Gene therapy for mitochondrial disorders
Abstract
In this review, we detail the current state of application of gene therapy to primary mitochondrial disorders (PMDs). Recombinant adeno-associated virus-based (rAAV) gene replacement approaches for nuclear gene disorders have been undertaken successfully in more than ten preclinical mouse models of PMDs which has been made possible by the development of novel rAAV technologies that achieve more efficient organ targeting. So far, however, the greatest progress has been made for Leber Hereditary Optic Neuropathy, for which phase 3 clinical trials of lenadogene nolparvovec demonstrated efficacy and good tolerability. Other methods of treating mitochondrial DNA (mtDNA) disorders have also had traction, including refinements to nucleases that degrade mtDNA molecules with pathogenic variants, including transcription activator-like effector nucleases, zinc-finger nucleases, and meganucleases (mitoARCUS). rAAV-based approaches have been used successfully to deliver these nucleases in vivo in mice. Exciting developments in CRISPR-Cas9 gene editing technology have achieved in vivo gene editing in mouse models of PMDs due to nuclear gene defects and new CRISPR-free gene editing approaches have shown great potential for therapeutic application in mtDNA disorders. We conclude the review by discussing the challenges of translating gene therapy in patients both from the point of view of achieving adequate organ transduction as well as clinical trial design.
Synopsis
Gene therapy approaches to treating primary mitochondrial disorders are still in early development for most translational pipelines. Advances in genetic engineering technologies have diversified the tools available to target a wider range of primary mitochondrial disorders.
1 INTRODUCTION
1.1 Mitochondrial function and dysfunction
Mitochondria are the main site of ATP production via the process of oxidative phosphorylation (OXPHOS).1 The OXPHOS system consists of five multi-subunit protein complexes (complexes I–V) located in the inner mitochondrial membrane.2 However, the role of mitochondria goes beyond that of just energy generation because they are the site of many other metabolic and signalling pathways. These include one carbon metabolism,3 iron–sulphur cluster biogenesis,4 production of reactive oxygen species, which are needed in multiple biological processes including antioxidant defence5 and in activating apoptosis.6 Intermediates produced in mitochondria are also needed in both purine and pyrimidine biosynthesis.7 Approximately 1500 proteins are thought to have a mitochondrial localization and function.8, 9 A majority of these are nuclear genome encoded since mitochondrial DNA (mtDNA), located within mitochondria, is a circular molecule ~16.5 Kb in size that contains only 37 genes encoding 13 polypeptides, 22 tRNAs, and 2 rRNAs.10 With the exception of complex II, which is encoded entirely by nuclear DNA,11 the OXPHOS complexes are reliant on mtDNA which encodes subunits located in complexes I, III, IV, and V.10
1.2 Primary mitochondrial disorders
Primary mitochondrial disorders (PMDs) are monogenic disorders resulting from either inherited or de novo pathogenic variants in genes that result in disturbances in OXPHOS, mitochondrial structure or function.12 They are individually rare but collectively common with an estimated incidence of 1: 4300.13 The genetics of mitochondrial disease is complex since it may result from pathogenic variants in either mtDNA or nuclear genes. While the inheritance of nuclear gene variants may be autosomal dominant,14 recessive or X linked,15, 16 mtDNA is exclusively maternally inherited.17 Furthermore, each cell contains several hundred copies of the mtDNA molecule, resulting in the concepts of ‘mtDNA heteroplasmy’ (the presence of both normal and mutated mtDNA in the same cell), and ‘heteroplasmy thresholds’ (the percentage heteroplasmy of a pathogenic variant required to cause mitochondrial dysfunction) and ‘homoplasmy’ (the presence of a homogenous population of mtDNA in a cell). Currently, there are thought to be ~400 nuclear and mtDNA genes that are associated with PMDs and with the advent of next generation sequencing, this number continues to rise.18 This genetic heterogeneity poses considerable challenges in terms of diagnosis but also for developing treatments.
Presently, there are no approved disease modifying treatments for most PMDs. A few disorders respond positively to specific vitamins or co-factors including SLC19A3 deficiency which responds to biotin and thiamine supplementation,19 ACAD9, FLAD1 and riboflavin transporter deficiencies which respond to riboflavin20, 21 and COQ2, COQ6 and COQ8B deficiency have responded to high-dose coenzyme CoQ10 (CoQ10) supplementation.22 In addition, several experimental small-molecule therapies are being trialled in patients with PMDs, although none of these address the primary genetic cause. Of these, only idebenone, a redox modulator and CoQ10 analogue, has been approved in Europe for Leber Hereditary Optic Neuropathy (LHON)23 and omaveloxolone (Skyclarys), which acts through a combination of activation of Nrf2 and inhibition of NF-κB, has recently received FDA approval for Friedreich Ataxia.
1.3 Genetic therapies
Gene therapy can be defined as a treatment which corrects the underlying genetic abnormality in affected cells or individuals with a monogenic disorder. The type of gene therapy applicable to PMDs is dependent on whether the genetic defect is in nuclear DNA or in mtDNA.
For nuclear gene defects, conventional ‘gene replacement’ approaches deliver a ‘correct’ (wild-type [WT]) copy of a mutated gene (gene of interest/ transgene) to the nucleus of target cells. The WT gene would then reconstitute normal protein function. This approach would be applicable to recessive disorders since the effect of gain-of-function pathogenic variants would not be abolished by the expression a WT copy of the gene. Another type of gene therapy is RNA-based therapy. Instead of providing the transgene in the form of DNA, an RNA version of the same therapeutic sequence can be provided. RNA therapies can function in a similar way to gene reconstitution or they can be engineered to ‘knockdown’ or ‘turn-off’ expression of other genes, for example through small interfering RNAs (siRNAs) or increase expression through small-activating RNAs. A third form of gene therapy is gene-editing, which aims to correct pathogenic variants in existing genes within cells without introducing new full-length copies of the target gene. This includes CRISPR-Cas9 gene editing to cut and recombine DNA and base editors.
For PMDs due to pathogenic variants in mtDNA, gene reconstitution approaches can also be undertaken, with the modification that the transgene is expressed within the nucleus, translated by cytosolic ribosomes and the protein product is modified to contain a mitochondrial targeting sequence (MTS) to enable mitochondrial import; an approach known as ‘allotopic expression’. However, it should be mentioned that mitochondrial import and correct assembly into OXPHOS complexes of allotopically expressed mtDNA-encoded proteins requires further experimental validation. Another method is the use of engineered enzymes such as zinc finger nucleases (ZFN), transcription activator-like effector nucleases (TALENs) and meganucleases to specifically recognise and introduce double stranded (ds) breaks in DNA containing pathogenic variants. While the induction of dsDNA breaks in conjunction with homologous recombination (HR) of a correct copy of a gene could, in principle, be applied to nuclear DNA in the context of treating PMDs, so far this approach has only been utilised to target and degrade mtDNA molecules which contain pathogenic variants resulting in a shift in heteroplasmy toward WT molecules. However, this technique cannot be applied to homoplasmic variants since this would cause mtDNA depletion. While CRISPR-Cas9 gene editing has not yet been adapted to target mtDNA variants associated with PMDs due to inefficient RNA import by human mitochondria, newer CRISPR-free base-editing technologies have been able to edit individual mtDNA bases without the need to import guide RNAs across the mitochondrial membranes.
Two different methods of gene therapy delivery are available. It may be accomplished in vivo where it is administered directly to the affected individual or ex vivo where relevant cells, for example, haematopoietic stem cells, are removed from the patient, treated with gene therapy and then infused back into the individual.24 For in vivo gene transfer various routes of administration can be exploited depending on which organ has to be targeted. For instance, for peripheral organs such as the liver, the intravenous (IV) route is preferentially used, intra-ocular or intracochlear routes for eye and ear disorders, respectively, while IV, intracranial or intrathecal routes can be used for targeting specific areas of the central nervous system (CNS).25 The choice of which delivery approach to take thus depends on the nature of the disease to be treated, including which organs are to be targeted.
The rationale for applying gene therapy to PMDs stems from them being usually cell-autonomous disorders, hence the missing gene product is not a secreted protein/enzyme and disease is not due to accumulation of a harmful substrate. Replacement of the missing protein into the circulation is unlikely to cross-correct disease in tissues so these disorders are not likely to be amenable to enzyme replacement therapy or bone marrow transplantation. An exception to this is mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) caused by biallelic pathogenic variants in TYMP where disease is in part due to harmful accumulation of thymidine (Thd) which can be cleared by enzyme replacement of the missing enzyme thymidine phosphorylase (TP) using allogeneic haematopoietic stem cell transplantation.26 Furthermore, in some PMDs, for example, DGUOK deficiency,27 TYMP deficiency,28 and ETHE1 deficiency,29 liver transplantation has had some success but does not correct disease in the brain.30 Gene therapy has the potential to target several organs simultaneously including the CNS, making it attractive since PMDs typically involve several different organ systems.
In this review, we will discuss advances in gene reconstitution approaches to treating PMDs, most of which are still in preclinical development. We also discuss recent developments using gene editing to manipulate both nuclear and mtDNA genes that are implicated in PMD. Finally, we also discuss the challenges of clinical translation of gene therapies for PMDs.
2 METHODS
2.1 Literature search terms
A literature search was performed in the Pubmed database on 2 January 2023 utilising the following search terms: 1. Gene Therapy (Title/Abstract) AND Mitochondrial Disease; 2. Mitochondrial disease AND Gene Editing. Some papers were also identified as secondary sources cited by papers identified in the literature search.
3 GENE THERAPY APPROACHES
3.1 Non-viral strategies
Non-viral approaches include physical methods of cell membrane bombardment (hydrodynamic injection of DNA, biolistic methods),31, 32 chemical methods (micelles of cationic surfactants, rhodamine nanoparticles, liposomes)32-34 and harnessing endogenous import machinery via MTS mediated import via the translocator of outer/inner membrane complexes into mitochondria.35 Many of the non-viral methods are at preliminary in vitro stages and are limited by poor transfection efficiency, weak specificity to mitochondria or failure due to cytotoxicity.36 Therefore, none of these strategies has advanced further in the translational pipeline for PMDs.
3.2 AAV strategies for mitochondrial disorders due to nuclear gene defects
Viral vectors are more efficient at achieving cell transduction than non-viral methods. Viral vectors are engineered to retain the scaffold of a naturally occurring virus, replacing the endogenous viral genome with the gene of interest, but retaining the virus's ability to infect cells efficiently. The most promising gene therapy vectors at present are recombinant adeno-associated viruses (rAAVs). Naturally occurring AAVs belong to the parvovirus family and do not usually cause disease in humans. They require co-infection with another replication competent virus such as adenovirus37 without which they will assume a state of latency by either integrating their genomes into a specific region of chromosome 1938 or remain episomal within the nucleus.39 The life cycle of naturally occurring AAVs is shown in Figure 1A. Naturally occurring AAV occurs in ~12 different serotypes each reflecting a unique pattern of tissue tropism (targeting).40, 41 Comparisons of IV biodistribution of AAV1-9 in adult mice has shown overlapping tissue tropism across the different serotypes.40 There are also several novel engineered AAV serotypes with superior ability to transduce certain tissues.42, 43 Tissue tropism of rAAVs is illustrated in Figure 1B.
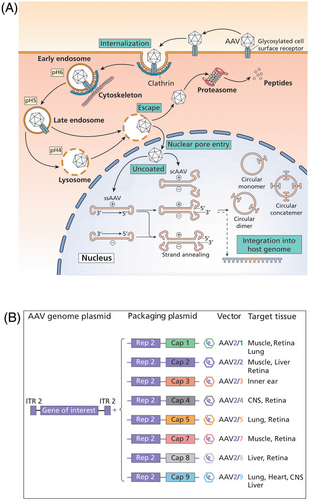
rAAVs are designed with the purpose of delivering a therapeutic transgene within an expression cassette instead of the original AAV genome and therefore lack the ability to replicate in the host. rAAVs have a packaging capacity that is limited to about 4.7 Kb. Promoter/enhancer sequences upstream of the transgene recruit host transcription factors within the nuclei of transduced cells thereby determining the transcriptional activity in the target cell type. Transgene expression is therefore determined by several variables including the species/strain of the subject,44 route of delivery,45 age at delivery,46 serotype of AAV, promoter47 and dose. Self-complementary AAVs (scAAVs) enable more rapid transgene expression since the cassette contains the transgene and its complement in cis thereby bypassing the need to undergo second strand synthesis.48
AAV-based gene therapy has been used to treat mouse models of PMDs caused by pathogenic variants in nuclear genes. This includes NDUFS3 deficiency,49 NDUFS4 deficiency,45 SURF1 deficiency,50 TYMP deficiency,51 TK2 deficiency,52 MPV17 deficiency,53 OPA1-dominant optic atrophy,54 AIFM1 deficiency,55 ANT1 deficiency,56 ethylmalonic encephalopathy,57 SLC25A46 deficiency,58 and Barth syndrome,59 as detailed in Table 1.
| Disorder | Human phenotype/animal model | Serotype-promoter-transgene | Route | Dose | Age | Target organ | Outcome | Ref |
|---|---|---|---|---|---|---|---|---|
| AIFM1 deficiency | Human phenotype: encephalomyopathy, peripheral neuropathy, demyelinating leukodystrophy. Mouse model: KO ‘Harlequin’ mice show phenotypic variability, ranging from early-onset ataxia associated with mortality to attenuated disease. 40% of KOs developed optic nerve atrophy in addition to neurological abnormalities. KOs developed poor weight gain and reduced survival as a group but in about 30% of cases these features were not seen. Most deaths occurred within the first month of life. Survivors developed progressive ataxia from 3 months. Complex I deficiency, decreased NDUFB8 expression seen in several brain regions, optic nerve, retina, skeletal muscle and to a lesser extent in kidney and spinal cord. There was also RGC degeneration and retinal neuroglobin deficiency. |
AAV2-CMV-mAifm1 | Ivit | 2.25 × 108, 7.1 × 108 vg/eye | 4–8 weeks | Eye | Dose-dependent improvement in complex I activity in optic nerves of injected eyes, preservation of RGC integrity, prevention of axonal losses and microglial inflammatory responses within the optic nerve, compared to untreated fellow eyes. | 55, 60, 61 |
| AAV2-CMV-mNgb (neuro-globin) or AAV2-CMV- mAifm1 | Ivit | 2 × 109 vg/eye or 5 × 108 vg/eye |
~16 weeks | Eye | Regardless of vector used, treated mice showed improvement in retinal electrophysiological performance (VEP/ERG) and in optomotor tracking. There was also reduced gliosis (GFAP expression) in retinas of gene therapy treated mice, rescue of complexes I and IV deficiency in optic nerves. While RGC cell counts did not improve, their connectivity was preserved. | 62 | ||
| ANT1 deficiency | Human phenotype: myopathy, PEO, ptosis Mouse model: loss of ANT1 expression, increased COX, NADH dehydrogenase, SDH histochemical staining in skm, morphological abnormalities of muscle fibres, ragged red fibres. Reduced ATP export from mitochondria |
AAV2-CMV-mAnt1 | IM | 2 × 109 infectious units/mouse | Birth | Skm | Improved ANT1 protein expression, amelioration of ATP export from skm mitochondria, correction of histochemical abnormalities and reduction in number of ragged red fibres seen. | 56 |
| ETHE1 deficiency | Human phenotype: Elevated levels of thiosulphate and inhibition of SCAD resulting in the formation of ethylmalonic acid, inhibition of COX activity and vascular endothelial damage. Early onset fatal necrotic and haemorrhagic brain lesions. Mouse model: The Ethe1 KO mouse model recapitulates the human phenotype closely since it shows extremely low liver SDO activity, elevated plasma thiosulphate and ethylmalonate levels and Complex IV deficiency in muscle and brain. KO animals have reduced survival (median 26 days). Animals can be kept alive using NAC from P18-P21. |
AAV8-TBG-hETHE1 | IV | 4 × 1013 vg/kg | 3 weeks | Liver, brain, skm | Dose-dependent improvement in liver ETHE1 protein expression, SDO activity, correction of plasma ethylmalonate and thiosulphate levels that correlated with survival. Improvement in brain and skeletal muscle complex IV activity and histochemical staining. Rescue of OXPHOS function in skm and brain likely to have been due to good liver transduction restoration of circulating H2S levels. KO mice survived without NAC. Survival correlated with liver transduction. | 57 |
| MPV17 deficiency | Human phenotype: neonatal/infantile-onset hepatocerebral disease Mouse model: Mpv17 KO mice have severe mtDNA depletion in liver, biochemical evidence of liver mitochondrial dysfunction, and lactic acidosis but normal survival. Administration of a high-fat (ketogenic) diet worsens the liver disease phenotype causing fulminant hepatic failure and mortality within 2 months. Importantly, no brain abnormality was demonstrated which implied insufficient modelling of neurological disease. However, the model also developed disease in other organs not relevant to the human phenotype including inner ear dysfunction, degeneration of the organ of Corti causing sensorineural hearing loss, and renal disease (focal segmental glomerulosclerosis). |
AAV8-TBG-hMPV17 | IV | 4 × 1012, 4 × 1013 vg/kg | 8 weeks | Liver | Exclusive liver transduction and normalisation of liver mtDNA copy number, rescue of RNA expression of mtDNA encoded transcripts, CI,CIII,CIV OXPHOS activities in liver, and serum ALT and AST levels. Prevention of ketogenic-diet induced cirrhosis resulting in improved survival. Similar improvement seen when AAV8 gene transfer was delivered at the higher dose following induction of liver cirrhosis, indicating ability to rescue this phenotype following symptom onset. Liver mtDNA rescued only to ~30% of WT levels, but this was sufficient to reverse liver disease. | |
| NDUFS3 deficiency | Human phenotype: Leigh syndrome Mouse model: KO mice developed weight loss from 3 months and all died by 15 months. Behavioural studies showed decreased ambulation and exercise tolerance on open field, treadmill and rota-rod testing respectively. Biochemistry: increased blood lactate, decreased complex I activity and increased complex IV activity in skeletal muscle. Histology: increased SDH and COX staining indicating compensatory upregulation. EM: abnormal cristae and lipid deposition. Western blot: upregulation of PGC1α indicating increased mitochondrial biogenesis and increased COXI and SDHA subunit expression. While this model presents an excellent model of skeletal muscle disease, the phenotype differs from that observed in human patients, where biallelic NDUFS3 variants have been associated with Leigh syndrome rather than a predominantly myopathic phenotype. |
AAV9-CMV-Ndufs3 | IV | 1.25 × 1015 vg/kg | 15–18 days, 2 months | skm | Complete restoration of body weight, muscle bulk, normalisation of behaviour (locomotion, exercise tolerance). Survival was greatly improved. Improvement of lactic acidosis, complex I activity in skeletal muscle and normalisation of COX activity, and reversion of the SDH/COX histochemical abnormalities seen in KOs. In follow-up, NDUFS3 expression persisted to 15 months in gene therapy treated KOs. | |
| NDUFS4 deficiency | Human phenotype: Leigh syndrome, mortality by 2 years. Mouse model: KO mice appear healthy initially but then develop poor growth, ataxia, motor dysfunction on rota-rod testing and progressive encephalomyopathy at ~5 weeks of age resulting in mortality by 7 weeks, consistent with human phenotype. Biochemical analysis in liver and brain showed complex I deficiency and KOs also develop lactic acidosis. |
AAV9-CMV-hNDUFS4 | IV + ICV | 3 × 1011, 2 × 1012, vg/mouse | Birth | Brain, skm, heart | IV delivery was able to target the visceral organs but not the brain and the converse was seen with ICV delivery. Dual route delivery was found to be superior to single IV or ICV delivery in ameliorating complex I deficiency in both brain and visceral organs, gait, rotarod assessments and survival which were demonstrated in a dose-dependent manner. Survival improved but not normalised | |
| AAV9-CMV-hNDUFS4 | IV ICV | IV: 1.2 × 1012 vg/mouse ICV: 1.5.3 × 1011 vg/mouse |
3 weeks | Brain, skm, heart | Greatest efficacy demonstrated with dual IV + ICV delivery. Reconstituted NDUFS4 protein expression and complex I activity in skeletal muscle, brain and heart. Rescue of body weight, improved rota-rod, and survival. | 45 | ||
| AAV PHP.B-CMV-hNDUFS4 | IV | 1 × 1012 or 2 × 1012 vg/mouse | Birth, 4 weeks | Brain, skm, liver | Satisfactory targeting of the brain, but only in adult gene transfer and not neonatal gene transfer. | 49 | ||
| OPA1 dominant optic atrophy | Human phenotype: optic neuropathy, RGC degeneration and loss of retinal nerve fibres, leading to reduced VA/blindness. A minority of patients also develop extraocular manifestations including deafness, peripheral neuropathy, parkinsonism, dementia (in adults) and myopathy. Mouse model: progressive loss of VA, RGC degeneration, worse in female mice. Reduced COX activity in retina. |
AAV2-CMV-hOPA1 | Ivit | 3.2 × 108, 1.6 × 1011 vg/eye | 12 weeks | Eye | Prevention of RGC degeneration, improved electrophysiology, slightly improved VA. | 54 |
| SLC25A46 Deficiency | Human phenotype: pontocerebellar hypoplasia, optic atrophy, peripheral neuropathy Mouse model: severe ataxia, difficulty feeding, reduced survival (all KOs die by 4 months). Cerebellar neuropathy including neuronal cell loss, astro- and microgliosis. Cerebellar EM: mitochondrial dysmorphology, enlargement and disorganization. Optic nerve atrophy. Peripheral neuropathy demonstrated on electrophysiology. Complex I deficiency in brain, heart, skm. COX deficiency in skm, heart. |
AAV PHP.B-CMV-Slc25a46 |
IV | 1.2 × 1014 vg/kg | Birth | Brain, periph.nerve. | Dose dependent improvement in weight and survival. Improvement in ataxic gait, posture. Histological rescue of cerebellar neuronal degeneration and EM abnormalities. Rescue of peripheral neuropathy. Amelioration of Complex I and IV deficiency in heart and skm. | 58 |
| SURF1 deficiency | Human phenotype: Leigh syndrome, median survival of 5.4 years. Mouse model: KO had prolonged survival. In skm, KOs displayed SDH and COX negative staining. COX deficiency in liver, brain, muscle, skin fibroblasts and heart. Reduced COXI subunit on western blot; complex I, II and III activities normal in all tissues. KOs had lactic acidosis in blood. Taken together, the viable model displayed more diffuse organ involvement than seen in patients but with overall decreased disease severity – prolonged survival compared to human patients. |
scAAV9-Cbh-hSURF1 | IT IV + IT |
IT: 2.8 × 1011 vg/mouse IV + IT: 8 × 1011 vg/mouse via both routes |
4 weeks | Brain, skm, liver | Dose-dependent improvement in COX activity in brain, skm and liver with no additional benefit of IT+IV administration even at higher dose. Improvement in COXI expression also observed in brain. Amelioration of exercise-induced lactic acidosis. The data suggest rescue of both brain and visceral organ mitochondrial dysfunction using IT delivery. | 50 |
| TAZ Deficiency | Human phenotype: Barth syndrome: cardiomyopathy, skeletal myopathy, neutropenia, poor feeding, growth, and 3-methylglutaconic aciduria. Mouse model: Total KO: neonatal mortality, impaired growth, skeletal, and cardiomyopathy. Fatiguability noted on treadmill test. Histology: Cardiomyocyte death and fibrosis. Abnormal mitochondria in heart and skm cells on EM. |
AAV9/ scAAV9- CBA- hTAZ scAAV2i8- cTNT- hTAZ scAAV2i8- MHCK7- hTAZ |
SC or IV | 1.2 × 1013 vg/kg | Birth, 3 months |
Heart, skm | Neonatal studies in complete KO model: AAV9/scAAV9 given SC improved survival, reduced cardiac fibrosis. Normal cardiac function maintained for 4 months but declined thereafter consistent with dropping longer term transduction. scAAV2i8 MHCK7-hTAZ (but not scAAV2i8 cTNT-hTAZ) also improved survival. Adult studies in complete KO model: AAV9 given IV at 3 months. Improved cardiac function, reversal of cardiomyopathy, reduction in fibrosis and cell death, EM abnormalities. Mild improvement in skm function, loss of skm transduction in long term |
59 |
| Cardiomyocyte-specific KO: dilated cardiomyopathy only progressing from 2 months onward. Histology as for complete KO. Normal survival. | AAV9- CBA- hTAZ |
IV | 1.2 × 1013 vg/kg | P20, 2 months |
heart | Juvenile mouse studies in cardiac-specific KO model: AAV9 given IV at P20 before cardiomyopathy onset prevented development of cardiomyopathy, fibrosis and cell death in a dose-dependent manner. Adult studies in cardiac-specific KO model: AAV9 given IV at 2 months: higher dose reversed cardiomyopathy, medium dose prevented disease progression. Dose-dependent improvement in cardiac fibrosis and cell apoptosis. |
59 | |
| TK2 deficiency | Human phenotype: Myopathic disease presenting in infants and children which normally spares the brain. mtDNA depletion in muscle. Mouse model: Tk2 knock-in (KI) mice demonstrate progressive myopathy from P10 and mortality at 2–3 weeks. KI mice showed reduced TK2 activity, and mtDNA depletion in skeletal muscle, brain, liver, heart, and kidneys, with multiple OXPHOS deficits observed mainly in the brain. There was also evidence of vacuolar degeneration and astrogliosis of the brain and spinal cord. KI mice also showed reduced locomotion on open field testing. The model differs from the human phenotype in that it has prominent neurological involvement including encephalopathy (head tremors). |
AAV9-CBA-hTK2 And AAV2-CBA-hTK2 |
IV | Neonatal AAV9: 4.2 × 1010, 4.2 × 1011 vg/mouse Neonatal AAV9 2.1 × 1011 vg/mouse + adult AAV2 1.05 × 1011 vg/mouse |
Birth, P29 |
Liver, brain, skm | Neonatal AAV9 injections showed dose-dependent partial improvement in weight and survival. Improved rota-rod performance and head tremors. Better transduction in brain and liver than in skm. TK2 enzyme activity and mtDNA copy number were rescued in these organs. Administration of a second IV dose of AAV (AAV2) at P29 was attempted to improve transduction in certain tissues, for example, liver. Higher liver transduction achieved. Overall slight improvement in survival but no significant improvement in weight compared to the higher single neonatal AAV9 dose group. IV delivery of different AAVs at two different time points undertaken without any negative effect seen in mice. |
|
TYMP deficiency |
Human phenotype: MNGIE. Leukoencephalopathy, GI dysmotility, peripheral neuropathy, myopathy, and ophthalmoplegia. Elevated blood Thd and dUrd levels. Liver mtDNA depletion. Mouse model 1: This requires additional knock-out of the gene Upp1 encoding uridine phosphorylase which can compensate for the loss of Tymp in mice. Double knock-out (dKO) mice displayed elevated Thd and dUrd levels in plasma, brain, heart, lung, muscle, spleen, small bowel, kidney and liver. TP activity was undetectable in all tissues except liver which showed 17% residual activity. Elevation of intramitochondrial dTTP was seen in both brain and liver and reduced dCTP was seen in brain, thought to be secondary to the high Thd levels. Disturbed dNTP pools caused mtDNA depletion (27–50% of WT levels in brain, with no significant differences seen in other organs). This resulted in brain complex I and IV deficiency. MRI and histology showed T2 hyperintense lesions and vacuolar degeneration in white matter indicating leukoencephalopathy. The model therefore recapitulates the neurological phenotype but not visceral disease. |
AAV8-TBG-hTYMP | IV | 2 × 1011–1 × 1013 vg/kg | 8–12 weeks | Liver | Dose-dependent increase in liver VCN, liver TP protein expression and activity. Improvement in plasma, brain, skeletal muscle, and liver Thd and dUrd levels despite persistently low TP activity in non-hepatic organs, implying that rescue of TP enzyme activity in the liver is sufficient to restore Thd/dUrd levels in other organs. |
|
AAV8-TBG- hTYMP |
IV | 2 × 1011–2 × 1012 vg/kg | 8–12 weeks | Liver | In prolonged follow-up to 21 months, liver VCNs showed a dilutional effect resulting in loss of restored enzyme activity in liver at lower doses, however sufficient liver TP activity was retained at a dose of 2 × 1012 vg/kg. Rescued plasma, liver, and brain Thd/dUrd levels. | |||
| Mouse model 2: Supplementation of the double KO model with additional oral Thd and dUrd resulted in a more overt neurological phenotype: ventriculomegaly on MRI and rota-rod abnormalities. | AAV8-AAT/HLP/TBG-hTYMP | IV | 5 × 1011–1 × 1013 vg/kg for AAV-TBG 2 × 1012, 13 for AAV-HLP/AAT |
8–11 weeks | Liver | The AAT promoter enabled the best rescue of the model. Rescued liver TP activity, plasma Thd/dUrd levels. Rescued ventriculomegaly on MRI and rota-rod testing. | 70 |
- Abbreviations: AAT, alpha 1 antitrypsin promoter; AAV, adeno-associated viral vector; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CAG, CMV enhancer + chicken beta actin/ rabbit beta globulin promoter; CBA, chicken-beta actin; CMV, cytomegalovirus; COX, Cytochrome c oxidase; cTNT, chicken troponin T promoter; d, days; dCTP, deoxycytidine triphosphate; dTTP, deoxythymidine triphosphate; dUrd, deoxyuridine; EM, electron microscopy; EMA Ethylmalonic acid; ERG, electroretinogram; GFAP, glial acidic fibrillary protein; GI, gastrointestinal; HLP, hybrid liver promoter; ICV, intracerebroventricular; IM, intramuscular; IT, intrathecal; IV, intravenous; Ivit, intravitreous; KI, knock-in; KO, knock-out; m, months; MHCK7, promoter containing enhancer/promoter regions of murine muscle creatine kinase (CK) and alpha-myosin heavy-chain genes; MNGIE, mitochondrial neurogastrointestinal encephalomyopathy; MRI, magnetic resonance imaging; mtDNA, mitochondrial DNA; MTS, mitochondrial targeting sequence; NAC, N-acetylcysteine; OXPHOS, oxidative phosphorylation; Px, postnatal day x; RGC, retinal ganglion cell; sc, self-complementary; SC, subcutaneous; SCAD, short chain acyl-CoA dehydrogenase; SDH, succinate dehydrogenase; SDO, sulphate dioxygenase; skm, skeletal muscle; TBG, thyroid binding globulin; Thd, thymidine; TP, thymidine phosphorylase; VA, visual acuity WT, wild-type; VCN, vector copy number; VEP, visually-evoked potential; vg, vector genomes.
A major benefit of an AAV-based gene reconstitution approach is that AAV vectors can transduce both dividing and non-dividing cells, and many are able to transduce a wide range of different organs. AAV8 and AAV9 have been the most frequently used vector serotypes in preclinical studies of gene therapy for PMDs due to their relatively wide tissue tropism. IV AAV 8 has been used to target the liver in ethylmalonic encephalopathy,57 MPV17,53 and TYMP deficient MNGIE.70 IV AAV9 has been used to target skeletal muscle in NDUFS3 deficiency49 and the heart in Barth syndrome.59 For disorders for which targeting a single organ is needed, customisation of the route or promoter used can help to restrict expression to the organ of interest. For example, in AIFM1 deficiency55 and OPA1 deficient optic atrophy,54 adequate transduction in the eye was achieved using a localised delivery route (intravitreal) to restrict the expression of a ubiquitously expressed promoter (cytomegalovirus [CMV]). In Barth syndrome, use of AAVs with transgene cassettes containing cardiac-specific promoters enabled specific expression within the heart.59
So far, however, these gene reconstitution approaches have not yet entered clinical trials. Possible explanations for slow clinical translation are as follows. One important limitation is that the phenotypes of animal models frequently differ from the corresponding human disease phenotypes, thereby limiting the conclusions that can be drawn regarding therapeutic efficacy. Examples of this include the MPV17 deficiency model in which mice did not develop cirrhosis or cerebral dysfunction compared to the human phenotype, and the SURF1 deficient mouse model which did not demonstrate reduced survival.50, 71 In TK2 deficiency,52 the model displayed encephalomyopathy and spinal cord disease that is not seen in patients and may explain why only partial improvement in the disease phenotype could be achieved. Conclusions regarding efficacy can thus be difficult to deduce when organ involvement is milder, since it is not possible to predict how the treatment would perform in humans, or if it is more severe or grossly different (e.g. if there is organ involvement that is not seen in humans), this may lead to an inability to detect a therapeutic effect.
A second limitation is that it can be difficult to achieve sustained transgene expression in desired tissues/organs. For the model of NDUFS4 deficient Leigh syndrome45 an invasive intracranial approach was needed to achieve adequate brain transduction. This poses an additional translational hurdle due to the route of delivery. In SURF1 deficiency,50 an intrathecal route was used instead, although the translatability of using this route to target the brain in humans with Leigh syndrome is yet to be demonstrated. In TYMP deficiency,69 vector dilution was seen in liver due to loss of transduction as the liver divides, despite adult gene transfer. This could only be sufficiently overcome by using a higher vector dose, which poses other challenges as it may give rise to toxic effects. Similar considerations apply to liver-targeted gene therapy in ethylmalonic encephalopathy.57 Multisystemic transduction is also necessary in most mitochondrial diseases and this has proved problematic in a number of preclinical studies. In AIFM1 deficiency,55 only eye manifestations were addressed, without attempting treatment of neurological and skeletal muscle manifestations. In SLC25A46 deficiency,58 brain and peripheral nerve were adequately targeted, but this was using IV AAV PHP.B which is not clinically translatable since AAV PHP.B does not transduce the human nervous system as it does in mice.
A third limitation of gene replacement approaches is the fact that a different gene therapy product would be needed for each individual disorder, making translation of this strategy for over 350 mitochondrial diseases unfeasible. In all but one of the AAV-based approaches, the transgene utilised was either the murine or human cDNA sequence of the knocked-out gene. The exception to this is the delivery of a vector containing the neuroglobin gene to mice with AIFM1 deficiency which showed therapeutic benefit.62 Neuroglobin is a mitochondrial localising protein expressed in the CNS which is thought to exert neuroprotective effects by preserving mitochondrial function. Knockdown of neuroglobin induces complex I and III dysfunction in rat retinas.72 Overexpression of neuroglobin in Aifm1 KO mice resulted in improved electrophysiological performance, reduced gliosis and better retinal ganglion cell connectivity (Table 1). It is yet to be determined whether neuroglobin overexpression may be beneficial in other neurological diseases. Since a major drawback of gene therapy pipelines is that each requires development of a bespoke therapy, the possibility of being able to treat multiple diseases with a single gene therapy product certainly warrants further investigation and independent proof of efficacy. A final consideration is the packaging capacity of AAVs which is limited to only 4.7 Kb. PMDs for which the cDNA of interest is large resulting in the payload exceeding this limit would not be amenable to AAV vector packaging.
3.3 AAV strategies for mitochondrial disorders due to mtDNA gene defects
Clinical trials of gene therapy have recently been undertaken for LHON. LHON is a PMD which causes blindness typically in males between 15 and 35 years. Several clinical trials recruited patients with m.11778G>A;p.Arg340His in MT-ND4 and used an ‘allotopic’ expression methodology (Table 2). The MT-ND4 gene is recoded according to the genetic code employed by nuclear genes, tagged to a non-native MTS and then delivered using an AAV2 vector via an intravitreal route of administration. Once the MT-ND4 gene enters the nucleus of transduced cells, it is then transcribed and the mRNA produced is translated in the cytosol, with the protein made being targeted to the mitochondrial compartment via the MTS.
| Study | Vector used | Route | Dose | Participants | Efficacy outcomes | AEs | Ref |
|---|---|---|---|---|---|---|---|
LHON NCT01267422 |
AAV2-CMV-COX10-MTS-hND4 | Ivit | 5 × 109, 1 × 1010 vg/eye | Nine children and adults, vision loss duration 1–17 y | Improvements in VA in 6/9 patients sustained at 7 years' follow-up. | Oral prednisolone given for a total of 9 weeks from 1 week before injection. No systemic AEs noted. No ocular AEs mentioned in reports | 73 |
LHON NCT02161380 Phase 1 |
scAAV2-CAG-P1-hND4v2 (P1 = MTS of P1 isoform of subunit c of ATP synthase) | Ivit | 5 × 109, 2.46 × 1010 vg/eye | 14 adults onset >12 months in 6 and <12 in 8 subjects | Improved VA in five participants, static in 8, deteriorated in 1. | Raised intraocular pressure, exposure keratitis, subconjunctival haemorrhage in one participant. Anterior uveitis in two patients-resolved spontaneously. | |
REVEAL Phase 1/2 |
AAV2-CMV-COX10 MTS-hND4 | Ivit | 9 × 109, 3 × 1010, 9 × 1010, 1.8 × 1011, vg/eye vs. untreated fellow eye | 15 adults, vision loss duration 8 –271 months | Improved VA in 6/14 patients' treated eyes. Better improvement seen in participants with disease duration ≤2 years and better baseline VA. | Most frequent intraocular AEs: anterior chamber inflammation, vitritis, ocular hypertension. Intraocular inflammation was managed with topical/oral steroids. All intraocular AEs eventually resolved. 40 systemic AEs reported over follow-up: all unrelated to treatment |
76 |
REVERSE Phase 3 RCT |
AAV2-CMV-COX10 MTS-hND4 | Ivit | 9 × 1010 vg/eye vs. sham treated fellow eye | 37 subjects ≥15 y. Vision loss duration of >6 months to 1 y | Mean VA improved in both AAV injected and sham-treated eyes. 92% of subjects showed a clinically relevant improvement in the AAV injected eye and 38% showed a clinically relevant improvement in both of their eyes. There was also an improvement in participant-reported quality of life outcomes. | Intraocular inflammation (92% of eyes) – predominantly anterior and intermediate uveitis. Topical/oral steroids used when needed. Mild rises in intraocular pressure – managed with standard topical treatment. | 77 |
RESCUE Phase 3 RCT |
AAV2-CMV-COX10 MTS-hND4 | Ivit | 9 × 1010 vg/eye vs. sham treated fellow eye | 39 subjects ≥15 y vision loss duration of ≤6 months | Over 96 weeks' follow-up, there was an initial deterioration in both AAV injected and sham injected eyes for first 24 weeks, stabilisation for next 24 weeks and then improvement thereafter. No significant differences in improvement seen between AAV injected and sham injected eyes. Significant improvement in some participant reported outcome measures, but not all. | Intraocular inflammation (74% of eyes). Topical/oral steroids were used effectively when needed. Mild rises in intraocular pressure were managed effectively with standard topical treatments. | 78 |
REFLECT Phase 3 RCT |
AAV2-CMV-COX10 MTS-hND4 | Ivit | 9 × 1010 vg/eye given unilaterally to 50 subjects (placebo of saline injected to fellow eyes) 9 × 1010 vg/eye given bilaterally to 48 subjects. |
98 subjects | Significant improvement in VA in all gene therapy-treated eyes from baseline but with greater benefits seen in patients receiving bilateral injections. However, improvement in VA was also seen in placebo injected eyes and there was no significant difference in the improvement in VA demonstrated in second eyes receiving gene therapy vs. placebo (primary endpoint). Patients also demonstrated improved perceived quality of life scores | All patients received oral steroids for 1 month following the injections. 14.3% of participants reported systemic AEs considered related to the study procedure. The most frequent were headache (4.1%), rash (3.1%), and nasopharyngitis (2.0%). Ocular AEs were reported in 77.6% of eyes, most frequently intraocular inflammation: vitritis-50%, iridocyclitis-25%, keratic precipitates-23%, and iritis-15%. Ocular AEs were more frequent in AAV injected eyes. Topical steroids were used to manage these effectively. | 79 |
RESTORE Phase 3 |
AAV2-CMV-COX10 MTS-hND4 | Ivit | 9 × 1010 vg/eye given unilaterally | 61 subjects previously enrolled in RESCUE and REVERSE in long-term follow-up | In long-term follow-up to 52 months post-onset of visual loss, there was sustained improvement of VA in both the AAV injected and sham treated eyes. Participant-reported quality of life composite score (VFQ-25) was also significantly improved compared to baseline. | N/A | 80 |
- Abbreviations: AAV, adeno-associated viral vector; AE, adverse event; CAG, CMV enhancer + chicken beta actin/rabbit beta globulin promoter; CMV, cytomegalovirus; COX, cytochrome c oxidase; Ivit, intravitreous; MTS, mitochondrial targeting sequence; RCT, randomised controlled trial; sc, self-complementary; VA, visual acuity; vg, vector genomes; y, year.
As the eye is immune privileged, relatively self-contained and accessible through intravitreal administration, this has enabled limitation of off-target extra-ocular transduction thereby limiting systemic toxicity. LHON is a suitable gene therapy target because most patients develop eye involvement only, although in rare cases they may develop additional neurological features (known as ‘LHON plus’). From a clinical trial design point of view, targeting of the eye was facilitated by a direct intravitreal route and there were also straightforward objective and disease-relevant outcome measures available to assess treatment response. Finally, it has been possible to recruit a more genetically homogenous population since the m.11778G > A pathogenic variant in MT-ND4 is responsible for approximately 60–70% of northern European and ~ 90% of Asian cases.81
The trials showed improvement in visual acuity in both AAV-injected eyes as well as sham injected eyes that were contralateral to AAV injected eyes, to a degree that far surpassed the natural history of LHON (wherein improvements in visual acuity in patients >15 years at the time of onset of visual loss does not occur). One possible explanation for the therapeutic benefit seen in sham injected contralateral eyes comes from non-human primate (NHP) studies82 in which AAV2 genomes were detected in the optic chiasm and also in contralateral retina, suggesting anterograde transport of vectors to the optic nerve, transfer across the optic chiasm to the contralateral optic nerve and then retrograde transport to the contralateral retina. However, the data are puzzling because in NHP studies, contralateral transduction was lower and short lived compared to ipsilaterally injected eyes whereas in clinical trials the degree of improvement seen in both gene therapy injected eyes and sham injected eyes was comparable. It has already been mentioned that this approach is highly controversial as import and incorporation of allotopically expressed mtDNA encoded proteins in functional respiratory complexes has not been convincingly supported by pre-clinical data.
Overall, the trials have demonstrated an improvement of visual acuity in LHON eyes carrying the m.11778G>A mtDNA pathogenic variant treated with AAV2 gene therapy surpassing that expected from the natural history of the disease. From a safety point of view, meta-analysis of 189 patients83 receiving AAV2 gene therapy (AAV2-CMV-COX10 MTS-hND4, lenadogene nolparvovec) for LHON from four different clinical trials showed that the treatment was generally well tolerated. Although intraocular inflammation was a frequent adverse event following gene therapy it frequently resolved spontaneously or with ocular corticosteroid drops. Systemic side effects were infrequent and mild. Furthermore, there was low viral load detectable in peripheral blood.
3.4 Lentiviral strategies
Retroviral vectors were some of the first vectors to be investigated in gene therapy. These are based on enveloped RNA viruses that require reverse transcription of their genome to make dsDNA following cellular transduction.84 Recombinant versions of these vectors offer several advantages including a large packaging capacity for the gene of interest,85 and integration into the host genome resulting in permanent retention of the transgene.86 Lentiviruses offer an advantage over earlier gamma retroviruses in that they transduce both dividing and non-dividing (i.e., terminally differentiated) cells.87 Their genome retains some components of the HIV1 genome but several elements of HIV1 have been removed, rendering them replication defective.88 As retroviruses, they retain the ability to integrate into the host cell genome, although at sites away from proto-oncogenes leading to a safer integration profile.89, 90 In 2020, Libmeldy (atidarsagene autotemcel), a lentiviral gene therapy product, was licensed in the EU for the ex vivo treatment of metachromatic leukodystrophy. Long-term follow-up data published in 202291 showed no evidence of clonal proliferation, providing much needed confidence in the safety of a lentiviral approach in treating neurometabolic diseases that are currently amenable to haematopoietic stem-cell transplantation.
Lentiviral gene therapy has been utilised to treat the mouse model of MNGIE. Experiments utilised an ex vivo gene transfer approach92 whereby lineage negative bone marrow cells from donor Tymp−/-Upp1−/− mice (dKOs) were transduced with lentiviral vectors containing hTYMP driven by a phosphoglycerokinase promoter. Transduced cells were infused into recipient dKOs preconditioned with total body irradiation. This resulted in a rescue of blood TP levels, lowering of Thd levels in plasma, the brain, and visceral tissues,93 as well as reversal of white matter oedema on MRI brain and spongy degenerative changes in brain,94 thereby suggesting efficacy of this approach. However, TYMP deficiency is an exception in PMDs, in that disease pathophysiology is due to a toxic elevation of Thd and deoxyuridine (dUrd) levels. This validates the use of an ex vivo lentiviral gene therapy approach to sufficiently catabolise these metabolites. This approach would not be applicable to other PMDs involving the brain, where in vivo transduction of relevant brain regions is necessary.
In summary, the benefits of a lentiviral approach include a larger packaging capacity compared to AAVs, and that vector integration would overcome vector dilution that is seen with AAVs in highly mitotically active organs. The drawbacks include that in vivo utilisation of lentiviruses has not been attempted in preclinical models of PMDs and there is a theoretical risk of insertional mutagenesis.
3.5 Destruction of mutant mtDNA to treat mtDNA disorders
3.5.1 Transcription activator-like effector nucleases
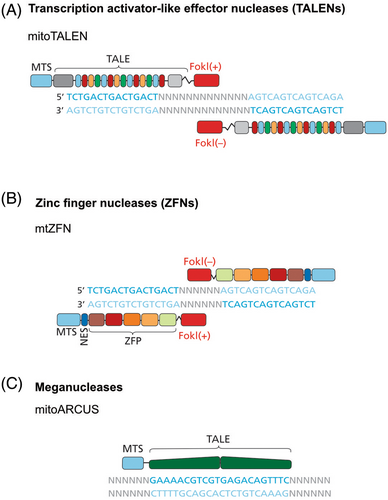
TALENs are nucleases which specifically cleave DNA of a target sequence. The TALE DNA-binding domains used in TALENs come from Xanthomonas bacteria, which invade plant cells and activate host promoters to enable efficient infection and propagation.95 The DNA sequence-specific binding of TALENs is provided by 34 aa modules. Each of these modules bind a 1-bp site through repeat variable diresidues (RVDs). By changing an RVD in each module and by linking several modules in a longer array, new DNA specificities can be achieved. TALENs are heterodimeric proteins: each monomer is designed to bind a specific target DNA sequence and is attached to a sequence-independent endonuclease domain from a type IIS enzyme FokI (Figure 2A). Successful TALEN binding and FokI dimerisation results in DNA cleavage. This is used to exploit the lack of repair mechanisms for dsDNA breaks in mtDNA, resulting in rapid degradation of cleaved mtDNA molecules.96 The aim is therefore to induce a shift in mtDNA heteroplasmy toward WT mtDNA.
Bacman et al.97 designed mitochondrially-targeted TALENs (mitoTALENs) that could be used to target mtDNA molecules containing mtDNA point mutations or large-scale deletions. To target the m.14459G>A point mutation in heteroplasmic cultured cells, a mutation-specific mitoTALEN monomer was designed recognizing the mutation site. When this monomer dimerizes with the companion monomer binding mtDNA on the opposite strand a dsDNA break is generated. The monomers do not cut WT mtDNA, as it is not recognized by the mutation-specific monomer, preventing dimerization. In another application, the mitoTALEN monomers were designed to bind WT DNA flanking either side of the 5 Kb deletion (the common deletion, m.8483_13459del4977).97 The FokI domains were therefore only in sufficient proximity to dimerize with one another in the presence of the 5 Kb deletion, cleaving mtDNA with the deletion and leaving normal mtDNA molecules intact. MitoTALEN expression within human 143B osteosarcoma cybrid cells heteroplasmic for the 5 Kb deletion resulted in a reduction in the percentage of mtDNA molecules containing the deletion and a corresponding increase in the percentage of normal mtDNA although there was also a transient reduction (depletion) in total mtDNA.
Reddy et al.98 explored the use of mitoTALEN in cells heteroplasmic for LHON with dystonia (LHOND) and neuropathy, ataxia, retinitis pigmentosa syndrome (NARP). The researchers used cybrid oocytes created from the fusion of mouse oocytes with patient fibroblasts. These cells were then fused with 143B osteosarcoma cybrid cells heteroplasmic for m.14459G>A. The resultant cells were then injected with mRNA coding for a mito-TALEN that targets the m.14459G>A variant. Analysis of cells showed a reduction in mtDNA containing the LHOND variant. A similar approach was taken to target both cybrid oocytes and an immortalised cell line with the m.9176T>C mutation associated with NARP. Utilisation of mito-TALENs targeting m.9176T>C showed a shift in heteroplasmy toward WT mtDNA at 72 h post-transfection.
Pereira et al. showed that mito-TALENs can by improved upon by utilisation of an alternative smaller monomeric nuclease (I-TevI) that can be engineered to recognise specific mtDNA variants (mitoTev-TALEs). This methodology was used to shift heteroplasmy toward WT mtDNA and rescue OXPHOS deficiency in patient-derived cybrids heteroplasmic for m.8344A>G, which is associated with myoclonic epilepsy with ragged-red fibres.99
mitoTALEN has also been applied successfully in vivo to rescue the m.5024C>T mouse model of mitochondrial cardiomyopathy. The m.5024C>T variant destabilises mitochondrial tRNAAla therefore affecting mitochondrial translation.100 Mice with the m.5024C>T variant develop reduced total body mass, cardiomyopathy, and COX deficiency in skeletal muscle and colon. Bacman et al.101 utilised AAV9 vectors to simultaneously drive expression of two mitoTALEN monomers, one of which recognises the pathogenic variant, which then dimerises with the second monomer, inducing mtDNA cleavage downstream of the variant locus. Intramuscular delivery of each AAV9 vector at 1.0–1.5 × 1012 vg/mouse resulted in restoration of WT mtDNA as well as tRNAAla expression. IV administration of the AAV9 vectors to juvenile mice at 2–3 weeks' age and intraperitoneal injections in neonatal mice at the same dose per mouse resulted in good transduction of both skeletal muscle and heart tissues, leading to a shift in heteroplasmy toward WT mtDNA without inducing mtDNA depletion in these organs.
3.5.2 Zinc finger nucleases
ZFNs are another type of nuclease that has been used to cleave mutant mtDNA molecules. ZFNs are composed of modular and engineerable, sequence-specific DNA-binding zinc-finger peptides linked to a FokI domain. To create DNA DSBs, the two ZFN monomers must be in close proximity, enabling FokI to dimerize (Figure 2B). Minczuk et al.102 reported an MTS-equipped mitochondrially targeted version – mtZFNs. Similarly to mitoTALENs, the dimeric architecture of mtZFNs allows for targeting of both mtDNA point mutations and deletions. In line with this, mtZFNs have been used to shift mtDNA heteroplasmy in cells with the m.8993 T>G point mutation and the 5 Kb common deletion (m.8483_13459del4977).103, 104 In these experiments, cells stably or transiently transfected with mtZFNs showed a substantial shift in heteroplasmy towards WT mtDNA, followed by the repopulation of mtDNA copy number, with the changes in the mutant level being stable over time and leading to improvements in mitochondrial function.
Following extensive experimentation in vitro, Gammage et al.105 investigated mtZFNs in vivo using the established m.5024C>T mouse model via an AAV vehicle. This therefore required co-expression of both monomers, each via a separate AAV9.45 vector (cardiotrophic serotype). A library of 24 possible ZFNs was generated to target the pathogenic variant. Based on in vitro data, the best pair of monomers was selected for in vivo studies. Next, AAVs were constructed to contain two different cassettes: one for each monomer, under control of a CMV promoter. The AAVs were administered to the mice IV at 5 × 1012 vector genomes (vg)/ mouse and then the animals were followed up until 65 days post-injection. This resulted in a specific reduction in mutated mtDNA in the heart of the animals as compared to skin fibroblasts taken from ear notching before injections were performed. Comparison of heteroplasmy levels in skin and heart was possible because the model has similar levels of mutant mtDNA heteroplasmy across these tissues. Treated animals showed rescue of mitochondrial tRNAAla stability in heart, improvements in tissue lactate, pyruvate and aspartate. Higher doses of 1 × 1013 vg/mouse resulted in induction of mtDNA depletion implying that careful dose selection will be essential for clinical translation. Importantly, no off-target effects on the nuclear genome were observed.
3.5.3 Meganucleases
Zekonyte et al.106 used a mtDNA targeted meganuclease called mitoARCUS to target the m.5042C>T variant in mice, utilising an AAV9 vector delivery method. The meganuclease is based on a bacterial endonuclease homodimer which can be engineered to create site-specific dsDNA breaks (Figure 2C). As for ZFNs and TALENs, creation of site-specific dsDNA breaks enables removal of mutant mtDNA molecules and a shift in mtDNA heteroplasmy favouring WT mtDNA molecules. Initial in vitro studies using mitoARCUS in mouse embryonic fibroblasts carrying the m.5042C>T variant showed a large reduction in mutant mtDNA content. However, total mtDNA also became significantly depleted and only restored to normal levels after 21 days. Next, AAV9 vectors containing the mitoARCUS coding sequence were injected IV into juvenile mutant mice at 2.5 weeks and adult mutant mice at 6 weeks. For juvenile mice, there was a progressive decrease in mutant mtDNA content in heart, skeletal muscle, kidney and liver, with no significant total mtDNA depletion over a period of 6 months' follow-up. In adult injected mice, stronger cassette expression was seen in heart and liver and almost complete elimination of mutant mtDNA in liver which was sustained in follow-up. Elimination of mutant mtDNA molecules was associated with significant improvement in liver expression of mt-tRNAAla compared to mice injected with an AAV9-GFP control. These data suggest that this methodology holds promise and warrants ongoing development.
3.6 Gene editing for mitochondrial diseases
3.6.1 CRISPR-Cas9 gene editing
CRISPR-Cas9 gene editing has enabled editing of nuclear-encoded genes. Editing genes encoded by mtDNA is thus far not possible due to inability to efficiently import editing components, specifically the guide RNA (sgRNA) across both mitochondrial membranes.107, 108
CRISPR-Cas9 gene editing has been applied to an iPSC model of OPA1-dominant optic atrophy.109 iPSCs were derived from patient fibroblasts containing the c.1334G>A; p.Arg445His pathogenic variant in OPA1. sgRNAs were constructed to enable Cas9-mediated dsDNA breaks proximal and distal to position c.1334 while ssDNA templates containing the WT sequence were introduced by transfection to the iPSCs. Correction of the variant was confirmed by Sanger sequencing and clones were cultured to assess functional outcome measures. This included rescue of mitochondrial network morphology and cell oxygen consumption rates, and restoration of staurosporine-induced apoptosis and mtDNA copy number to WT control levels.
A trial of an in vivo CRISPR-Cas9 gene editing approach110 was undertaken to integrate hTYMP into an intron of either the endogenous Tymp gene in the mouse model or the gene encoding albumin (Alb), thereby placing the transgene under direct control of the endogenous Tymp and Alb loci promoters respectively. This was achieved by delivering hTYMP cDNA within an AAV8 vector and delivering CRISPR/Cas9 either as mRNA contained within lipid nanoparticles or DNA via another AAV8 vector. Treated mice showed long-term restoration of TP activity in blood and a decrease in levels of Thd and dUrd to normal levels, with the best efficacy seen when using the lipid nanoparticle method of delivering CRISPR/Cas9 mRNA. These data expand the possible methods by which AAVs and newer delivery systems can achieve long-term transgene expression in liver in mitochondrial disease.
CRISPR-Cas9 gene therapy in the context of PMDs is still in its infancy. More in vivo work is needed using existing well characterised preclinical models to assess its application to PMDs caused by pathogenic variants in nuclear genes. As stated previously, it would not be applicable to disorders caused by point mutations and large-scale deletions/rearrangements of mtDNA due to the difficulty of importing guide RNA across the mitochondrial membranes. Off-target editing also remains a risk.
3.6.2 CRISPR-free gene editing
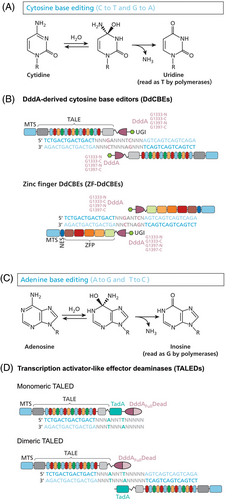
Another promising technology is a cytidine deaminase which enables CRISPR-free dsDNA base editing.111 DddA is a cytidine deaminase which converts cytosine to uracil in dsDNA, leading to C>T base changes. In nature, it is part of a secreted bacterial toxin produced by Burkholderia cepacia to induce mutagenesis in neighbouring bacteria. Delivery of the intact protein to HEK293T cells caused toxicity in vitro, but when DddA is split into two inactive parts, it is less toxic than if delivered to cells in its natural state. Early in vitro experimental work demonstrated that this split-expression methodology can induce base editing of cytosine residues that follow thymine. To achieve mitochondrial gene editing, DddA halves were fused to TALE array proteins containing an MTS. These DddA base editors were termed DddA-derived cytosine base editors (DdCBEs). (Figure 3). This enabled site-specific mtDNA editing of several genes in vitro, including MT-ND1, MT-ND2, MT-ND4, MT-ND5, and MT-ATP8 with efficiencies of up to 40%.
Building upon the DdCBE technology further, Silva-Pinheiro et al.112 showed that it is possible to induce de novo variants at specific desired loci in mtDNA in vivo in somatic tissues. This was achieved using AAV9.45 vectors encoding the DdCBE monomers to target the heart of neonatal and adult mice via IV delivery. In follow-up, for both neonatal and adult mice, high levels of edited mtDNA molecules were detectable in mouse heart tissue, with better efficiency seen in neonatally injected mice. However, significant off-target editing was seen in mtDNA, especially in neonatal compared to adult-injected mice, implying a need for further refinement of this technology to improve specificity.
Following an initial proof of concept for mtDNA editing in mouse embryos using custom-designed DdCBEs,113 Lee et al. used DdCBEs to base edit the mt-Nd5 gene at a single-cell embryonic stage and generate transgenic mice harbouring the m.12918G>A variant114 (analogous to the m.13513A>G variant in humans which is associated with mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes [MELAS] and Leigh syndromes). Furthermore, co-injecting mitoTALENs to degrade any unedited mtDNA molecules increased % heteroplasmy for the induced pathogenic variant. Mutant mice developed weight loss, ultrastructural abnormalities in mitochondria on electron microscopy and histological abnormalities in brain including ventriculomegaly and hippocampal atrophy.
Single-cell embryo DdCBE injection was used also by Silva-Pinheiro et al.115 to introduce the m.8069G>A nonsense variant to inactivate the mouse Mt-Atp6 gene. This work was a part of the effort to generate a library of DdCBEs – MitoKO – optimized for the precise ablation of each mtDNA protein-coding gene in the mouse mitochondrial genome.
Mok et al.116 refined the design of DdCBEs to broaden the sequence context of base editing, as well as improve editing efficiency. These new DdCBE variants were able to edit C residues that follow any nucleotide, not just T. The group demonstrated that new DdCBEs could be used in mutagenesis to create mtDNA variants in human cells in the MT-ND4 gene that are associated with LHON such as m.11696G>A which is in an AC sequence context.
Further work with DdCBEs led to the development of zinc finger DdCBEs (ZF-DdCBEs)117 and a subsequent improvement of their editing performance through engineering their architectures, defining improved ZF scaffolds, and installing DddA activity-enhancing mutations.118 Leveraging the small size of ZF-DdCBEs, Willis et al.118 used a single AAV9 vector (rather than two vectors necessary for the delivery of TALE-based DdCBEs) to install pathogenic variants by base editing into heart, liver, and skeletal muscle in postnatal mice.
Cho et al.119 broadened the approach even further by designing (TALE)-linked deaminases, which link to an adenine deaminase rather than cytidine deaminase (Figure 3). Adenine deaminases convert adenine residues to inosine which is paired with cytosine, enabling A-to-G conversions in mtDNA in human cells. This would therefore enable mutagenesis of a much larger number of known pathogenic variants in mtDNA for which A>G base changes are frequently seen in patients.
DdCBEs have not yet been utilised to correct pathogenic variants in mtDNA either in vitro or in vivo. For example, cytidine deaminase activity enabling C>T transitions on the antisense strand would enable G>A transitions of the sense strand which would be therapeutic in correcting pathogenic A>G variants. However, as discussed above, refinements of this technology to improve specificity, enable in vivo delivery and organ targeting, as well as utilise different nucleotide deaminases has expanded the therapeutic potential of this technology. What remains to be determined is whether this will be sufficient to rescue a majority of mutant mtDNA molecules of cells within desired tissues to achieve long-lasting efficacy. Furthermore, off-target base editing in either mtDNA or nuclear DNA is a theoretical possibility and would need to be interrogated in future preclinical studies.
3.7 Challenges of clinical translation of gene therapy for mitochondrial disorders
Some of the challenges to be considered in clinical translation of gene therapy include achieving adequate organ transduction, safe dosing, clinical trial design, establishing outcome measures, and recruitment of trial participants.
3.8 Achieving adequate organ transduction
PMDs frequently cause multisystemic involvement. From a gene therapy point of view, this creates a need to target multiple different organs/tissues simultaneously. The visceral organs can be targeted via the IV route but CNS involvement is a major consideration since many PMDs involve the brain.
3.8.1 Current experience using IV AAV9 gene therapy to transduce the CNS
Advances in AAV gene reconstitution approaches have enabled development of serotypes which have improved CNS transduction via the IV route. Early studies by Foust et al. using scAAV showed that it was possible to target the brain in newborn mice using IV AAV9.46 Immunohistochemical findings in this study demonstrated high GFP expression in brain following neonatal and adult IV gene transfer. However, Gray et al.120 showed that adult IV scAAV gene transfer in NHPs resulted in low brain transduction compared to mice at similar doses/kg, implying that onward clinical translation using this type of construct and route to target the brain is likely to be problematic. The rescue of the disease phenotype of SMN deficient mice121 provided confidence for using IV AAV9 gene therapy for targeting the spinal cord which led to successful clinical trials in SMA type 1.122 However, preclinical studies46, 121 also indicated that brain targeting was inferior to spinal cord targeting via the IV route. As discussed previously, AAV-based gene therapy has been applied to other mouse models of PMD. Neonatal IV AAV9 injections were unable to rescue the neurological phenotype of the NDUFS4 deficient model of Leigh syndrome.45 These studies therefore do not support the use of IV AAV9 to target the brain. Alternative approaches need to be considered.
3.8.2 Alternative AAV serotypes
Recently, newer synthetic recombinant AAV serotypes have been generated using capsid libraries by selecting vectors with enhanced CNS transduction. AAV PHP.B showed greater brain transduction compared to AAV9 via the IV route in C57/BL6 mice.123 It also rescued brain abnormalities in NDUFS4 deficient mice.124 However, biodistribution findings were not replicated in other mouse strains and NHPs, implying that clinical translation of this approach is unlikely.125, 126 Another novel serotype, AAV-F, demonstrated superior brain targeting following adult IV gene transfer in mice compared to AAV9.43 This included enhanced transduction of neurons and astrocytes. So far, confirmation of these findings in NHPs is pending, although data from a recent study comparing AAV9 to AAV-F via intrathecal delivery in NHPs showed no significant brain biodistribution differences between the two serotypes.127
Two new promising AAV serotypes, AAV.CAP-B10 and AAV.CAP-B22, have recently been described.128 AAV.CAP-B10, showed enhanced brain (in particular neuron) transduction following IV gene transfer in 3-week-old mice. IV studies in NHPs (marmosets) showed that both AAV.CAP-B10 and AAV.CAP-B22 had higher brain transgene expression than AAV9, in cortex, striatum, and brainstem among other regions. Furthermore, AAV.CAP-B22 predominantly transduced astrocytes. These new AAV serotypes provide hope that it may be possible to achieve sufficient brain transduction following IV vector administration.
3.8.3 Alternative routes of gene transfer to target the CNS
To date, the FDA has not licensed any in vivo IV gene therapy products seeking to target the brain despite many clinical trials investigating this approach.129 A majority of clinical trials instead utilise an intracranial route.130 The benefits of this approach are that they bypass the blood brain barrier enabling better transduction. The brain is an immune privileged site, implying a reduced likelihood of evoking an immune response or transduction being affected by neutralising anti-AAV antibodies.131
Possible intracranial routes are intrathecal (intracisternal, lumbar intrathecal), intracerebroventricular and intraparenchymal. Intrathecal delivery involves injection into cerebrospinal fluid either at the level of the cisterna magna or lumbar intrathecal delivery. The intracisternal route has been shown to achieve reasonably good brain biodistribution and to be effective in ameliorating neurological disease in dogs with Sanfilippo disease.132, 133 The main drawback is the high risk of injury to the medulla in humans.134 An AAV9 lumbar intrathecal approach was able to ameliorate brain mitochondrial dysfunction in murine SURF1 deficiency, however biodistributional studies in NHPs yielded conflicting results,50, 132, 135 therefore it is unclear whether this approach could be translated in humans.
The intracerebroventricular route is invasive since it requires access to the ventricular system. Intracerebroventricular AAV9 injections showed excellent biodistribution throughout the brain,133 including transduction of both neurons and glia.132, 136 This approach ameliorated the neurological phenotype in large animal models of Sly disease132 and CLN5 Batten disease,137 making it a viable option for transducing the brain.
The intraparenchymal route involves direct brain injections which enables localised delivery and reduces off-target neurotoxicity.138 Brain intraparenchymal gene therapy clinical trials are underway for AADC deficiency and Sanfilippo disease and have demonstrated efficacy in Batten disease.130, 139 The difficulty with PMDs, where multiple brain regions are affected, is that large numbers of injections are likely to be needed, reducing feasibility of this approach.
The drawbacks of the intracranial routes are that they require neurosurgical intervention, which may entail peri-operative complications, but also the possibility of local immune responses.140 Furthermore, for many PMDs where visceral involvement is also present, it is likely that IV delivery would still be needed to target these organs.
Further preclinical studies are needed utilising novel vector serotypes and/or combined routes of delivery to refine existing gene therapy approaches for PMD.
3.9 Safety considerations
Much has been learned from recent AAV clinical trials about dosing, toxicity and immunogenicity of AAVs. Toxicity of high-dose AAV in humans has generated concern in the past few years.141 High dose IV AAV9 gene therapy has been associated with severe liver failure in two patients with spinal muscular atrophy type 1 at doses of 1.1 × 1014vg/kg,142 and thrombotic microangiopathy (thrombocytopaenia, anaemia, complement activation) and acute renal impairment in two patients with Duchenne muscular dystrophy at doses ≥5 × 1013 vg/kg.143 High dose AAV8 at ≥1 × 1014 vg/kg given to patients with X-linked myotubular myopathy was associated with gastrointestinal infection, liver failure, sepsis, elevated cardiac troponin, and death in two cases even though these toxicities were not observed following administration of comparable doses in mice and NHPs.144 Scale-up of dosing required to bridge between preclinical and clinical studies is not straightforward and requires exploratory dose escalation to determine minimum effective dose.
Pre-existing humoral and T cell-mediated immunity are a well-recognised concern, with a high percentage of the healthy population demonstrating seropositivity to naturally occurring AAVs and memory T cell responses to a slightly lesser extent.145, 146 Trials typically exclude patients with detectable antibodies as part of their eligibility criteria147 since this is associated with a loss of transduction. This would reduce the number of patients who would potentially benefit from treatment.
3.10 Clinical trial design
Clinical trials of novel therapies for rare diseases are frequently not placebo controlled or blinded and therefore utilisation of natural history cohort data of historical patients is likely to be helpful.148 Trial participants could also act as their own ‘control’,149 particularly if they were already symptomatic prior to gene transfer. Identification of existing patients through mitochondrial patient registries, for example, the UK Mitochondrial Disease patient cohort150 or its international equivalents, is likely to be helpful. Administration of gene therapy vector to healthy volunteers to assess safety (phase I study) would not be expected.151 Instead, safety and efficacy are frequently undertaken together in a small number of patients, thereby fulfilling the aims of both Phase I and II together.
3.11 Establishing trial outcome measures and clinical trial readiness
Outcome measures can be defined as ‘elements that measure change in health or quality of life’.152 Consideration needs to be given to which biochemical, molecular, and clinical outcome measures could be used in clinical trial design, according to the disease phenotype. For example, in one international Delphi workshop seeking to establish clinical trial readiness in primary mitochondrial myopathies, clinical PMD experts agreed on the need to use standardised, validated, and reproducible outcome measures in clinical trials recruiting adults and children with mitochondrial myopathies.153 This included establishing consensus on which clinical scales, functional tests, performance outcome measures, patient-reported outcome measures including quality of life indices and biomarkers should be used in future trials.
Blood biomarkers that meaningfully reflect disease severity are needed, especially since tissue molecular and biochemical analyses that many preclinical gene therapy studies use to demonstrate efficacy are unlikely to be used in a clinical trial setting due to the invasiveness of biopsies. The utility of FGF21 and GDF15 has been explored in recent years154 but neither is specific to PMD and certainly not for any particular genetic defect. More exploratory biomarker work is needed and use of preclinical animal models and multi-omic approaches may help with this.12
For clinical trials of gene therapy in LHON, for example, best corrected visual acuity and optical coherence tomography have been used as outcome measures. Neurological examination and developmental outcomes could be monitored using validated scales such as the Newcastle Paediatric Mitochondrial Disease Scale.155 Although some of these scoring systems were not developed for mitochondrial disorders, they may still be able to accurately capture neurological features but would ideally need to be validated for this patient cohort. In a clinical trial of idebenone in MELAS, the Fatigue Severity Scale score in addition to venous and cerebral lactate (on MR spectroscopy) were used as outcome measures (NCT00887562). Finally, natural history data could be utilised in view of the likely lack of a control arm in future trials but this is likely to only be useful if data are collected prospectively in age and disease-severity matched patients.148
3.12 Recruitment of trial participants
Eligible patients would need to be identified for a gene therapy clinical trial. Many PMDs present with classical clinical phenotypes for which the differential diagnosis could include several different underlying genetic causes. Phenotypes may overlap with non-mitochondrial diseases thereby making a genetic diagnosis essential to determine eligibility for gene therapy. In recent years, this has been made possible through next generation sequencing which is the preferred diagnostic modality.
PMDs are individually ultra-rare; therefore, the number of patients who could be recruited to a gene therapy clinical trial would be small. Patient recruitment will therefore require international collaboration to recruit as many patients as possible via international patient registries. Inclusion criteria for a trial will need to be agreed. In future, should newborn genomic screening be made available, this may lead to early identification of a larger number of individuals who could benefit from gene therapy.
4 FINAL CONSIDERATIONS
In future, while different gene therapy products will share common design elements, the presence of a different therapeutic transgene or sequence elements will necessitate separate regional regulatory approvals for each gene therapy product. This is because each could potentially possess a different toxicological profile and the merits of different preclinical efficacy data packages would need to be appraised individually. This is likely to introduce delays in approval but is an essential regulatory step. Other challenges in clinical translation include a need to attract adequate investment from pharmaceutical companies. For some rare diseases, this is a real concern, especially since there may be few patients who could benefit from the therapy because of low disease prevalence and high early childhood mortality that is typical of many PMDs. There are also concerns about the affordability of licensed gene therapy products by global health budgets and equitable access to therapies for those who need them, especially in countries with large socioeconomic disparities.
5 CONCLUSION
Advances in gene therapy design have resulted in an explosion in preclinical AAV-based gene-reconstitution approaches in preclinical models of PMDs. This has also been made possible by the creation of a wider range of disease models, especially mouse models which are able to recapitulate human disease phenotypes. However, a translational bottleneck is evident since only one of these gene therapies has entered clinical trials, that is, AAV2 gene therapy for LHON. Issues related to AAV vector toxicity at high IV doses have hampered clinical trials for other metabolic and neurological disorders and indicated a need to exercise caution. As many PMDs involve the CNS, targeting of the CNS through other routes of delivery needs to be considered. Administration of AAV via intracranial routes introduces further complexities on account of significant risks of these procedures in humans. Gene editing approaches are currently at extremely early stages of development. CRISPR-Cas9-based gene editing and newer RNA-free base editors have had limited therapeutic application so far and the possibility of off-target base editing also needs to be addressed. Nucleases that introduce dsDNA breaks into mtDNA are also still in early stages of development. Clinical translation will need to address the safety of induction of mtDNA depletion even if temporary, issues of off-target nuclease activity and whether therapeutic shifts in mtDNA heteroplasmy are sustained long term.
In future, in view of the rarity of most PMDs, any forthcoming clinical trials will require multinational collaboration. Participant recruitment, the timing of gene transfer, and the selection of relevant and accessible outcome measures will need to be considered, facilitated by a thorough understanding of the natural history of disease strengthened by prospective characterisation of relevant patient cohorts. Refinement of translational pipelines will benefit the scientific community and inspire gene therapy studies for other PMDs for which effective disease-modifying therapies are much needed.
AUTHOR CONTRIBUTIONS
Nandaki Keshavan: Concept and drafting of manuscript. Shamima Rahman: Concept, review, and editing of manuscript. Michal Minczuk: Review of manuscript, figures. Carlo Viscomi: Review of manuscript, figures. Guarantor: Shamima Rahman.
FUNDING INFORMATION
Nandaki Keshavan acknowledges funding from Action Medical Research. Shamima Rahman acknowledges grant funding from Great Ormond Street Hospital Children's Charity, the Lily Foundation and the National Institute of Health Research (NIHR) Great Ormond Street Hospital Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the United Kingdom Department of Health. Carlo Viscomi acknowledges grant funding from AFM-Telethon, France; Telethon, Italy; Department of Biomedical Sciences—UNIPD; PNRR Mission 4, Component 2, Investment 1.4-CN00000041 Spoke 1 funded by the European Union – NextGenerationEU. Michal Minczuk acknowledges grant funding from the UKRI Medical Research Council, UK; Associazione Luigi Comini, Italy; the CureMito Foundation, USA; AFM-Telethon, France; Cancer Research, UK. The authors confirm independence from the funders. The content of the article has not been influenced by the funders.
CONFLICT OF INTEREST STATEMENT
Nandaki Keshavan and Carlo Viscomi declare no competing interests. Shamima Rahman declares that she is an editor-in-chief of the Journal of Inherited Metabolic Disease and has provided consultancy on primary mitochondrial diseases for pharmaceutical companies as listed in the IJCME conflict of interest form. Michal Minczuk is a founder, shareholder and member of the Scientific Advisory Board of Pretzel Therapeutics, Inc, a company developing treatments for primary mitochondrial diseases as listed in the IJCME conflict of interest form.
INFORMED CONSENT AND ANIMAL RIGHTS
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Open Research
DATA AVAILABILITY STATEMENT
All relevant data are included in the manuscript. No additional data are applicable.




