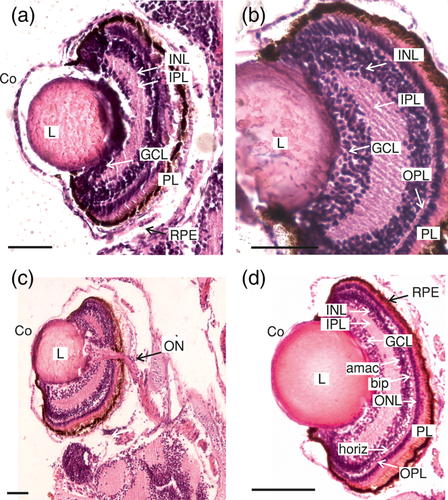
Toward the end of the larval stage, DS VI and DS VII, 9 of the 10 layers defined for the vertebrate retina could be visualized (Figure 6a–d), from the outside to the inside: (1) RPE, (2) photoreceptor (cones and rods) layer (PL), (3) OLM, (4) ONL, (5) OPL, (6) INL, (7) IPL, (8) GCL, and (9) ONFL (Figure 6a,b). The outer and ILMs are thin, and were not seen in all samples. The location of the OLM is shown in Figure 6d. At DS VI and even more clearly in DS VII, the nuclei of the three types of cells in the INL were in different strata from the outside to the inside: horizontal cells, bipolar cells, and amacrine cells (Figure 6d). Also, it was not possible distinguishing the two types of photoreceptor cells in the photoreceptor layer. The external segment could be distinguished in relation to the pigmented epithelium, the internal segment, as well as its nuclei forming the ONL. A regular pattern of RPE was observed (Figure 6d).
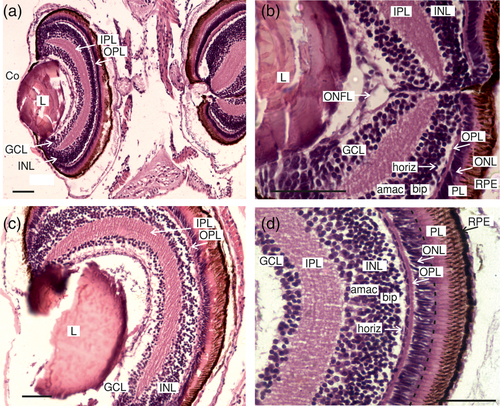
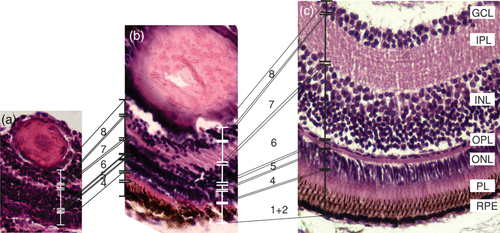
When comparing the conformation of anchovy larvae retina throughout the development, we observed an increase of the tissue complexity and the thickness of their retina layers, given the importance of this sense in the teleost fish and according to the growth of the eye (Figure 7). During initial stages, we observed the following layers (Figure 7): (4) ONL, (5) OPL, (6) INL, (7) IPL, and (8) GCL, but the IPL and the GCL showed little development. The thickness of these layers increased toward intermediate developmental stages (Figure 7b). It highlights the rapid increase of the INL, inner plexiform and the photoreceptor layer between the intermediate and advanced developmental stages (Figure 7c). Figure 8 summarizes the development of the retinal layers at each developmental stage.
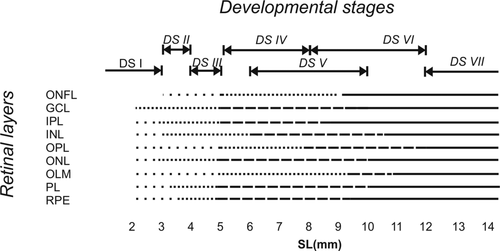
 , present but at initial developmental stage;
, present but at initial developmental stage;  , beginning development;
, beginning development;  , intermediate development;
, intermediate development;  , advanced development
, advanced development4 DISCUSSION
The retina of Engraulis anchoita larvae presents the typical histological conformation of the eye of teleost fish (Haacke et al., 2001). In general, the ontogenetic progression of the anchovy larvae allowed observing an increase of complexity of the retina with increasing differentiation, development, and thickness of all the defined layers of the typical vertebrate eye (Morote et al., 2011; Roo et al., 1999; Yahaya et al., 2011; Zaunreiter, Junger, & Kotrschal, 1991). We observed this increase in complexity of the retina in parallel to the increase in the focal distance and according to the growth of the eye. Given that visual acuity is directly related to the focal distance, knowing this parameter throughout the development could estimate the variation of visual acuity during growth. However, there are other parameters to calculate the optical resolution such as the photoreceptor density and the photoreceptor/ganglion cell ratio (Haacke et al., 2001; Land & Nilsson, 2002; Morote et al., 2011).
Vision plays an important role in the survival of fish larvae to capture prey or detect predators (Yahaya et al., 2011). The visual capacity of fish larvae is determined by the structure of the retina, and it shows a wide range of structural adaptations to the environment and lifestyles (Rodríguez & Gisbert, 2002). Retinal cell density and distribution are species-specific and determine the visual resolution and the ultimately how the fish perceive the environment (Collin & Shand, 2003). Feeding success in marine fish larvae depends on several biotic and abiotic factors, such as concentration and type of prey, turbulence, temperature, and light conditions (Kristiansen, Lough, Werner, Broughton, & Buckley, 2009). The light intensity is important because most of the larvae are visual predators, as is the case of E. anchoita (Sato et al., 2011). The intensity and quality of the light spectrum affect the feeding capacities of the larva, altering the search behavior of prey and the relative distances to them (Morote et al., 2011).
In general, at the beginning of the exogenous feeding, the larvae have a limited visual acuity and the retina presents only single cones as photoreceptors; but as the larva develops, the retinal thickness and the focal distance increase. In a typical fish retina, the spatial distribution of photoreceptor cells varies according to the quality of ambient light. It has been determined in several species that in the larvae the cones appear first and the presence of the rods occurs toward the end of the larval stage (Evans & Browman, 2004; Haacke et al., 2001).
At the beginning of the larval period (DS I) of E. anchoita an unpigmented eye was observed with a poorly differentiated retina, which would indicate that the eyes were not functional yet. Toward the end of the DS I, approximately 4 mm SL, the eyes were pigmented and the GCL and the PL were present in the retina, indicating that the eyes might be functional. This stage of acquisition of the functionality coincided with the absorption of the yolk and the beginning of the exogenous feeding. Cohen, Diaz, and Díaz (2014) observed that since the beginning of their ontogeny E. anchoita larvae have the necessary morphological equipment to face the digestive and absorptive processes successfully. Early anchovy larvae showed enterocytes with microvilli, secretory mucous cells of a great variety of glycoproteins, pancreatic cells with small granules of zymogen and ducts that connect accessory glands to the intestine. In this way, both the digestive and visual systems of anchovy larvae acquire their functionality at the early stages of their development, increasing their complexity and size throughout the larval period. The progression of ontogeny leads to an increase in the visibility of a transparent larva, due in part to the pigmentation of the eye among other structures, which could increase its vulnerability to its predators (Kvenseth, Pittman, & Helvik, 1996). The rapid development of these structures ensures rapid detection and escape to predators and, consequently, the survival of larvae in the early stages (Collin & Shand, 2003). After the eye is pigmented, a reflective argentea develops. This silvery stratum contains guanine and serves to camouflage the eye (Haacke et al., 2001).
The pattern of development of the different layers of the retina was similar to that of other fish species with pelagic larvae (Morote et al., 2011; Roo et al., 1999; Yahaya et al., 2011; Zaunreiter et al., 1991). We highlight the rapid increase of the INL, inner plexiform layer and the photoreceptor layer between the intermediate and advanced stages. Toward the end of the larval period and the beginning of the transition to the juvenile stage (approximately 20 mm), a remarkable stratification of the retina was observed, as well as a clear differentiation of the cells that make it up, resembling the typical structures of the adult stage. However, the differentiation of photoreceptor cells in the cone and rod layer was not undertaken. This is partly due to the technical limitations of the optical instruments used or suggestion that the rods appear in more advanced stages of the development of this species, as reported in other Engraulididae (Haacke et al., 2001), coinciding with the observed in other clupeiform larvae such as E. mordax, Sardina pilchardus, and Clupea harengus in which the appearance of the rods, as well as their functionality, is given to the end of the larval period following with the metamorphosis toward the adult stage (Matsuoka, 1999).
Engraulis anchoita has a much smaller eye and lens diameter compared to the eye of larvae of other species such as Merluccius hubbsi (Betti, 2011) and M. merluccius (Morote et al., 2011). Having a relatively large eye and lens increases the visual capacity of the species (Bohórquez, Chavarro, Ramírez, Bulla, & Giraldo, 2013). The larvae of M. merluccius and M. hubbsi have a highly selective diet composed of calanoid copepods and do not include other species in their diet that are more abundant in the environment (Morote et al., 2011; Temperoni & Viñas, 2013). In contrast, the diet of anchovy larvae is composed of eggs and nauplii of copepods and copepodites that are the most abundant planktonic components (Sato et al., 2011). The low visual resolution of the anchovy larvae probably leads to the fact that these are not specific when selecting their food as compared to hake larvae.
The acidification of the oceans, caused by a decrease in the pH of seawater due to an increase in the atmospheric concentration of CO2, is one of the most serious threats to marine life (Gattuso et al., 2015; Gattuso, Mach, & Morgan, 2013). Pathological changes occur in different tissues of fish larvae as a consequence of the increasing environmental acidity. Frommel et al. (2014, 2016) found alterations specifically in the retina of the larvae associated with a pH reduction (e.g., an abnormal shape in the pigmented epithelial layer), and also observed vacuolation in the cornea, and damage to the lens. Alterations in larval retina could damage the vision producing severe effects, among them, affecting the way of perceiving the intensity of light influencing its orientation (Leis, 2018). The ocean acidification may act as an additional source of natural mortality, affecting fish populations, particularly those subject to fishing exploitation because the population is destabilized and its ability to respond to climate change is reduced (Le Quesne & Pinnegar, 2012).
Recent estimates suggest that, due to the high absorption of anthropogenic CO2, the pH in the Argentine Sea has decreased by an average of 0.1 units since the preindustrial period (Kahl, 2018; Kahl, Bianchi, Osiroff, Pino, & Piola, 2017; Orselli et al., 2018). This rate, equivalent to −0.002 year−1, is in congruence with observed in other regions of the globe (Bates et al., 2014). However, at the EPEA the mean pH values were 7.943 ± 0.11during late austral summer and autumn 2015/2018, with variable observations as expected for a coastal area (unpublished data, Carla Berghoff, INIDEP). It is known that coastal areas are sensitive to ocean acidification, deoxygenation, and eutrophication, these factors are multiple stressors in the context of climate change that affect larval development and thus impact fisheries. Monitoring these conditions in situ and maintaining sustained observations over time is crucial. In the present work, we have described the development of the larval eye of Engraulis anchoita collected at the EPEA. This study may represent baseline information necessary for future research, aimed at determining the possible effect of acidification on the larvae of this species.
The temporal continuity of the investigations developed with the information obtained in the EPEA will allow monitoring effects of long-term environmental and biological variables on the early ontogeny of anchovy life traits. The commercial fishing potential of E. anchoita relies on its high abundance, as well as its essential role in the food web of the Argentine sea. These facts reinforce the need to continue deepening knowledge about this species especially in the context of climate change.
5 CONCLUSIONS
Because of the progress of climate change, ocean acidification is expected, and this can cause severe damage to the different organs of the marine fish. Pathological changes have been observed in the eye of some fish species. To study the possible damage that acidification can cause on the visual system, it is necessary to know the normal structure of the eye. However, the morphology of the eye of Engraulis anchoita, a species with a high fishing potential and with an essential role in the trophic network of the other fishing resources of Argentina, is unknown. This study has demonstrated that the retina of E. anchoita larvae presents the typical histological conformation of the eye of teleost fishes. The visual system of E. anchoita larvae, as well as the digestive system, is functional from the early larval stages. Besides, the development of both systems is synchronized and an increase in their complexity and size was observed throughout the larval period. Engraulis anchoita has a much smaller eye and lens diameter compared to the eye of other species at equivalent developmental stages.
ACKNOWLEDGMENTS
We wish to thank the captains and crews of Dr. Bernardo Houssay research vessel for their help during sample collection. Special thanks to the authorities of Instituto Nacional de Investigación y Desarrollo Pesquero and Prefectura Naval Argentina for these samplings. Thanks to Marta Estrada for technical support at Ecología Reproductiva Cabinet from the INIDEP, special thanks to Gustavo Macchi. This is INIDEP N°2200 contribution.
AUTHOR CONTRIBUTIONS
Valeria Miranda: Formal analysis; investigation; methodology; writing-original draft. Stefania Cohen: Investigation; supervision; writing-review and editing. Alcira Díaz: Investigation; supervision; writing-review and editing. Marina Vera Diaz: Formal analysis; funding acquisition; investigation; methodology; resources; supervision; writing-original draft; writing-review and editing.
CONFLICT OF INTEREST
The authors have no competing interests to declare.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding authors upon reasonable request.




